Abstract
Background & Aims
The insulin-like growth-factor 2 (IGF2) mRNA binding protein p62 is highly expressed in hepatocellular carcinoma tissue. Still, its potential role in liver disease is largely unknown. In this study we investigated pathophysiological implications of p62 overexpression in mice.
Methods
We generated mice overexpressing p62 under an LAP-promotor. mRNA expression levels and stability were examined by real-time RT-PCR. Allele-specific expression of Igf2 and H19 were assessed after crossing mice with SD7 animals. The Igf2 downstream mediators pAKT and PTEN were determined by Western Blot.
Results
Hepatic p62 overexpression did neither induce inflammatory processes or liver damage. However, 2.5 week old transgenic animals displayed a steatotic phenotype and improved glucose tolerance. p62 overexpression induced the expression of the imprinted genes Igf2 and H19 and their transcriptional regulator Aire (autoimmune regulator). Neither monoallelic expression nor mRNA stability of Igf2 and H19 was affected. Investigating Igf2 downstream signalling pathways showed increased AKT activation and attenuated PTEN expression.
Conclusion
The induction of a steatotic phenotype implies that p62 plays a role in hepatic pathophysiology.
Keywords: PTEN, IMP, NAFLD, NASH, CMV promoter activity, p65
Introduction
During the past 20 years a rise in HCC incidence has been noticed, which is associated with metabolic risk factors like obesity, diabetes mellitus, non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH) [1].
The oncofetal protein p62 was originally identified as a 62 kDa autoantigen from a patient suffering from HCC [2]. p62 belongs to the family of IGF2 mRNA-binding proteins (IMPs) and represents a splice variant of IMP2. p62 expression is absent in adult livers, but can be found in HCC nodules and in fetal liver [3]. IMPs have been shown to be implicated in growth promotion, carcinogenesis, and tumor progression [4–6]. The interaction of p62 and IGF2 might be of special interest with regard to the tumor-promoting nature of IGF2: reduced IGF2 expression was shown to enhance survival from HCC [7], and a dysregulation of the haploidic imprinting status of IGF2 and H19 is associated with metabolic diseases and cancer development [8].
IGF2 plays a key role in mammalian growth through metabolic and growth-promoting effects [9]. The induction of downstream signal transduction pathways is mediated mainly through the activation of phosphoinositide 3 (PI3)-kinase/AKT [10] and AKT inactivation is facilitated by the tumor-suppressor PTEN (phosphatase and tensin homolog [10,11].
Since a potential role of p62 in liver disease is largely unknown, we characterized a mouse model in which p62 was exclusively overexpressed in the liver. The animals developped fatty livers at an early age, paralleled by a non-inflammatory phenotype.
Materials and Methods
Animals
All animal procedures were performed in accordance with the local animal welfare committee.
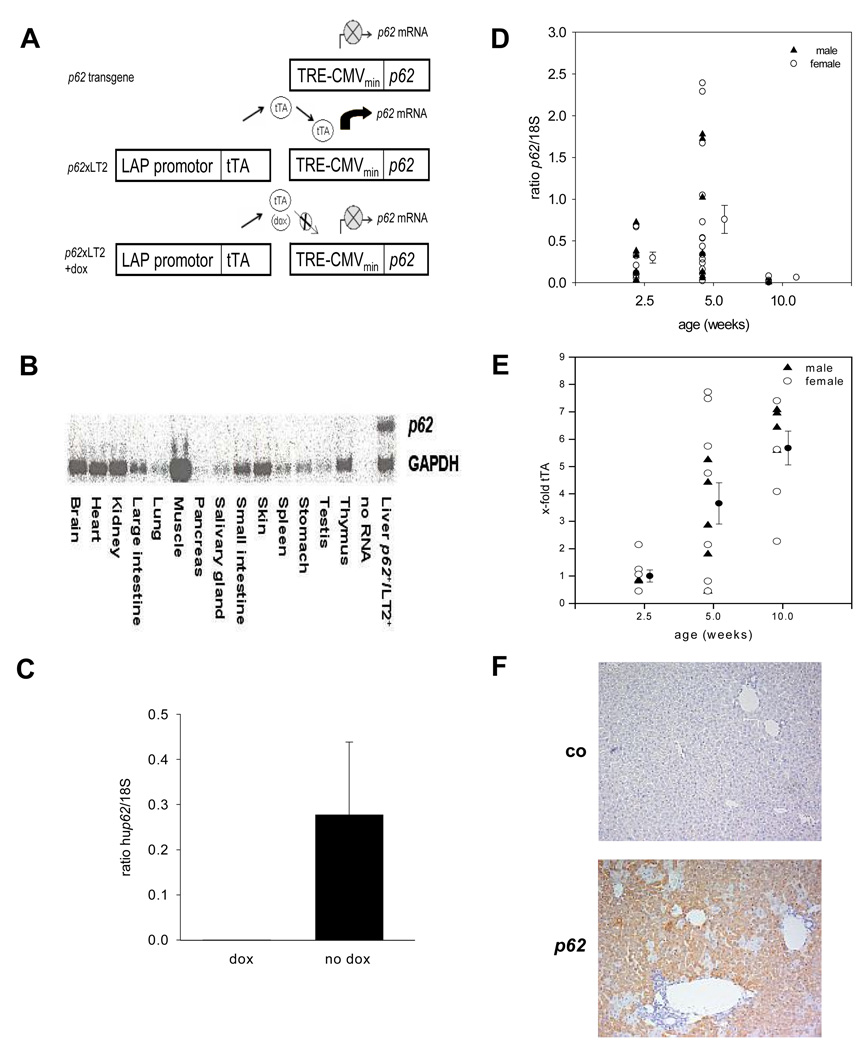
The targeting vector contained the human p62 protein under control of the transrepressive responsive element cytomegaly virus (TRE-CMVmin) promotor (Fig. 1A). In order to induce p62 expression, transgenic mice were bred with LT2 mice, which carry a liver enriched activator protein (LAP) under control of a tetracycline transactivator (tTA) [12]. Liver-specific expression of the transgene can be switched off by the application of doxycycline (Fig. 1A).
Fig. 1. Hepatic p62 overexpression.
(A) Generation of p62 transgenic mice. No expression of p62 mRNA under TRE-CMV promotor control (upper panel). Liver-specific expression of p62 mRNA in double-positive p62+/LT2+ mice (middle panel). Application of doxycycline inhibits transgene expression (lower panel). TRE-CMVmin: transrepressor responsive element cytomegalovirus; tTA: tetracycline transactivator; LAP: liver enriched activator protein; dox: doxycycline (B) p62 expression in different mouse organs. The Northern Blot detects the 2.0 kb band of p62 mRNA only in livers of p62 transgenic mice. (C) p62 mRNA expression after doxycycline (dox) administration. (D) Hepatic p62 mRNA expression (n=14/2.5 weeks, n=21/5 weeks, and n=8/10 weeks). (E) tTA expression in LT2+ mice (n=7/2.5 weeks, 12/5 weeks, and 8/10 weeks). (F) p62 immunohistochemistry.
p62+/LT2+ (p62) were compared to p62 −/LT2+ (co) in all experiments. Primer sequences for genotyping are given in supplementary data.
After microinjection, two mouse lineages founded on a different background were maintained. In lineage 23, only males displayed the transgene. If not stated otherwise, experiments were performed on lineage 50 to consider gender-specific differences.
p62/SD7 mice
LT2 mice were bred to homozygous SD7 animals to produce reciprocal F2 progeny (LT2 × Mus spretus) [13]. 2.5 week old p62+/LT2+ and p62 −/LT2+ mice being heterozygous for the Mus spretus allele were analysed.
Real-time quantitative polymerase chain reaction
Experiments and quantification were performed as described in detail in [14, 15]. Sequences and conditions are given in supplementary data.
Allele-specific expression analysis using single-nucleotide primer extension (SNuPE)
Primer extension was performed employing SNuPE primers placed adjacent to the polymorphic sites. All steps are described in detail in [16]. The allele-specific expression index was assessed by calculating the ratio h(C)/[h(C)+h(T)].
IP-Glucose Tolerance Test (IP-GTT)
2.5 week old mice were starved before they were given a single i. p. injection (10 µl/g body weight) of glucose (B. Braun, Melsungen, Germany). Circulating glucose levels were measured with an Accu-Check Aviva glucometer (Roche Diagnostics, Mannheim, Germany).
Serum analysis
2.5 week old mice were starved and sacrificed. Serum levels were determined at the “Zentrallabor des Universitätsklinikums des Saarlandes” (Homburg, Germany).
Hepatocyte isolation and mRNA stability
Hepatocytes were isolated using a modified two-step collagenase perfusion method [17] with a viability exceeding 80 %.
Cells were cultured on collagen-coated plates and the next day treated with 10 µg/ml ActD at different time points [18] (see supplementary data).
Histology and immunohistology
Staining was performed either on cryosections or paraffin-embedded tissues. Detection for immunohistochemistry was done with the CSA II kit (DAKO, Hamburg, Germany).
Western blot analysis
Western blots were performed according to [19]. Antibodies used were specific to phosphoAKT (Ser473), PTEN (New England Biolabs, Frankfurt a. M., Germany and α-tubulin (Sigma, Thermo Fisher Scientific, Karlsruhe, Germany).
Statistical analysis
Groups were compared using student´ s t-test for independent, normally distributed samples. Data represent the mean ± standard error of the mean (SEM). P values less than .05 were considered significant.
Results
Liver specific expression of p62
Solely double-positive (p62+/LT2+) mice expressed the transgene in the liver (Fig. 1A+B). Doxycyclin administration abrogated p62 expression (Fig. 1C). Expression levels of p62 showed rather high interindividual variability and strongly decreased at the age of 10 weeks (Fig. 1D) although LAP activity increased with age, as shown by increased tTA expression in LT2+ mice (Fig. 1E). p62 expression is restricted to the cytoplasm (Fig. 1F).
Induction of a fatty liver in 2.5 week old p62 transgenic mice
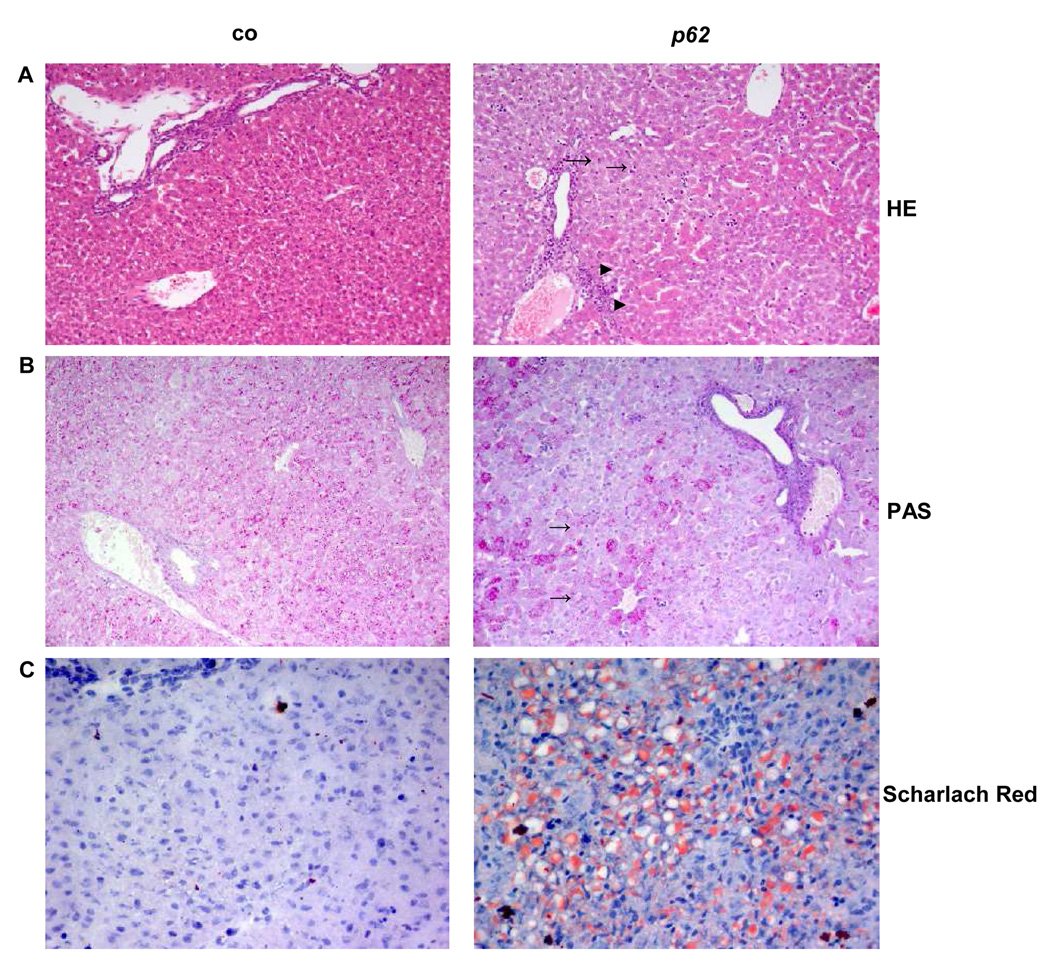
Livers displayed an accumulation of basophilic cells around the central veins (Rappaport zone 1, Fig. 2A). Leukocyte infiltration was not observed.
Fig. 2. Liver histology of 2.5 week old animals.
(A) HE-stained liver tissue. Pericentrally located basophilic cells (→) were only detected in p62 transgenic animals. (►) displays eosinophilic cells. (B) Glycogen staining. (→) show the accumulation of glycogen around the central vein. (C) Scharlach Red stained cryosections. A microvesicular distribution of fatty acids occurred in hepatocytes of transgenic animals. (co, n=11, p62, n=21). (A/B: 20×, C: 40× original magnification)
A decrease in glycogen staining indicated metabolic alterations (Fig. 2B).
Liver architecture gave hints of an accumulation of neutral lipids. Specific fat staining demonstrated a steatotic phenotype with a significant rise in fat droplets without a preferred zonal distribution in 58% of animals (Fig. 2C). Fatty livers occurred with a higher frequency in females (66%) when compared to males (44%).
Livers of animals at older age displayed no histological alterations.
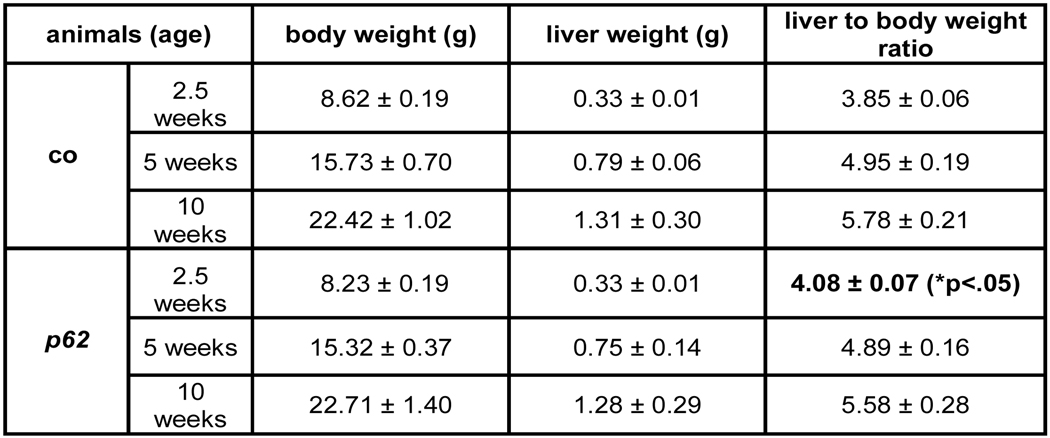
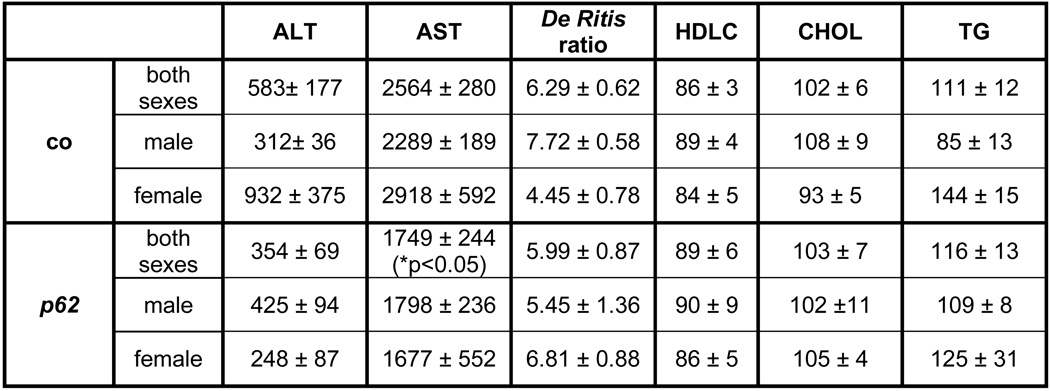
p62 transgenic animals at 2.5 and 5 weeks of age displayed a non-significant difference in liver weight, with a tendency towards lower body weights. A slight but significant increase in the liver to body weight ratio was only observed at the age of 2.5 weeks (Fig. 3A). Serum cholesterol and HDLC did not differ, neither with regard to gender nor the experimental groups (Fig. 3B). However, a slight but significant increase in triglyceride (TG) levels was found in males (Fig. 3B).
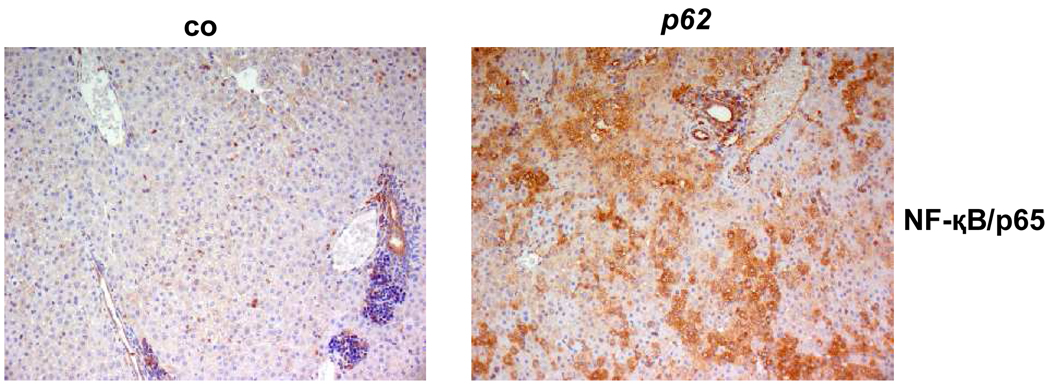
Fig. 3. Fatty liver and serum parameters.
(A) Weight parameters of 2.5 (n=co:26/p62: 29), 5 (n=co: 12/p62: 12), and 10 (n=co: 6/p62: 3) week old mice. (B) Serum parameters of 2.5 week old p62 transgenic mice (p62: n=10, of which n=6 male and n=4 female) vs. controls (n=16, of which n=9 male and n=7 female). Lipids expressed in mg/dl, transaminases expressed in U/l. (C) Immunostaining of the NF-κB subunit p65 (left: co, n=7, right: p62, n=15).
Absence of liver damage and inflammation
In order to determine characteristics of NASH, i.e. liver alterations encompassing inflammation, a potential activation/translocation of NF- κB (p65 subunit), which plays a pivotal role in the inflammatory response, was determined.
Nuclear p65 in immune cells, indicating inflammatory activity, was detected to a very low extent in both experimental groups (Fig. 3C). Interestingly, however, an increased cytoplasmic staining of hepatocytes was revealed in 80% of transgenic animals compared to controls.
The lack of an increase in serum transaminases underlined the absence of inflammation (Fig. 3B).
Increased expression of Igf2 and H19
Since p62 belongs to the family of IGF2 mRNA-binding proteins and due to the fact that IGF2 shares an imprinting control region with H19 [20], potential expression changes were determined.
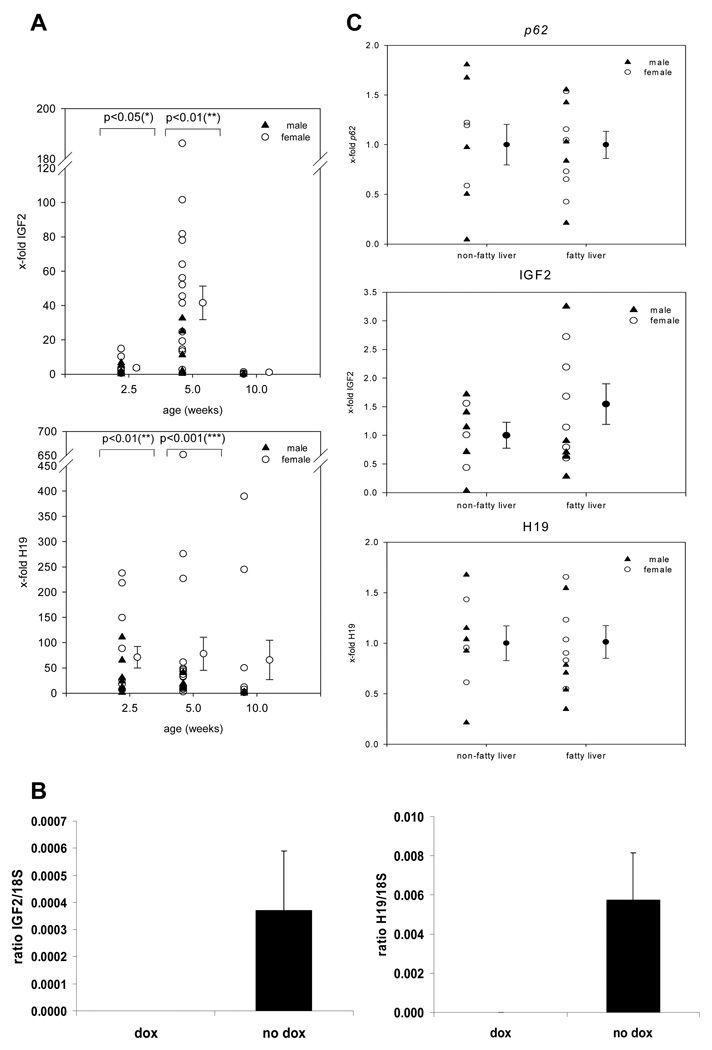
A significant upregulation of Igf2 and H19 could be shown (Fig. 4A). This effect of p62 overexpression on Igf2 and H19 was not due to genetic predisposition: after administration of doxycycline, Igf2 and H19 levels declined, also seen in another lineage (Fig. 4B and supplementary Fig. 1). p62 transgenic females displayed higher Igf2 and H19 mRNA expression levels in comparison to males, further supported by the detection of a lower p62 expression in another lineage, where only males express the transgene (Fig. 4B and supplementary Fig. 1). When we grouped 2.5 week old animals into fatty liver and non-fatty liver transgenic mice neither differences in p62 nor H19 expression were observed (Fig. 4C). However, Igf2 levels were higher in fatty livers than in phenotypically normal transgenic tissues (Fig. 4C).
Fig. 4. H19 and Igf2 expression.
(A) Time course of Igf2 and H19 mRNA expression (n=14/2.5 weeks, 21/5 weeks, 8/10 weeks). (B) Igf2 and H19 mRNA expression after doxycyclin (dox) administration. (C) p62, Igf2 and H19 mRNA expression in non-fatty transgenic (n=8) vs. fatty transgenic livers (n=11) with values for non-fatty transgenic livers set as 1.
Mechanisms of Igf2 and H19 induction
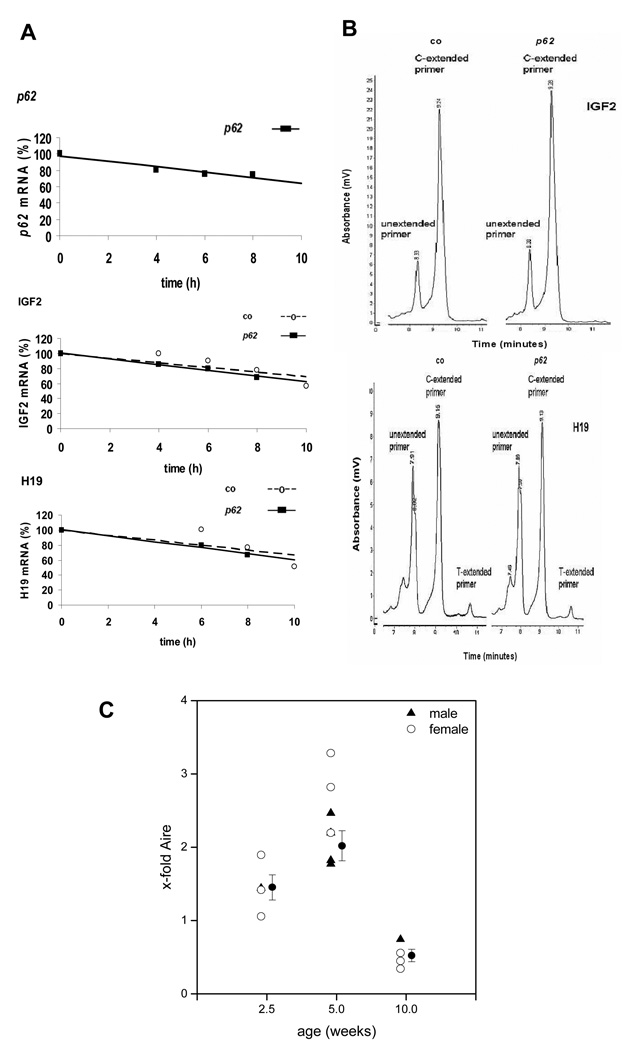
With p62 being an mRNA binding protein it might regulate mRNA stability [21]. Igf2 and H19 mRNA stability was estimated in actinomycin D-treated hepatocytes. Fig. 5A shows that steady-state levels of all mRNAs decreased similarly over time and more than 50 % of mRNA levels were left after 10 h.
Fig. 5. Mechanism of Igf2 and H19 induction.
(A) mRNA stability in p62 transgenic (n=3) vs. control (n=3) hepatocytes, expressed relative to t=0 h. (B) Allele-specific expression of Igf2 and H19. Representative HPLC chromatogram showing amplification products for Igf2 (above) and H19 (below). (C) Time course of Aire mRNA expression (n=4 at 2.5 weeks, 7 at 5 weeks and 4 at 10 weeks).
Since no stabilizing action of p62 on Igf2 and H19 mRNA was observed, we investigated allele-specific expression of Igf2 and H19. A mono-allelic Igf2 and H19 expression could be demonstrated in both groups (Fig. 5B), corresponding to an allele-specific index of 1.0.
Both Igf2 and H19 have been reported to be strongly induced by the transcriptional regulator Aire (autoimmune regulator) [22]. Interestingly, Aire expression was significantly increased in p62 transgenic animals at the age of 2.5 and 5 weeks, whereas it was downregulated at the age of 10 weeks (Fig. 5C). Female animals showed a slightly higher expression of Aire.
Activation of IGF2 downstream targets
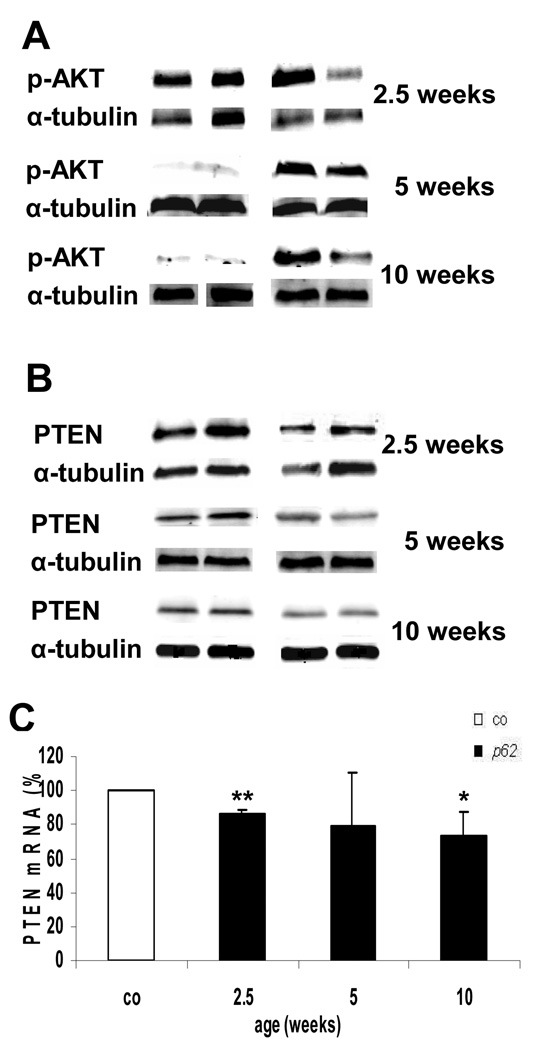
Enhanced AKT phosphorylation at Ser473 was observed at the age of 5 and 10 weeks, whereas 2.5 week old animals showed no changes (Fig. 6A). A reduction of both PTEN protein and mRNA levels could be demonstrated for 2.5 and 10 week old p62 transgenic mice (Fig. 6B/C).
Fig. 6. Igf2 downstream target activation.
(A) pAKT and (B) PTEN at 2.5 (n=co:6/p62: 7), 5 (n=co: 4/p62: 5) and 10 weeks (n=co: 7/p62: 6). Protein normalized to α-tubulin. Representative blots are shown. (C) PTEN mRNA expression of 2.5 (n=co:8/p62: 13), 5 (n=co: 4/p62: 6) and 10 week (n=co: 8/p62: 12), mean percentages ± SEMs are shown. P<.01 (**) vs. controls of the respective age.
Improved glucose tolerance
Since histological analyses suggested metabolic changes, an intraperitoneal glucose tolerance test (IP-GTT) was performed.
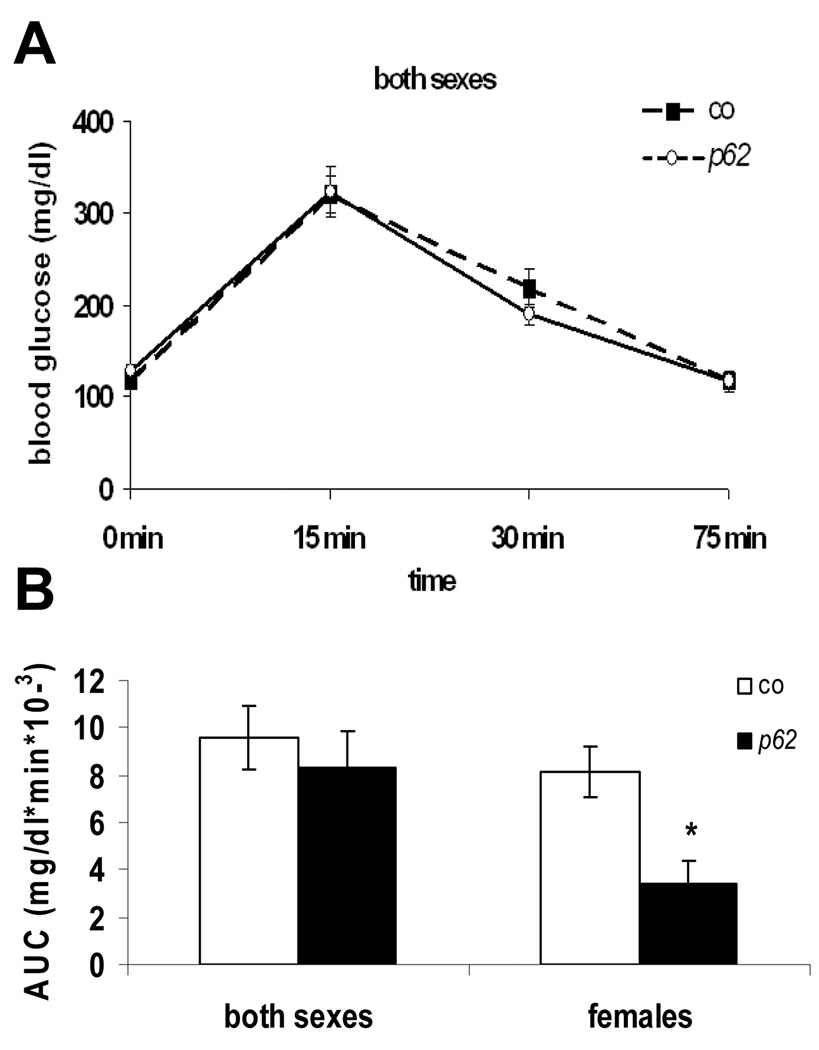
Fasting levels of glucose and end point values corresponded in both experimental groups. The time course revealed a slightly improved glucose clearance of p62 transgenic animals at 30 min after glucose administration (p>.05) (Fig. 7A).
Fig. 7. Glucose tolerance test.
(A) Blood glucose values (mg/dl) (co: n=17, p62: n=11). (B) AUC (area under the curve) of glucose levels. Values integrated over 75 min vs. gender-specific controls.
The glucose tolerance distribution curve in female p62 transgenic mice revealed a significant reduction of glucose levels at 30 min (69.5 ± 10.8 %) and at 75 min (75.2 ± 15.1%) (p <.05, both) in comparison to controls. Differences in glucose tolerance were further supported by a significant decrease in the AUC (area under the curve: glucose concentration over time) of females only (Fig. 7B). In summary, the results indicate a gender-specifically enhanced glucose clearance in the presence of p62.
Discussion
The exclusive expression of p62 in HCC cancer nodules [2] together with its appearance in fetal liver make it an oncofetal protein [3]. Functional implications of the protein have as yet been completely unknown. We herein present the first phenotypic characterization of liver-specific p62 overexpression in transgenic mice.
Although no evidence of spontanous tumor formation upon p62 overexpression was detected, a hint on an impact of p62 on cell malignancy was given by HE-staining due to the appearance of basophilic cell foci in p62 transgenic liver tissue, a phenotype suggesting a progressive cellular dedifferentiation [23].
Histological fat staining revealed the phenotype of a fatty liver with a microvesicular fat distribution in p62 transgenic mice, as found in other genetic mouse models and in cases of human NAFLD [24]. Interestingly, fat accumulation was accompanied by increased Igf2 expression, as also found in human fatty livers [25].
A steatotic phenotype is considered to be benign with little risk of disease progression unless inflammation is detected [26]. The lack of an increase in serum transaminases was also observed in dietary models of fatty livers [27]. Higher TG (triglyceride) levels as observed in male p62 transgenic mice are not toxic per se and were also found in mice with a liver-specific nuclear respiratory factor 1 deletion [28] and the significant increase of the ratio of the liver to body weight is consistent with observations made in fatty liver models [29].
The decrease of glycogen in p62 transgenic animals might represent an early stage of liver dysfunction, as it has been described in humans with alcohol-induced liver cirrhosis [30].
Since fatty livers are often connected to the establishment of insulin resistance leading to impaired glucose tolerance, IP-GTT was performed. A significant decrease in the area under the curve (AUC) in p62 transgenic females could be demonstrated, indicating an increased ability to clear glucose. This observation is in concordance with improved glucose tolerance after liver-specific PTEN deletion in mice [24]. Interestingly, the effect of decreased PTEN is less pronounced at 5 weeks compared to 2.5 weeks of age, when actual fat depositions occur. This suggests PTEN downregulation as a critical feature in p62-induced steatosis.
The increase of pAKT, being known as a promotor of cell malignancy and a metabolic regulator [31], can be explained by p62-mediated Igf2 induction. Also PTEN downregulation might support the increase in pAKT via the attenuated ability of PTEN to dephosphorylate the AKT activator PIP-3 [10]. Since AKT phosphorylation was not increased at the age when steatosis occurred, however, its pathophysiological role is suggested to be of minor relevance.
The gender differences in liver phenotypes reflect the observation that females express higher Igf2 levels although p62 levels were similar in males and females. Interestingly, the transcriptional regulator of Igf2, Aire [22] is also higher expressed in transgenic females and it is known to act gender-specifically [32]. Both fatty livers and improved glucose tolerance were more pronounced in females, suggesting a causal interaction between Aire-induced Igf2 and the metabolic phenotype.
The shared regulation of IGF2 and PTEN has been shown in several cancer cells [33] and a direct inhibition of PTEN expression by Igf2 has been reported [34]. The p62-induced downregulation of PTEN might contribute to the increase in fatty acids as shown in mice with a liver-specific PTEN deletion [24]. Vice versa since fatty acids are able to downregulate PTEN [35], they might contribute to further declined PTEN expression.
The question whether the fatty liver phenotype in p62 transgenic animals is accompanied by NASH, i. e. the additional occurrence of inflammation, was addressed by different approaches. No leukocyte infiltrates could be detected. The lack of transaminase increases, also observed in a genetic mouse model of NAFLD [28], as well as the absence of NF-κB translocation confirmed the absence of a pro-inflammatory phenotype. Interestingly, p62 transgenic animals showed a distinct increase in cytosolic p65. Very few studies address changes in the expression levels of non-activated, i.e. cytosolic p65 [36], but an association with tumor diseases has been demonstrated in malignant epithelial cells from colorectal tissue [37]. Constitutive overexpression of the p65 protein has also been shown in thyroid carcinoma cells [38] and the oncogene MDM2 induces p65 protein expression in acute lymphoblastic leukemia [39]. Although functional implications of increased levels of p65 are as yet largely unknown, they might enhance an inflammatory response upon respective stimuli [36]. Whereas the microvesicular fat distribution in p62 transgenic mice forms the borderline from a benign to a morbid condition [40], our results suggest that the “second hit” towards the progression of NASH, resulting from inflammation, is missing.
Our data report that p62 induces both IGF2 and H19 [41] expression, which are known to play opposite roles in tumor development. Despite strongly increased Igf2 levels, our animals did not develop tumors. This might be connected to high levels of the tumor suppressor gene H19 [13]. Also the decline of transgene expression at the age of 10 weeks most likely contributes to the lack of the development of a malignant disease. Our data do not indicate that LAP-induced gene expression is downregulated with age, which is why we suggest that CMV promoter activity declines.
Since p62 is a member of the IMP family [3] and IMPs have been reported to bind to H19 [42], IMPs are potential candidates to influence mRNA stability [21]. However, our results revealed no influence of p62 on mRNA stability of Igf2 and H19. Since both mRNAs turned out to be rather stable mRNAs (t1/2>10 h) the regulation via stabilizing mechanisms is rather unlikely since stability-regulated genes mostly represent short-lived mRNAs [21].
The counter-regulatory actions between IGF2 and H19 are very complex [43]. The imprinted genes IGF2 and H19 often show coordinate, reciprocal regulation [44, 45]. On the other hand, Li et al. found parallel expression of IGF2 and H19 in HCC [46] and loss of imprinting (LOI) of IGF2 in HCC has been associated with coexpression of H19 and IGF2 [47]. Therefore, investigations of the chromosomal expression of IGF2 and H19 evoked by p62 were done. However, neither a change in allele-specific nor biallelic expression was detected for Igf2 and H19. Our result that LOI of the IGF2 locus is not involved in increased Igf2 gene expression in p62 transgenic mice is in concordance with the observation made by Feinberg et al. [48].
The increased expression of the transcriptional regulator Aire is most likely responsible for the high expression of both Igf2 and H19 in p62 transgenic animals: both Igf2 and H19 are among the genes most highly regulated by Aire expression [22]. Although Aire has been described to be found in hepatocytes to a high extent [49], a functional implication of Aire expression in the liver has as yet been completely unknown. Therefore, further studies need to establish an insight into the connection between the autoantigen p62 and Aire, the latter being known to be an important regulator of autoimmunity [49].
Taken together, our data provide evidence that p62 exhibits a distinct upregulation of the metabolic growth factor Igf2 via induction of the transcriptional activator Aire. p62 seems to play a pathophysiological role in liver disease through its induction of a fatty liver phenotype. p62 might therefore serve both as a diagnostic marker as well as a pharmacological target.
Supplementary Material
Acknowledgement
We thank Nikolaus Gladel, Marga Sand-Hill, Sieglinde Wagner, Gertrud Walter, and Daorong Liu for excellent technical assistance. We thank the Kistner group for providing LT2 mice. We are especially indebted to Dr. Frank Chisari of The Scripps Research Institute for his help and advice with induction of the p62 transgenic mice.
The project was funded by the Deutsche Krebshilfe (#107751) and by grant CA 56956 from the National Institutes of Health, U.S.A. A.K.K. was supported by the Alexander von Humboldt foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
E. Tybl, S.M. Kessler, and A.K. Kiemer designed experiments, analysed data and wrote the manuscript. A.K. Kiemer and E.M. Tan initiated and directed the study. The others generated p62 transgenic mice, designed experiments, and participated in data acquisition. There are no conflicts of interest to disclose.
References
- 1.Takamatsu S, Noguchi N, Kudoh A, Nakamura N, Kawamura T, Teramoto K, et al. Influence of risk factors for metabolic syndrome and non-alcoholic fatty liver disease on the progression and prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2008;55:609–614. [PubMed] [Google Scholar]
- 2.Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–1110. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu M, Nakamura RM, Dent ED, Zhang J-Y, Nielsen FC, Christiansen J, et al. Aberrant Expression of Fetal RNA-Binding Protein p62 in Liver Cancer and Liver Cirrhosis. Am J Pathol. 2001;159:945–953. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeng YM, Chang CC, Hu FC, Chou HY, Kao HL, Wang TH, Hsu HC. RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology. 2008;48:1118–1127. doi: 10.1002/hep.22459. [DOI] [PubMed] [Google Scholar]
- 5.Hansen TV, Hammer NA, Nielsen J, Madsen M, Dalbaeck C, Wewer UM, et al. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol Cell Biol. 2004;24:4448–4464. doi: 10.1128/MCB.24.10.4448-4464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson L. Medical oncology: IMP3 is a novel prognostic marker for colon cancer. Nat Rev Clin Oncol. 7:123. [Google Scholar]
- 7.Yao X, Hu JF, Daniels M, Shiran H, Zhou X, Yan H, et al. A methylated oligonucleotide inhibtis IGF2 expression and enhances survival in a model of hepatocellaular carcinoma. J Clin Invest. 2003;111:265–273. doi: 10.1172/JCI15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saho WJ, Tao LY, Gao C, Xie JY, Zhao RQ. Alterations in methylation and expression levels of imprinted genes H19 and Igf2 in the fetuses of diabetic mice. Comp Med. 2008;58:341–346. [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen FC. The molecular and cellular biology of insulin-like growth factor II. Prog Growth Factor Res. 1992;4:257–290. doi: 10.1016/0955-2235(92)90023-b. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, Nakano T, Mak TW, Sasaki T. Portrait of PTEN: Messages from mutant mice. Cancer Science. 2008;99:209–213. doi: 10.1111/j.1349-7006.2007.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinciguerra M, Sgroi A, Veyrat-Durebex C, Rubbia-Brandt L, Buhler LH, Foti M. Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog (PTEN) via microRNA-21 up-regulation in hepatocytes. Hepatology. 2009;49:1176–1184. doi: 10.1002/hep.22737. [DOI] [PubMed] [Google Scholar]
- 12.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimizu T, Miroglio A, Ripoche MA, Gabory A, Vernucci M, Riccio A, et al. The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci USA. 2008;105:12417–12422. doi: 10.1073/pnas.0801540105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiemer AK, Senaratne RH, Hoppstädter J, Diesel B, Riley L, Tabeta K, et al. Attenuated activation of macrophage TLR9 by DNA from virulent mycobacteria. J Innate Immun. 2009;1:29–45. doi: 10.1159/000142731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouayed J, Desor F, Rammal H, Kiemer AK, Tybl E, Schroeder H, et al. Effects of lactational exposure to benzo[alpha]pyrene (B[alpha]P) on postnatal neurodevelopment, neuronal receptor gene expression and behaviour in mice. Toxicology. 2009;259:97–106. doi: 10.1016/j.tox.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Tierling S, Dalbert S, Schoppenhorst S, Tsai CE, Oliger S, Ferguson-Smith AC, et al. High-resolution map and imprinting analysis of the Gtl2-Dnchc1 domain on mouse chromosome 12. Genomics. 2006;87:225–235. doi: 10.1016/j.ygeno.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Kulhanek-Heinze S, Gerbes A, Gerwig T, Vollmar AM, Kiemer AK. Protein kinase A dependent signalling mediates anti-apoptotic effects of the atrial natriuretic peptide in ischemic livers. J Hepatol. 2004;41:414–420. doi: 10.1016/j.jhep.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Kiemer AK, Vollmar AM. Autocrine Regulation of Inducible Nitric-oxide Synthase in Macrophages by Atrial Natriuretic Peptide. J Biol Chem. 1998;273:13444–13451. doi: 10.1074/jbc.273.22.13444. [DOI] [PubMed] [Google Scholar]
- 19.Duenschede F, Tybl E, Kiemer AK, Dutkowski P, Erbes K, Kircher A, et al. Bcl-2 upregulation after 3-nitropropionic acid preconditioning in warm rat liver ischemia. Shock. 2008;30:699–704. doi: 10.1097/SHK.0b013e31816f6562. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Ishihara K, Kato R. Mechanisms of Igf2/H19 imprinting: DNA methylation, chromatin and long-distance gene regulation. J Biochem. 2000;127:711–715. doi: 10.1093/oxfordjournals.jbchem.a022661. [DOI] [PubMed] [Google Scholar]
- 21.Eberhardt W, Doller A, Akool E-S, Pfeilschifter J. Modulation of mRNA stability as a novel therapeutic approach. Pharmacology & Therapeutics. 2007;114:56–73. doi: 10.1016/j.pharmthera.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Johnnidis JB, Venanzi ES, Taxman DJ, Ting JPY, Benoist CO, Mathis DJ. Chromosomal clustering of genes controlled by the aire transcription factor. Proc Natl Acad Sci USA. 2005;102:7233–7238. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Q, Benner A, Hofmann WJ, Otto G, Pichlmayr R, Bannasch P. Human hepatic preneoplasia: phenotypes and proliferation kinetics of foci and nodules of altered hepatocytes and their relationship to liver cell dysplasia. Virchows Arch. 1997;431:391–406. doi: 10.1007/s004280050116. [DOI] [PubMed] [Google Scholar]
- 24.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity. Proc Natl Acad Sci USA. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiappini F, Barrier A, Saffroy R, Domart MC, Dagues N, Azoulay D, et al. Exploration of global gene expression in human liver steatosis by high-density oligonucleotide microarray. Lab Invest. 2006;86:154–165. doi: 10.1038/labinvest.3700374. [DOI] [PubMed] [Google Scholar]
- 26.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 27.Lee L, Alloosh M, Saxena R, Alstine Wv, Watkins BA, Klaunig JE, et al. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology. 2009;50:56–67. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci USA. 2005;102:4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otogawa K, Kinoshita K, Fujii H, Sakabe M, Shiga R, Nakatani K, et al. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: implications for the pathogenesis of human nonalcoholic steatohepatitis. Am J Pathol. 2007;170:967–980. doi: 10.2353/ajpath.2007.060441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen OE, Reichle FA, Mozzoli MA, Kreulen T, Patel MS, Elfenbein IB, et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981;68:240–252. doi: 10.1172/JCI110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O´Connor R. Survival factors and apoptosis. Adv Biochem Eng Biotechnol. 1998;62:137–166. doi: 10.1007/BFb0102309. [DOI] [PubMed] [Google Scholar]
- 32.Hassler S, Ramsey C, Karlsson MC, Larsson D, Herrmann B, Rozell B, et al. Aire-deficient mice develop hematopoetic irregularities and marginal zone B-cell lymphoma. Blood. 2006;108:1941–1948. doi: 10.1182/blood-2006-04-019679. [DOI] [PubMed] [Google Scholar]
- 33.Perks CM, Vernon EG, Rosendahl AH, Tonge D, Holly JM. IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene. 2007;26:5966–5972. doi: 10.1038/sj.onc.1210397. [DOI] [PubMed] [Google Scholar]
- 34.Moorehead RA, Hojilla CV, De Belle I, Wood GA, Fata JE, Adamson ED, et al. Insulin-like growth factor-II regulates PTEN expression in the mammary gland. J Biol Chem. 2003;278:50422–50427. doi: 10.1074/jbc.M306894200. [DOI] [PubMed] [Google Scholar]
- 35.Vinciguerra M, Carrozzino F, Peyrou M, Carlone S, Montesano R, Benelli R, et al. Unsaturated fatty acids promote hepatoma proliferation and progression through downregulation of the tumor suppressor PTEN. J Hepatol. 2009;50:1132–1141. doi: 10.1016/j.jhep.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Oerlemans R, Vink J, Dijkmans BAC, Assaraf YG, van Miltenburg M, van der Heijden J, et al. Sulfasalazine sensitises human monocytic/macrophage cells for glucocorticoids by upregulation of glucocorticoid receptor and glucocorticoid induced apoptosis. Ann Rheum Dis. 2007;66:1289–1295. doi: 10.1136/ard.2006.060509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charalambous MP, Lightfoot T, Speirs V, Horgan K, Gooderham NJ. Expression of COX-2, NF-kappaB-p65, NF-kappaB-p50 and IKKalpha in malignant and adjacent normal human colorectal tissue. Br J Cancer. 2009;101:106–115. doi: 10.1038/sj.bjc.6605120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkappaB p65 protein expression. Oncogene. 1997;15:1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 39.Gu L, Findley HW, Zhou M. MDM2 induces NF-kappaB/p65 expression transcriptionally through Sp1-binding sites: a novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood. 2002;99:3367–3375. doi: 10.1182/blood.v99.9.3367. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed KM, Nantajit D, Fan M, Murley JS, Grdina DJ, Li JJ. Coactivation of ATM/ERK/NF-kappaB in the low-dose radiation-induced radioadaptive response in human skin keratinocytes. Free Radic Biol Med. 2009;46:1543–1550. doi: 10.1016/j.freeradbiomed.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leighton PA, Saam JR, Ingram RS, Stewart CL, Tilghman SM. An enhancer deletion affects both H19 and Igf2 expression. Genes Dev. 1995;9:2079–2089. doi: 10.1101/gad.9.17.2079. [DOI] [PubMed] [Google Scholar]
- 42.Runge S, Nielsen FC, Nielsen J, Lykke-Andersen J, Wewer UM, Christiansen J. H19 RNA binds four molecules of insulin-like growth factor II mRNA-binding protein. J Biol Chem. 2000;275:29562–29569. doi: 10.1074/jbc.M001156200. [DOI] [PubMed] [Google Scholar]
- 43.Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 45.Ohlsson R, Hedborg F, Holmgren L, Walsh C, Ekstrom TJ. Overlapping patterns of IGF2 and H19 expression during human development: biallelic IGF2 expression correlates with a lack of H19 expression. Development. 1994;120:361–368. doi: 10.1242/dev.120.2.361. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Nong Z, Ekstrom C, Larsson E, Nordlinder H, Hofmann WJ, et al. Disrupted IGF2 promoter control by silencing of promoter P1 in human hepatocellular carcinoma. Cancer Res. 1997;57:2048–2054. [PubMed] [Google Scholar]
- 47.Ariel I, Ayesh S, Perlman EJ, Pizov G, Tanos V, Schneider T, et al. The product of the imprinted H19 gene is an oncofetal RNA. Mol Pathol. 1997;50:34–44. doi: 10.1136/mp.50.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983;111:47–54. doi: 10.1016/s0006-291x(83)80115-6. [DOI] [PubMed] [Google Scholar]
- 49.Halonen M, Pelto-Huikko M, Eskelin P, Peltonen L, Ulmanen I, Kolmer M. Subcellular Location and Expression Pattern of Autoimmune Regulator (Aire), the Mouse Orthologue for Human Gene Defective in Autoimmune Polyendocrinopathy Candidiasis Ectodermal Dystrophy. J Histochem Cytochem. 2001;49:197–208. doi: 10.1177/002215540104900207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.