Abstract
Corticotropin-releasing factor (CRF) functions as one of the major mediators of the mammalian stress response and appears to play a key role in the pathophysiology of mood and anxiety disorders. Small molecule CRF1 receptor antagonists may represent a novel form of pharmacotherapy for these disorders. The therapeutic success of CRF1 receptor antagonists will depend, in part, upon whether tolerance develops to the actions of these compounds and whether appropriate patterns of HPA axis function is maintained. This study evaluated the effects of long term (~4 week) treatment with the CRF1 receptor antagonist R121919, on CRF receptor function, HPA axis activity, behavioral measures, adrenal gland size, and body weight gain.
Animals treated with 20 mg/kg/day of R121919 spent significantly more time in the open field in a defensive withdrawal test (138±36 seconds for R121919 vs 52±12 seconds for vehicle, p=0.01). No significant effect of chronic CRF1 receptor blockade on basal ACTH or corticosterone concentrations were detected, nor were significant changes detected in an elevated plus maze test. Both vehicle- and R121919- treated rats showed increases in AUC and peak ACTH and corticosterone concentrations following air puff startle stress, without any overall group differences, although a clear but non-significant attenuation in HPA axis response was observable in R121919 treated animals. Chronic CRF1 receptor blockade increased CRF peptide mRNA expression in the PVN and decreased CRF peptide mRNA expression in the central nucleus of the amygdala. Overall our results suggest that anxiolytic effects of chronic CRF1 receptor antagonism persist following chronic administration without significant attenuation of the HPA axis’s ability to mount a stress response.
Keywords: CRF, CRH, R121919, NBI-30775, CRF Antagonist, animal models
1. Introduction
Corticotropin-releasing factor (CRF) is a 41 amino acid-containing neuropeptide neurotransmitter/ hypothalamic hypophysiotropic factor that functions as the major physiological mediator of the mammalian stress response. CRF neurons in the paraventricular nucleus of the hypothalamus as well as extrahypothalamic CRF neuronal populations throughout the brain coordinate the behavioral, endocrine, autonomic and immune aspects of the stress response.
Over the past two decades, a burgeoning database derived from several laboratories have revealed that CRF administered directly within the central nervous system (CNS) produces many of the behaviors reminiscent of those observed in patients with major depression and anxiety disorders. These include, but are not limited to, increased anxiety, decreased appetite, decreased sexual activity, disrupted sleep and altered locomotor activity (see (Holsboer and Ising, 2008; Ising and Holsboer, 2007; Stahl and Wise, 2008) for reviews). Clinical studies have generated results consistent with the preclinical studies implicating the involvement of hypothalamic and extrahypothalamic CRF systems in the pathophysiology of depression. Numerous studies have demonstrated elevated CRF concentrations in the cerebrospinal fluid (CSF) of patients suffering from depression (Arborelius et al., 1999). Elevated cisternal CSF CRF concentrations have also been detected in depressed suicide victims (Arato et al., 1989). Postmortem studies of CRF receptors (Merali et al., 2004; Nemeroff et al., 1988), CRF (Merali et al., 2006), and CRF mRNA expression (Bao et al., 2007; Raadsheer et al., 1995) also provide evidence of CRF hypersecretion in depression. Moreover, there are a number of studies showing evidence for hypothalamic-pituitary-adrenal (HPA) axis hyperactivity in depression (Coplan et al., 1996; Gutman and Nemeroff, 2003; Holsboer et al., 1995; Holsboer et al., 1987; Nemeroff, 1999).
By the late 1980s, a number of research groups, including our own, had hypothesized that a small molecule CRF receptor antagonist with oral bioavailability that readily penetrates the blood-brain barrier might represent a novel class of antidepressant and/or anxiolytic agents. CRF1 receptor antagonists possess activity in many different laboratory animal paradigms for detecting clinically effective antidepressants or anxiolytics including foot shock (Mansbach et al., 1997), restraint stress (Gully et al., 2002; Gutman et al., 2003; Heinrichs et al., 2002; Li et al., 2003), learned helplessness (Mansbach et al., 1997), defensive withdrawal (Arborelius et al., 2000; Gutman et al., 2003; Heinrichs et al., 2002; Li et al., 2003), and increases in alcohol consumption following acute stress (Gilpin et al., 2008; Lowery et al., 2008). A limited number of studies have investigated the behavioral effects of CRF1 receptor antagonists in humans. An initial open-label study in humans suggested that CRF1 receptor antagonists can indeed be efficacious in the treatment of depression with reductions observed in severity of both depressive and anxiety symptoms (Zobel et al., 2000). A later clinical study with the CRF1 receptor antagonist CP-316,311 was terminated early due to lack of efficacy of this compound in the treatment of major depression (Binneman et al., 2008).
If CRF antagonists are efficacious in the treatment of depression and anxiety, they will be administered both acutely and utilized chronically. Although a number of laboratory animal studies have suggested that acute blockade of CRF1 receptors produces anxiolytic and antidepressant effects, it is important to determine that chronic administration does not lead to tolerance to the therapeutic, i.e. anxiolytic/antidepressant effects, of this novel class of agents. Pharmacological theory suggests that persistent blockade of a receptor may cause alterations (e.g., upregulation) of either the neurotransmitter and/or its receptor density which could limit the usefulness of this class of compound and/or lead to potential serious side effects if the drug were abruptly withdrawn. Because corticosterone concentrations are under tight physiologic regulation, it is crucial to determine what HPA axis changes, if any, occur following prolonged blockade of the CRF1 receptor. The goal of these studies was to evaluate the effects of long term (4 week) blockade of CRF1 receptors on CRF receptor function, HPA axis activity, behavioral measures, adrenal gland size, and weight gain. It is clearly of paramount importance to establish whether chronic administration of R121919 results in tolerance to its anxiolytic effects and/or produces disturbances in HPA axis function. Chronic complete blockade of the HPA axis could have significant deleterious consequences, resulting in Addisonian-like crisis in times of acute stress. Tissue from these animals was also analyzed to determine if chronic CRF1 receptor blockade produces alterations in the pattern of mRNA expression of CRF, urocortin, and the CRF1 and CRF2 receptors. These results are a logical follow up to our previous studies with R121919 in which acute administration did not alter basal HPA axis measures but reduced ACTH and corticosterone responses to an air puff stressor and produced an anxiolytic-like effect in the defensive withdrawal paradigm (Gutman et al., 2003).
2. Materials and Methods
2.1 Experimental Design
Animals were administered R121919 (NBI-30775) via an Alzet minipump (2ML4) for 31 days based on estimated final body weights. Though these pumps are rated for 4 weeks of administration, we routinely use them in our lab for 4.5–5 weeks as the reservoir volume of 2 mL’s and a flow rate of 60 µL/day allows for reliable drug delivery past the nominal 4 weeks. Drug was loaded into the pumps at a concentration designed to deliver 20 mg/kg/day based on a final body weight of ~ 425 g.
On day 22, rats were implanted with an intravenous (iv) jugular catheter as described below, and following recovery from the anesthetic, housed singly until the end of the experiment. Five days later the animals were subjected to an air puff startle stressor, and blood samples were collected via the iv catheter over a 90-minute period following the air puff. Basal serum ACTH and corticosterone (CORT) concentrations were also measured in an additional blood sample obtained 30 minutes before the initiation of the air puff. Animals were tested in the defensive withdrawal paradigm three days later, and then killed on the 31 st day after pump implantation. Animals were killed via iv euthanasia solution (FatalPlus, Vortech Pharmaceuticals, Dearborn, MI), and trunk blood collected to determine serum R121919 concentrations. The adrenal glands were dissected, carefully removed from all adjoining fatty tissue, and weighed.
2.2 Animals
Male Sprague-Dawley rats (~225 g on arrival; Harlan, Alabama) were housed two per cage unless otherwise noted, with food and water available ad libitum, in an environmentally controlled animal facility with a 12 hour light/dark cycle (lights on at 0730 hours). Animals were allowed at least 7 days to acclimate to the animal facility prior to initiation of experimentation. There were a total of 12 vehicle treated animals and 12 R121919 treated animals in the initial experimental groups. The Emory University Institutional Animal Care & Use Committee approved all of the animal protocols utilized in these studies. Animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.3 Drug Preparation
R121919 was dissolved in a vehicle consisting of 5% v/v polyethoxylated castor oil (Alkamuls EL-620; Rhone-Poulenc) in 0.3% tartaric acid (Sigma, St. Louis) and placed into Alzet 2ML4 osmotic minipumps (Alza Corp., Cupertino, Calif., USA) at a concentration sufficient to deliver 20 mg/kg per day for 31 days calculated based on an estimated final animal weight of ~ 425 g. The solution was sonicated for at least an hour with the addition of glacial acetic acid (approximately 15–20 µL in a volume of 4–5 mL of solution) until the powder was completely in solution. Both the vehicle and the antagonist used in the studies were adjusted to a final pH ~ 4.5. R121919 was a gift of Janssen Pharmaceuticals (Beerse, Belgium).
2.4 Minipump Implantation
Prior to implantation, the minipumps were primed overnight in 0.9% saline at room temperature. Under methoxyflurane anesthesia, the minipumps were implanted subcutaneously, through an incision made between the scapulae, as described in detail by Owens et al. (1991). The rats were handled and weighed daily, and the pumps manipulated to prevent the growth of fibrous adhesions around the minipump (Tanay et al., 1996).
2.5 Air Puff Startle
Rats were prepared with an indwelling jugular catheter 5 days prior to the air puff startle and subsequently housed singly. The procedure has been described in detail elsewhere (Thrivikraman et al., 2002). Three days after surgery, the animals were moved from their cage into polyethylene buckets (28 cm in diameter and 37 cm high) with food and water available ad libitum. The animals were weighed and then allowed to acclimate to the bucket for 2 days prior to testing. The bucket was used to allow convenient access to the jugular cannula for blood sampling.
Experiments were performed between 09:00 and 13:00h. On the morning of the experiment, jugular cannula were connected to 50 cm tubing extension (PE50; VWR, Atlanta) containing heparanized saline (20 IU/ml), connected to 1-cc syringes, and extended outside the bucket. Prior to the initiation of the air puff startle (APS), a blood sample (300 µL) was obtained to determine basal serum ACTH and CORT concentrations. APS was applied in repeated blocks from a pressurized air can (50–65 psi), directed towards the side of the head of the rat, and applied from a distance of 10–20 cm. Each air puff consisted of three 1-second bursts over a 5-s period; a total of three sets of bursts, separated by one minute, were applied resulting in a total of 9 bursts of air. Additional blood samples were collected at 5, 10, 15, 30, 60, and 90 minutes after beginning the air puff.
2.6 Defensive Withdrawal
The rats were handled daily prior to the experiments in order to rotate the Alzet mini pumps. All experiments were conducted between 08 00 and 13 00 h which allowed the blood sample for serum ACTH and CORT concentrations to be obtained at a similar time of day as the air puff experiments and before the normal rise in corticosterone that occurs in the evening in rodents.
For the defensive withdrawal experiments, a 100 cm X 100 cm white-plexiglass arena with 50 cm high walls was used. The bottom of the arena was painted a flat-gray and grid lines were drawn at 20 cm intervals to facilitate scoring. On testing day, the light level was adjusted to 600–750 lux across the entire arena using a lightmeter (VWR Scientific, Atlanta, GA). To begin the trial, the rat was placed in front of a black PVC tube (10 cm in diameter X 21 cm in length, closed at one end) and allowed to walk in unassisted. The tube was then placed into the field at a distance of 20 cm from a corner, with the open end of the tube facing the corner. Each trial lasted 15 minutes and was videotaped. Following the conclusion of the trial, blood was collected via the indwelling IV cannula to evaluate the stress responsivity of the HPA axis
Whole blood was collected in 1.5 mL microcentrifuge tubes and glass Vacutainer tubes containing EDTA (Beckon Dickinson, NJ) on ice for CORT and ACTH measurements, respectively. Blood samples were centrifuged (3100 × g CORT/ 1900 × g ACTH, 10 min, 4°C), and the plasma and serum was collected and stored at −80°C until ACTH and CORT determinations were performed (Thrivikraman and Plotsky, 1993).
ACTH was measured in samples of rat plasma by a two site immunoradiometric assay (Nichols Diagnostics, San Juan Capistrano, CA) with coefficient of variation of 5% and sensitivity of 1 pg/ml. Corticosterone was assayed in samples of rat serum by a double antibody RIA (ICN Biomedical, Costa Mesa, CA) with a coefficient of variation of 6% and a sensitivity of 1.2 ng/ml.
2.7 Elevated plus maze
The elevated plus maze consisted of two 50 cm enclosed arms (i.e., arms with 40 cm high walls at the sides), and two 50 cm open arms (i.e., arms with no walls at the sides). The four arms were arranged in a cross pattern extending out from a common 10cm × 10cm platform at the center where all arms met. The floor of the maze was mounted at a height of 50 cm above the floor of the room. Testing was conducted under low ambient lighting, and was approximately 75 lux. All testing on the elevated plus maze took place late in the light phase of the light/dark cycle (i.e., between 14:00 and 17:00 h) as we have found these conditions to produce the most consistent behavioral results in our laboratory. Animals were tested between 12 and 14 days after pump implantation. The test was begun by placing the animal on the central platform of the maze facing into a closed arm. Animals remained on the plus maze for 5 minutes. Between testing of each animal, the maze was thoroughly cleaned with 70% ethanol. All sessions were videotaped for subsequent analysis.
2.8 In situ Hybridization
Serial coronal brain sections (15 or 20 µm) were sliced on a cryostat at −17°C, thaw mounted onto SuperFrost Plus slides (Fisher, Pittsburgh PA) under RNAse-free conditions, and stored with Humi-Cap desiccant capsules (Gibco BRL Products, Grand Island NY) at −80°C until the assay. In situ hybridization was performed according to the procedures described by Simmons et al. (1989) with minor modifications. We have described the specifics of the protocol in detail elsewhere (Skelton et al., 2000).
2.9 CRF Receptor Autoradiography
Using the atlas of Paxinos and Watson (1986), brains from the restraint stress and defensive withdrawal studies were sectioned at the level of the prefrontal cortex and lateral septum in order to determine CRF1 and CRF2 receptor binding, respectively. The prefrontal cortex has been shown via in situ hybridization to predominantly express CRF1 receptors, whereas the lateral septum only expresses CRF2 receptors in rodents (Van Pett, 2000). For both experiments, 15 µm brain sections containing the prefrontal cortex and the lateral septum were sectioned at approximately −20°C and mounted on Superfrost Plus Slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C until the assay which is described in detail elsewhere (Gutman et al., 2003).
2.9 Image Analysis
Images from the receptor autoradiography films were digitized with a Dade-MTI CCD-72 (Michigan City, IN) image analysis system equipped with a Nikon camera. Semiquantitative analysis was performed using AIS software (version 4.0, Imaging Research, Inc.; Ontario, Canada). Optical densities were calibrated against coexposed [125I]-microscale standards (Amersham, Piscataway, NJ) and expressed in terms of nCi/g of tissue equivalent. In all cases, 3 to 4 sections per region were matched for rostrocaudal level according to the atlas of Paxinos and Watson (1986) and used to produce a single value for each animal. For each region analyzed, 3–5 sections were analyzed and averaged together to generate a single optical density reading for each region for each animal.
2.10 Defensive Withdrawal Behavioral Analysis
Latency to exit the tube was determined by recording the time of onset of the first 4-paw transition from inside the tube into the arena. The rat was considered to have returned to the tube when it had completely returned to the tube interior. The time of each excursion was then summed and subtracted from the number of seconds of the experiment (900) to determine the total time spent inside the tube. The sessions were videotaped, and then analyzed separately by two experienced raters who were blind to treatment. The interrater reliability was greater than 0.9.
2.11 Statistics
Significant differences were evaluated by one- or two-tailed t-tests or one-way ANOVA followed by Student-Newman-Keuls (SNK) post hoc analysis as appropriate. ACTH and CORT concentrations were log10 transformed before statistical analysis. All data are expressed as the mean ± SEM. All statistics were performed using SigmaStat (V2.03, SPSS Science, Chicago, IL).
3. Results
3.1 ex vivo receptor binding
Receptor autoradiography was performed on brain tissue at the level of the lateral septum. Because the animals were treated with R121919 for several weeks, sauvagine binding would be expected to be reduced if R121919 is present in sufficient concentrations to occupy CRF1 receptors in vivo. There was a significant reduction in [125I]-sauvagine binding in animals treated with R121919 (115.4 ± 13.6 Relative Optical Density Units (RODU) for control vs 63.12 ± 16.8 RODU for R121919 treated animals, ~50% mean reduction in CRF1 receptor binding, p=0.02, two tailed Students t-test) suggesting that R121919 was present in concentrations sufficient to attenuate 125I-sauvagine binding. Sauvagine binding was not restored even after the sections were preincubated for 4 hours at 37°C (data not shown), suggesting bound R121919 does not readily dissociate from the receptor. There were no significant changes in CRF2 receptor binding in the lateral septum (p=0.3678).
3.2 Body Weight Gain
There was no effect of R121919 on final body weight with a final weight for the vehicle treated animals of 392.1g ± 8.9 (n=10) and 387.0g ± 5.8 (n=14) for the 20 mg/kg group (p=0.64).
3.3 Adrenal Gland Weights
R121919 treatment did not significantly alter adrenal gland weight after 31 days of treatment. Mean adrenal gland weight in the vehicle group was 59.8 mg ± 2.9 mg (n=10) and 64.9 ± 3.0 mg (n=12) in animals treated with 20 mg/kg of R121919 for 31 days (p=0.24).
3.4 Hormone Measures
3.4.1 Basal Values
Basal serum ACTH and CORT concentrations were measured in blood samples obtained from the iv catheter 30 minutes prior to the air puff stress, administered on the 25th day of R121919 administration. Chronic R121919 administration did not significantly alter basal serum ACTH or CORT concentrations (See Figure 1). Mean ACTH concentrations were 8.4 ± 1.8 pg/ml and 9.5 ± 1.8 pg/ml for the vehicle and 20 mg/kg groups, respectively (p=0.67). Mean corticosterone concentrations were for 6.4 ± 3.0 ng/ml and 7.6 ± 4.6 ng/ml for the vehicle and 20 mg/kg group, respectively (p=0.84)
Figure 1.
Basal and Stimulated Endocrine Values after Defensive Withdrawal. There were no significant changes in either basal or stimulated endocrine values after a defensive withdrawal in animals treated with R121919 (20 mg/kg/day) for 30 days.
3.4.2 ACTH and CORT response to a behavioral stressor (Defensive Withdrawal)
Blood samples were obtained via the iv catheter immediately after the defensive withdrawal. R121919 did not significantly attenuate either the ACTH (95.9 ± 24.7 pg/ml for vehicle and 154.5 ± 51.8 pg/ml for the 20 mg/kg group, p=0.321) or corticosterone (130.3 ± 30.6 ng/ml for vehicle and 137.5 ± 20.ng/ml for the 20 mg/kg group, p=0.84) response to defensive withdrawal (See Figure 1).
3.4.3 ACTH/Corticosterone response to air puff startle
The ACTH and corticosterone responses to an air puff startle are presented in Figure 2. ACTH and CORT responses to air puff startle were non-significantly attenuated by R121919, although the overall curve suggests a non-significant decrease in both these parameters following R121919 administration. A repeated measures ANOVA analyzing the CORT response to airpuff startle with time and drug exposure as factors indicated a significant effect of time (p<0.0001, F=13.98, df=7), no significant effect of drug (p=0.16, F=2.148, df=1) and no interaction between drug exposure and time (p=0.66, F=0.72, df=7). Similarly, a repeated measures ANOVA analyzing the ACTH response to airpuff startle with time and drug exposure as factors indicated a significant effect of time (p=0.002, F=3.53 df=7), no significant effect of drug (p=0.49, F=0.50, df=1) and no interaction between drug exposure and time (p=0.50, F=0.91, df=7). An area under the curve analysis (AUC) failed to detect any significant differences in ACTH (p=0.60) or CORT (p=0.44) response to the air puff startle.
Figure 2.
ACTH and corticosterone response to an air puff startle following 25 days of R121919 (20 mg/kg/day) treatment.
3.5 Behavioral Experiments
3.5.1 Defensive Withdrawal
The results from the defensive withdrawal tests are presented in Figures 3. R121919 significantly increased the total time spent in the arena 52.2 ± 12.6 s for vehicle treated animals vs 138.6 ± 32.6 s for R121919 treated animals (p=0.016). R121919 did not significantly decrease latency to exit the defensive withdrawal tube (372.9 ± 63.5 s for vehicle treated animals vs 235.9 ± 46.0 s for R121919 treated animals, p=0.11).
Figure 3.
[A] Total time spent out of the tube during a 15 minute defensive withdrawal. Chronic R121919 (20 mg/kg/day) administration significantly increased the total time spent in the arena (p=0.016). [B] R121919 did not significantly attenuate the latency to enter the arena (p=0.108) s=seconds
3.5.2 Elevated plus maze
Total % of time spent on the open arms in the elevated plus maze was not signicicantly different between the groups (2.43% ± 1.0 for control animals vs 4.75% ± 1.7 for R121919 treated animals, p=0.30).
3.5.2 In situ hybridization
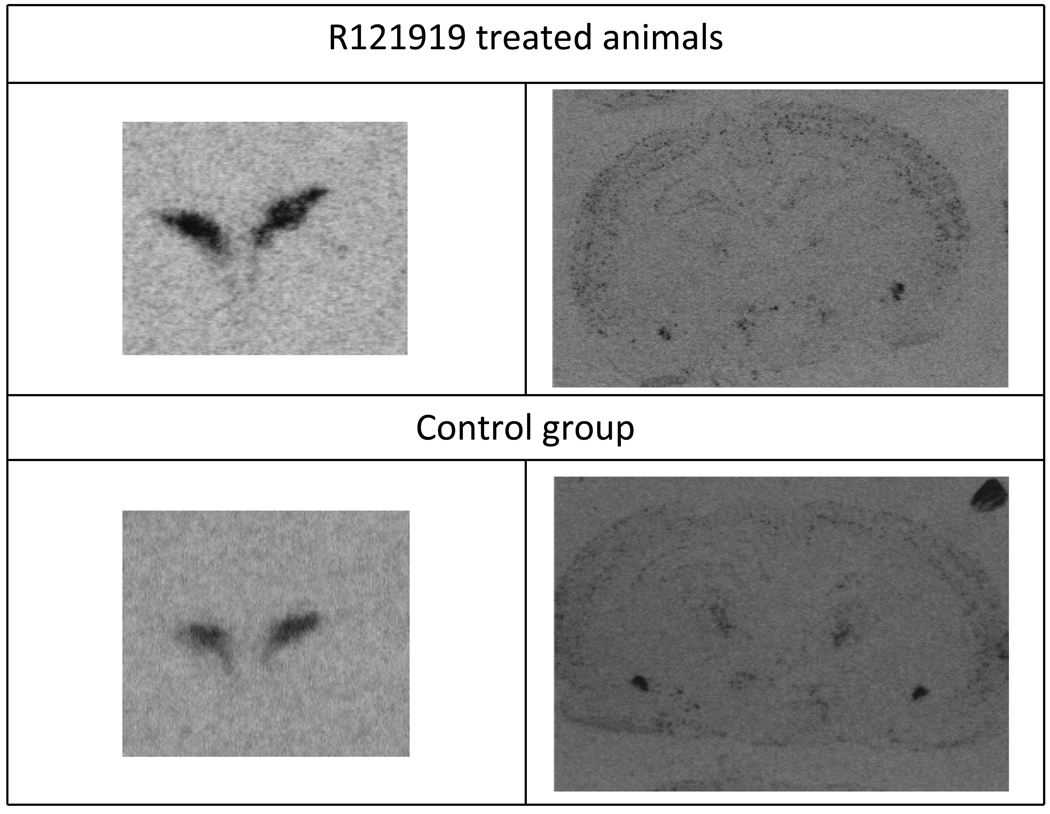
Several key regions known to contain CRF, urocortin, CRF1 or CRF2 receptors were analyzed for changes in mRNA concentrations. The regions surveyed are presented in Table 1 and representative in situ hybridization images are presented in Figure 4. A significant increase in CRF peptide mRNA expression was detected in the PVN (759.6±42.1 Relative Optical Density Units (RODU) for control animals vs 921.6±31.6 RODU for R121919 treated animals, ~20% increase, p= 0.005), and a significant decrease in CRF peptide mRNA was measured in the central nucleus of the amygdala (260.7±12.3 RODU for control animals vs 223.1±17.8 RODU for R121919 treated animals ~15% decrease, p=0.045). No significant alterations in CRF1 mRNA (107.9±8.8 RODU for control vs 111.4±5.7 RODU for R121919 treated ainmals) were detected in the parietal cortex , no urocortin mRNA (1101.6 ± 121.8 RODU for control vs 1104.9±98.4 RODU for R121919 treated animals) changes were detected in the Edinger Westphal nucleus, nor were any CRF2 receptor mRNA changes detected in the ventromedial hypothalamus (54.7±3.9 RODU for control vs 58.8±8.4 RODU for R121919 treated animals).
Table 1.
Survey of CRF system mRNA expression PVN = Paraventricular Nucleus of the Hypothalamus, CEA = Central Nucleus of the Amygdala,
| Region | Transcript | Two way t-test |
|---|---|---|
| Paraventricular nucleus of the hypothalamus | CRF mRNA | p=0.016 * |
| Central Nucleus of the Amygdala | CRF mRNA | p=0.045* |
| Parietal Ctx | CRF1 mRNA | p=0.40 |
| Basolateral Amygdala | CRF1 mRNA | p=0.76 |
| Ventral Medial Hypothalamus | CRF2 mRNA | p=0.37 |
| Edinger Westphal Nucleus | Urocortin mRNA | p=0.23 |
Figure 4.
Representative in situ hybridization images showing an increased (upper left image) density of CRF mRNA in the PVN (left,top) following chronic administration of R121919 and decreased CRF mRNA in the central nucleus of the amygdala (CeA) (right, top image)
4. Discussion
Chronic CRF1 receptor antagonism via R121919 reduced measures of anxiety after chronic administration as assessed in the defensive withdrawal paradigm. While both ACTH and CORT concentrations appeared attenuated following an air-puff startle, this effect was not significant. Basal plasma ACTH and serum CORT concentrations were also unaffected as a result of persistent CRF1 receptor antagonism. The importance of these results cannot be underestimated with respect to the ultimate clinical utility of this medication class. Overall our results are similar to an earlier similarly designed study where we investigated the results of acute treatment with R121919; in that study similar anxiolytic-like effects were observed (i.e., increase in time spent in the defensive withdrawal arena), although in the previous study the decreases in ACTH and CORT responses to the air puff startle reached significance (Gutman et al., 2003). Of note, no change in time spent on the open arms in the elevated plus maze were detected following chronic R121919 administration, although as we and others have noted previously (Gutman et al., 2008), there is considerable variability in the activity of CRF receptor antagonists in this particular paradigm.
The results from our initial acute dosing study would suggest concentrations of R121919 sufficient to produce behavioral responses should also blunt HPA-axis responsiveness. Though it did not reach significance, the endocrine response to the air-puff startle stress in animals treated with 20 mg/kg/day of R121919 was attenuated. One possible explanation for the near normal HPA-axis function is that the upregulation of hypothalamic CRF mRNA (~20%) resulted in increased CRF release from the hypothalamus, overcoming any effects of pituitary CRF1 receptor blockade in the PVN to produce sufficient ACTH release. Concomitant increases in CRF1 receptor density in the pituitary and/or increased sensitivity of pituitary corticotrophs can also not be ruled out. Increased sensitivity of the adrenal gland to ACTH can also not be ruled out, although adrenal gland weight was not significantly increased in chronically treated animals suggesting no significant adrenal hyperplasia. This may also simply reflect a lack of power due to the relatively large variability in individual animal ACTH/CORT responses. However of note the ACTH/CORT responses largely parallel the results seen in a clinical trial with the CRF1 receptor antagonist NBI-34041 in humans. In this study a TRIER Social Stress Test was administered to study participants, and the resulting ACTH/CORT responses were monitored following this psychological stressor (Ising et al., 2007). Similar to our results, basal ACTH/CORT were also not significantly altered following CRF1 receptor antagonist treatment. Zobel et. al (2000) also observed that the ACTH and cortisol response to an exogenous CRF challenge remained intact in depressed inpatients treated with R121919 (Zobel et al., 2000).
The observed decrease in CRF mRNA in the CeA observed in the chronically dosed animals may also be responsible, in part, for the anxiolytic effects seen in the current study. The central nucleus of the amygdala is the primary output nucleus of the amygdala, and reduced CRF mRNA production and likely decreased CRF output, would be expected to reduce measures of anxiety (Funk et al., 2006; Liang et al., 1992). However the specific target nucleus associated with the reduction in anxiety seen in the defensive withdrawal paradigm can not be directly inferred from this study, as the dosing method used in this study produces CRF1 receptor blockade throughout the entire brain. The mean receptor occupancy determined in this study (~50% receptor occupancy) is consistent with the receptor occupancy needed to produce significant effects in the defensive withdrawal paradigm in a study that directly measured brain receptor occupancy with the CRF1 receptor antagonist DMP696 (Li et al., 2003), suggesting our dosing regimen was appropriate. Of note, following acute treatment with R121919, Post et al (2005) showed upregulation of CRF1 mRNA in the amygdala, however this was after a single dose and was only noted at 1 out of the 3 doses tested, making direct comparison to these results difficult.
Our results are also largely consistent with previously published reports with CRF receptor antagonists after chronic administration. We have previously reported (Arborelius et al., 2000) that CP-154,526 significantly decreased defensive withdrawal behavior after 9 days of treatment (3.2 mg/kg/day). No changes in CRF1 mRNA expression were observed in parietal cortex, basolateral amygdala, or cerebellum after chronic treatment; though a dose-dependent decrease in CRF mRNA expression was observed in the PVN and Barrington’s nucleus. It is unclear if this discrepancy is related to nonspecific or unique effects of CP-154,526, because the highest dose of this drug was associated with toxicity.
Several other studies in which CRF receptor antagonists were administered chronically have also not detected major alterations in body weight or endocrine parameters. Long term treatment with antalarmin, a methyl analog of CP-154526, (20 mg/kg intraperitoneally once per day for 11 days) decreased basal plasma ACTH and corticosterone concentrations, but did not alter body weight, blood glucose, or leptin levels (Bornstein et al., 1998). Similarly, antalarmin treatment (20 mg/kg; ip; twice daily × 8 weeks) decreased basal plasma ACTH and corticosterone concentrations in rats (Wong et al., 1999). Eight week treatment did not attenuate the ACTH/CORT response to an acute stressor, indicating it does not cause stress-induced adrenal insufficiency. Ten days of treatment with CRA1000 significantly decreased locomotor activity in the dark phase of the diurnal cycle, without altering weight gain, food or water intake, or plasma ACTH or CORT concentrations in rats (Ohata et al., 2002). Moreover, HPA axis reactivity was not altered following an acute immobilization stress in chronically treated (10 days) rats. Oshima et. al (2003) also reported that chronic administration of R121919 in mice using similar doses to those reported in this study (10–20 mg/kg/day) resulted in no significant changes in HPA axis reactivity to a novel stressor (swim stress) following 15 days of treatment.
Conclusion
In conclusion, our results suggest that anxiolytic effects of chronic CRF1 receptor antagonism persist following chronic administration, i.e. that there is no therapeutic tolerance to the behavioral effects of this compound after repeated administration. Moreover, chronic treatment does not significantly alter basal or stress-induced HPA axis activity, suggesting there is a low likelihood that long term administration of this class of compounds will lead to an undesirable outcome of HPA suppression, such as adrenal insufficiency.
Acknowledgements
This work was supported by NIH MH 42088 and MH-58299. The funding sources had no role in collection, analysis, or interpretation of the data and/or subsequent analysis. We would also like to thank Dr. Kelly Skelton and Susan Plott for their technical assistance.
Abbreviations
- CeA
Central nucleus of the amygdala
- CNS
Central nervous system
- CRF
Corticotropin-releasing factor
- HPA
Hypothalamic-pituitary-adrenal
- PVN
Paraventricular nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Gutman and Dr. Thrivikraman have no financial disclosures.
Michael J. Owens, Ph.D.
Research Grants: NIH, Eli Lilly, Pfizer, GlaxoSmithKline, Lundbeck, Cyberonics, Ortho-McNeil Janssen, AstraZeneca, Dainippon Sumitomo Pharma, SK Life Sciences.
Consultant: H. Lundbeck A/S, Takeda
Honorarium: Eli Lilly.
Patents: Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters (US 7,148,027 B2).
Charles B. Nemeroff, M.D. Ph.D.
Research Grants
NIH
Scientific Advisory Board
AFSP; AstraZeneca; Forest Laboratories; NARSAD; Quintiles; Janssen/Ortho-McNeil, PharmaNeuroboost, Mt. Cook Pharma, Inc
Stockholder or Equity
Corcept; Revaax; NovaDel Pharma; CeNeRx, PharmaNeuroboost
Board of Directors
American Foundation for Suicide Prevention (AFSP); George West Mental Health Foundation; NovaDel Pharma, Mt. Cook Pharma, Inc
Patents
Method and devices for transdermal delivery of lithium (US 6,375,990 B1)
Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters (US 7,148,027 B2).
REFERENCES
- Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989;25:355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of Endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Skelton KH, Thrivikraman KV, Plotsky PM, Schulz DW, Owens MJ. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. Journal of Pharmacology and Experimental Therapeutics. 2000;294:588–597. [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Webster EL, Torpy DJ, Richman SJ, Mitsiades N, Igel M, Lewis DB, Rice KC, Joost HG, Tsokos M, Chrousos GP. Chronic effects of a nonpeptide corticotropin-releasing hormone type I receptor antagonist on pituitary-adrenal function, body weight, and metabolic regulation. Endocrinology. 1998;139:1546–1555. doi: 10.1210/endo.139.4.5938. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gully D, Geslin M, Serva L, Fontaine E, Roger P, Lair C, Darre V, Marcy C, Rouby PE, Simiand J, Guitard J, Gout G, Steinberg R, Rodier D, Griebel G, Soubrie P, Pascal M, Pruss R, Scatton B, Maffrand JP, Le Fur G. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1,3-thiazol-2-amine hydrochloride (SSR125543A): a potent and selective corticotrophin-releasing factor(1) receptor antagonist. I. Biochemical and pharmacological characterization. Journal of Pharmacology and Experimental Therapeutics. 2002;301:322–332. doi: 10.1124/jpet.301.1.322. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Coyer MJ, Boss-Williams KA, Owens MJ, Nemeroff CB, Weiss JM. Behavioral effects of the CRF1 receptor antagonist R121919 in rats selectively bred for high and low activity in the swim test. Psychoneuroendocrinology. 2008;33:1093–1101. doi: 10.1016/j.psyneuen.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003;79:471–478. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. Journal of Pharmacology and Experimental Therapeutics. 2003;304:874–880. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben U, Wiedemann K, Muller OA, Stalla GK. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression. Implications for pathophysiology of DST nonsuppression. Biol Psychiatry. 1987;22:228–234. doi: 10.1016/0006-3223(87)90237-x. [DOI] [PubMed] [Google Scholar]
- Ising M, Holsboer F. CRH-sub-1 receptor antagonists for the treatment of depression and anxiety. Exp Clin Psychopharmacol. 2007;15:519–528. doi: 10.1037/1064-1297.15.6.519. [DOI] [PubMed] [Google Scholar]
- Ising M, Zimmermann US, Kunzel HE, Uhr M, Foster AC, Learned-Coughlin SM, Holsboer F, Grigoriadis DE. High-affinity CRF1 receptor antagonist NBI-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology. 2007;32:1941–1949. doi: 10.1038/sj.npp.1301328. [DOI] [PubMed] [Google Scholar]
- Li YW, Hill G, Wong H, Kelly N, Ward K, Pierdomenico M, Ren S, Gilligan P, Grossman S, Trainor G, Taub R, McElroy J, Zazcek R. Receptor occupancy of nonpeptide corticotropin-releasing factor 1 antagonist DMP696: correlation with drug exposure and anxiolytic efficacy. Journal of Pharmacology and Experimental Therapeutics. 2003;305:86–96. doi: 10.1124/jpet.102.045914. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJ, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci. 1992;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol. 1997;323:21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Bedard T, Anisman H. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The preeminent role of early untoward experience on vulnerability to major psychiatric disorders: the nature-nurture controversy revisited and soon to be resolved [news; comment] Molecular Psychiatry. 1999;4:106–108. doi: 10.1038/sj.mp.4000512. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Ohata H, Arai K, Shibasaki T. Effect of chronic administration of a CRF(1) receptor antagonist, CRA1000, on locomotor activity and endocrine responses to stress. Eur J Pharmacol. 2002;457:201–206. doi: 10.1016/s0014-2999(02)02663-8. [DOI] [PubMed] [Google Scholar]
- Oshima A, Flachskamm C, Reul JM, Holsboer F, Linthorst AC. Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology. 2003;28:2148–2159. doi: 10.1038/sj.npp.1300267. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Ritchie JC, Nemeroff CB. The 5-hydroxytryptamine2 agonist, (+)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane stimulates the hypothalamic-pituitary-adrenal (HPA) axis. I. Acute effects on HPA axis activity and corticotropin-releasing factor-containing neurons in the rat brain. Journal of Pharmacology and Experimental Therapeutics. 1991;256:787–794. [PubMed] [Google Scholar]
- Paxinos GWC. The rat brain in stereotaxic coordinates. San Diego: Harcourt Brace Jovanovich; 1986. [Google Scholar]
- Post A, Ohl F, Almeida OF, Binder EB, Rucker M, Welt S, Binder E, Holsboer F, Sillaber I. Identification of molecules potentially involved in mediating the in vivo actions of the corticotropin-releasing hormone receptor 1 antagonist, NBI30775 (R121919) Psychopharmacology (Berl) 2005;180:150–158. doi: 10.1007/s00213-004-2134-x. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechn. 1989;12:169–181. [Google Scholar]
- Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. Journal of Neuroscience. 2000;20:1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Wise DD. The potential role of a corticotropin-releasing factor receptor-1 antagonist in psychiatric disorders. CNS Spectr. 2008;13:467–483. doi: 10.1017/s1092852900016709. [DOI] [PubMed] [Google Scholar]
- Tanay VA, Glencorse TA, Greenshaw AJ, Baker GB, Bateson AN. Chronic administration of antipanic drugs alters rat brainstem GABAA receptor subunit mRNA levels. Neuropharmacology. 1996;35:1475–1482. doi: 10.1016/s0028-3908(96)00065-2. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Huot RL, Plotsky PM. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc. 2002;10:84–94. doi: 10.1016/s1385-299x(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Plotsky PM. Absence of glucocorticoid negative feedback to moderate hemorrhage in conscious rats. American Journal of Physiology. 1993;264:E497–E503. doi: 10.1152/ajpendo.1993.264.4.E497. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. Journal of Comparative Neurology. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wong ML, Webster EL, Spokes H, Phu P, Ehrhart-Bornstein M, Bornstein S, Park CS, Rice KC, Chrousos GP, Licinio J, Gold PW. Chronic administration of the non-peptide CRH type 1 receptor antagonist antalarmin does not blunt hypothalamic-pituitary-adrenal axis responses to acute immobilization stress. Life Sciences. 1999;65:L53–L58. doi: 10.1016/s0024-3205(99)00268-4. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. Journal of Psychiatric Research. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]