Abstract
Purpose
Over-expression of Inhibitors of apoptosis proteins (IAPs) contributes to therapeutic resistance. Smac promotes caspase activation by binding to IAPs upon release from the mitochondria. IAP antagonists, also called SMAC mimetics, are promising anticancer agents modeled after this mechanism. We investigated the role and mechanisms of Smac- and Smac mimetic-mediated chemosensitization in HNSCC cells.
Experimental Design
The effects of SMAC knockdown, SMAC over-expression and a small molecule Smac mimetic on the chemosensitivities of HNSCC cells were determined. The mechanisms of Smac- and Smac mimetic-mediated chemosensitization were investigated by analyzing growth suppression, the mitochondrial apoptotic pathway, caspase activation and IAP proteins. The therapeutic responses of HNSCC cells with different levels of Smac were compared in xenograft models.
Results
We found that Smac mediates apoptosis induced by several classes of therapeutic agents through the mitochondrial pathway. SMAC knockdown led to impaired caspase activation, mitochondrial membrane depolarization and release of cytochrome c. A small molecule Smac mimetic, at nanomolar concentrations, significantly sensitized HNSCC cells to gemcitabine-induced apoptosis and restored gemcitabine sensitivity in SMAC-knockdown cells, through caspase activation, XIAP dissociation and mitochondria-associated events, but not the TNF-α pathway. Furthermore, Smac levels modulated the therapeutic response of HNSCC cells to gemcitabine in xenograft models.
Conclusions
Our results establish a critical role of Smac in mediating therapeutic responses of HNSCC cells, and provide a strong rationale for combining Smac mimetics with other anticancer agents to treat HNSCC.
Keywords: Smac, IAP antagonists, apoptosis, mitochondria, Head and Neck Cancer
Introduction
Patients with head and neck squamous cell carcinoma (HNSCC) are often diagnosed with advanced diseases that respond poorly to chemo and radiation therapy. As a result, the overall survival of HNSCC patients has not been significantly improved over the past two decades (1). Gemcitabine is a chemotherapeutic agent commonly used to treat HNSCC, and often in combination with other modalities such as surgery, radiation or additional chemotherapeutic agents including cisplatin. The chemo- andradio-sensitization properties of gemcitabine are associated with severe mucositis in the majority of patients (2–4). Therefore, novel strategies are needed to improve efficacy and reduce side effects in HNSCC treatment.
Deregulation of programmed cell death (apoptosis) is a major cause of therapeutic resistance (5–7). Apoptosis is blocked through a variety of mechanisms in HNSCC cells. The tumor suppressor p53 is frequently mutated or targeted for degradation by human papillomavirus (HPV) oncoproteins (8), which prevents the induction of pro-apoptotic BH3-only proteins, such as PUMA and Noxa, in response to DNA damage (9). Over-expression of anti-apoptotic Bcl-2 family members (10), such as Bcl-xL and Bcl-2, is also common in HNSCC (11). Widespread over-activation of growth factor pathways, such as EGFR and STATs, can suppress apoptosis by affecting the expression of several Bcl-2 family members (12). Over-expression of inhibitor of apoptosis proteins (IAPs), such as c-IAP2 and XIAP, has been reported in HNSCC (13, 14) and other malignancies (15, 16).
Second mitochondria-derived activator of caspase (Smac) is an endogenous inhibitor of IAPs (17). Upon release into the cytosol, Smac binds to IAPs through its N-terminal AVPI domain and relieves the inhibition of caspases by IAPs. However, induction of apoptosis in response to various anticancer agents is not affected by Smac deficiency in murine models (18). Studies using human colon cancer cells revealed a role of Smac in mediating apoptosis to selective classes of agents, such as Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (Trail) (17, 19). Several small molecules mimicking the AVPI domain of Smac, also called Smac mimetics, have been developed and showed antitumor effects in combination with other conventional chemotherapeutic agents in preclinical models (20–22). In some cancer cells, Smac mimetics alone promote apoptosis by engaging TNF-α signaling through IAP degradation (23–26). However, the role of Smac in mediating therapeutic responses and the mechanism of Smac mimetic-induced sensitization of cancer cells remain to be defined.
In this study, we investigated the role of Smac in mediating chemosensitivity of HNSCC cells in vitro and in vivo. Our results suggest that Smac regulates the sensitivity of HNSCC to several classes of anticancer agents, and modulates gemcitabine-induced apoptosis through the intrinsic/mitochondrial pathway. Smac over-expression, or Smac mimetic compounds, effectively enhanced apoptosis induced by gemcitabine in HNSCC cells through the mitochondrial pathway, but not the alternative IAP/TNF-α autocrine or paracrine pathway.
Materials and Methods
Cell culture and drug treatment
Head and neck cancer cell lines were obtained from the University of Pittsburgh Cancer Institute (UPCI) Head and Neck Cancer program. All cell lines were maintained at 37°C in 5% CO2. Cell culture media included DMEM (Mediatech, Herdon, VA) for 1483 cells, and RPMI 1640 (Cellgro, Herdon, VA) for JHU cells, and were supplemented with 10% FBS (HyClone, Logan, UT), 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Gemcitabine was purchased from Eli Lilly and Company (Indianapolis, IN). Other anticancer agents used in the study include Cisplatin, 5-fluorouracil (5-FU), TNF-related apoptosis-inducing ligand (Trail) (PeproTech, Rocky Hill, NJ), and human TNF-α and its neutralizing antibody (R&D system, Minneapolis, MN, USA). TetraLogic Pharmaceuticals supplied the Smac mimetic, GT-A, and control compound, GT-C (19). Stock solutions of all small compounds were prepared in DMSO. For treatment with adenovirus, Ad-PUMA or the BH3-deleted form (Ad-ΔBH3), (27) cells were infected with adenovirus (40 MOI) for 48 h. The Noxa expression vector was constructed in pcDNA3.1, using a PCR-mediated method, and confirmed by sequencing and Western blotting.
Western blotting
Antibodies used for Western blotting included those against caspase-8, caspase-3, Myc (Cell Signaling Technology, Beverly, MA), cytochrome c, α-tubulin (BD Biosciences), caspase-9 (Stressgen Bioreagents, Ann Arbor, MI), cytochrome oxidase subunit IV (Cox IV; Invitrogen), Bcl-2 (Dako, Carpinteria, CA, USA), PUMA (27), p53 (DO1), cIAP-1, cIAP-2 (R&D system), XIAP (Invitrogen), Survivin (Cell Signaling), Bim, Noxa and Smac (EMD Biosciences, Gibbstown, NJ). Western blotting analysis was performed as previously described (28).
Immunoprecipitation (IP)
Cells were harvested 24 h with or without Smac mimetic treatment (150 nM) in T-75 flasks, and resuspended in 1 ml of EBC buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.5% Nonidet P-40) supplemented with protease inhibitors cocktail (Roche Applied Sciences). The cells were disrupted by sonication and then spun at 10,000 g for 10 min to collect the cell lystate. For immunoprecipitation (IP), 2 μg of antibodies or control IgG were added to protein G dynabeads (Invitrogen) for 1 h followed by incubation with 400 μl cell lysates according to manufacturer’s instructions. After the final wash, the beads were mixed with 50 μl of 1x Laemmli sample buffer, heated at 95 °C for 10 min, and analyzed by Western blotting.
Apoptosis assays
Adherent and floating cells were harvested, stained with Hoechst 33258 (Invitrogen), and analyzed for apoptosis by nuclear staining assay and flow cytometry(28). For detection of mitochondrial membrane potential change, harvested cells were stained by Mito Tracker Red CMXRos (Molecular Probes) for 15 min at 37°C and analyzed by flow cytometry using the FL3 channel, according to the manufacturer’s instructions. For colony formation assays, equal numbers of cells were subjected to various treatments and plated into 12-well plates at different dilutions. Colonies were visualized by crystal violet staining 11 to 14 days after plating as previously described (28). Each experiment was performed in triplicate and repeated at least twice.
Analysis of cytochrome c release
Mitochondrial and cytosolic fractions were isolated from treated cells by differential centrifugation as previously described (27, 29). Concentrations of cytosolic fractions obtained from different samples were normalized using a protein assay dye reagent from Bio-Rad (Benicia, CA). All fractions were mixed with equal volumes of 2x Laemmli sample buffer and subjected to Western blotting analysis.
Stable SMAC knockdown (KD) and SMAC over-expressing (SO) cells cells
SMAC shRNA was constructed using the pSUPER vector (Oligoengine, Seattle, WA), as described (30). Puromycin-resistant clones were isolated as previously described (31). Western blotting was used to identify stable clones with significant down-regulation of Smac in HNSCC lines JHU-012, JHU-019, JHU-022 and 1483. For Smac SO cells, JHU-012 and 1483 cells were transfected with an expression construct encoding either Myc-tagged wild-type Smac (AVPI) or mutant Smac with deletion of alanine in the AVPI domain (ΔA) (32), and were selected by G418 (1 mg/ml for JHU-012; 1.2 mg/ml for 1483). Stable clones expressing Smac were identified by Western blotting. Drug resistant transfectants without KD or SO behaved similarly to the parental cells in response to chemodrugs tested. The parental (P) cells were therefore chosen as the controls.
Xenograft tumors
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. JHU-012 and 1483 xenografts were established and measured, as described (28). In brief, 5–6 week old female athymic nude mice (Harlan, Indianapolis, IN) were inoculated with JHU-012 or 1483 (5×106 cells per site) on both flanks. Tumors were allowed to establish for 10 days. The tumor volumes were measured in two dimensions using a vernier caliper. Mice were randomized into groups (7 mice per group), such that the average tumor volume across the groups was the same. Gemcitabine or vehicle (ddH2O) treatments were administered intraperitoneally (i.p.) at 80 mg/kg thrice on days 10, 13 and 16 (33). For all in vivo experiments, tumor volumes were measured every other day in 2 dimensions and volumes were determined in mm3 using the formula l × b2 ×0.52 (where l is the larger diameter and b is the smaller diameter of the tumor). Mice were injected i.p. 2 h before sacrifice with a single dose of bromodeoxyuridine (BrdU) at 150 mg/kg to label cells in S phase. BrdU was dissolved in PBS to a final concentration of 30 mg/mL. Histologic and immunofluorescence analysis for apoptosis and proliferation were performed on 5-μM frozen sections, as described (28).
Statistical analysis
Statistical analysis was carried out using GraphPad Prism IV software. All P-values were calculated by the student’s t-test, and P<0.05 was considered significant. Means ± one standard deviation (SD) were displayed in figures where applicable.
Results
Smac mediates apoptosis induced by therapeutic agents in HNSCC cells
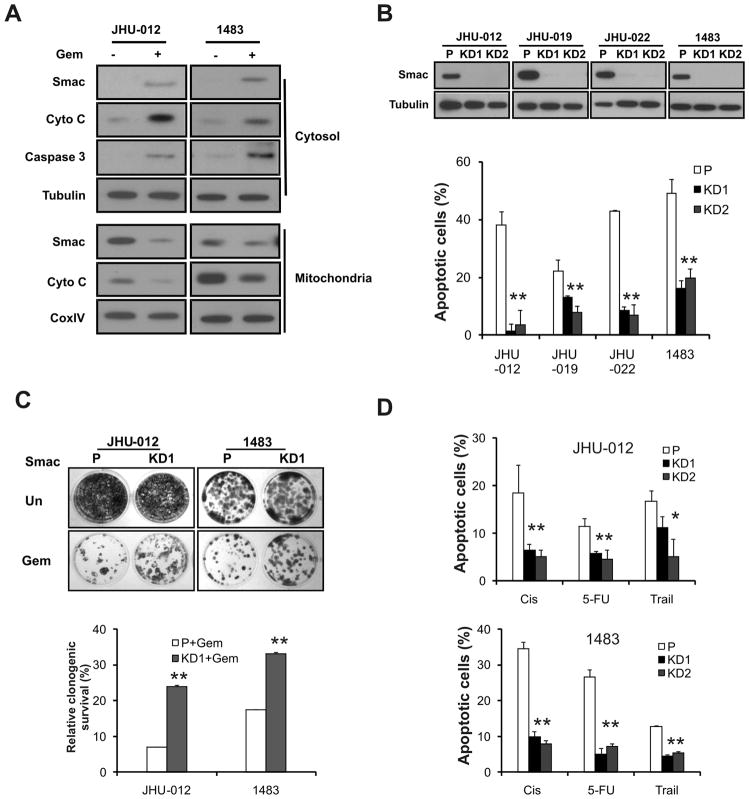
To determine a potential role of Smac in chemotherapeutic agents-induced apoptosis in HNSCC cells, we first analyzed several biochemical markers of apoptosis following gemcitabine treatment. Gemcitabine was found to induce cytosolic release of cytochrome c and Smac, and caspase 3 activation in 4 HNSCC lines, including JHU-012, 1483, JHU-019 and JHU-022 cells (Fig. 1A and data not shown). We then generated stable SMAC knockdown (KD) cells in these 4 lines by small hairpin RNA (shRNA)-mediated gene silencing. Two independent SMAC-KD clones of each line were produced. SMAC-KD cells were found to be resistant to gemcitabine-induced apoptosis, compared with parental cells (Fig. 1B and S1). SMAC knockdown partially rescued long-term cell growth suppression induced by gemcitabine in JHU-012 and 1483 cells (Fig. 1C). In addition, SMAC knockdown significantly blocked apoptosis induced by other therapeutic agents, including cisplatin, 5-FU and Trail in HNSCC cells (Fig. 1D). These data demonstrate that Smac mediates apoptosis induced by several classes of anti-cancer agents in HNSCC cells.
Figure 1. Smac mediates apoptosis induced by therapeutic agents in HNSCC cells.
(A) Gemcitabine induced release of Smac and cytochrome c, and activation of caspase 3. JHU-012 and 1483 cells were treated with 50 μM gemcitabine for 48 h. Smac and cytochrome c in the cytosolic and mitochondrial fractions were analyzed by Western blotting. Tubulin and CoxIV were used as controls for fraction and loading. (B) SMAC knockdown blocked gemcitabine-induced apoptosis. Upper, examples of stable knockdown of SMAC clones in indicated HNSCC lines were identified by Western blotting. Lower, apoptosis was analyzed by nuclear fragmentation assay. P-parental cells; KD1, KD2- two independent knockdown clones. (C) SMAC knockdown enhanced clonogenic survival of HNSCC cells following gemcitabine treatment. JHU-012 and 1483 cells were treated by 10 μM gemcitabine or left untreated for 6 h, then plated at 1:500 dilution (~400 cells per well) in 12-well plates and allowed to form colonies for 14 days. Upper panel, representative pictures of the colonies. Lower panel, the colonies containing >50 cells were enumerated and relative survival calculated with untreated cells set at 100%. (D) SMAC knockdown blocked apoptosis induced by multiple therapeutic agents. JHU-012 and 1483 cells were treated with indicated agents for 48 h. Apoptosis was measured by nuclear fragmentation assay. Cis, cisplatin (50 μM), 5-FU, 5-Fluorouracil (50 μg/mL) and Trail (100 ng/mL). **, P < 0.01, *, P < 0.05, KD vs. P.
Smac mediates gemcitabine-induced apoptosis through the mitochondrial pathway
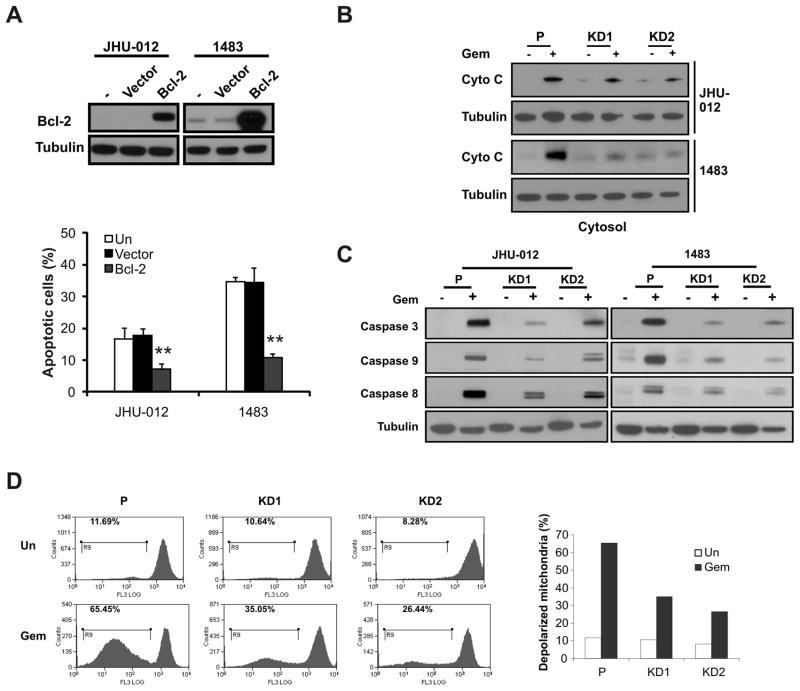
We further examined the potential mechanism of Smac-mediated and gemcitabine-induced apoptosis. Over-expression of Bcl-2 blocked apoptosis induced by gemcitabine in both JHU-012 and 1483 cells (Fig. 2A), suggesting an important role of the mitochondrial pathway. We then compared biochemical markers of the mitochondrial pathway in parental and SMAC-KD cells following gemcitabine treatment. Consistent with reduced apoptosis, release of cytochrome c and activation of caspases 3, 8 and 9 were significantly attenuatedin SMAC-KD cells, compared with parental cells(Fig. 2B and 2C). In addition, mitochondrial membrane depolarization was significantly blocked in SMAC-KD cells (Fig. 2D).
Figure 2. Smac mediates gemcitabine-induced apoptosis through the mitochondrial pathway.
(A) Bcl-2 suppressed gemcitabine-induced apoptosis. JHU-012 or 1483 cells were transfected with a Bcl-2 expression construct or empty vector followed by 50 μM gemcitabine treatment for 48 h, respectively. Upper, the expression of Bcl-2 was analyzed by Western blotting. Lower, apoptosis was analyzed by nuclear fragmentation assay. **P<0.01, Bcl-2 vs. vector or mock (un) transfected group. (B) Cytochrome c release in parental and SMAC-KD cells treated as in (A) was analyzed by Western blotting in the cytosolic fractions. Tubulin was used as control for loading. (C) Activation of caspases 3, 8 and 9 was analyzed by Western blotting in indicated cells treated with 50 μM gemcitabine for 48 h. (D) Left, Mitochondrial membrane depolarization in JHU-012 parental and SMAC-KD cells was analyzed by flow cytometry 48 h after 50 μM gemcitabine treatment. Right, quantitation of depolarized cells
DNA damage is known to activate the expression of several BH3-only proteins and the mitochondrial apoptosis pathway through p53 stabilization. We found that BH3-only proteins PUMA, Bim and Noxa were strongly induced by gemcitabine, while p53 was not consistently induced (Fig. S2A). SMAC knockdown blocked apoptosis induced by exogenous expression of PUMA or Noxa (Fig. S2B). In addition, elevated expression of PUMA or Noxa sensitized HNSCC cells to apoptosis induced by gemcitabine (Fig. S2C). These data suggest that regulation of the Bcl-2 family of proteins is likely to be upstream of Smac, and Smac mediates gemcitabine-induced apoptosis and caspase activation by promoting mitochondrial damage, such as membrane depolarization and cytochrome c release, in a positive feedback loop (19, 34, 35).
SMAC over-expression potentiates gemcitabine-induced apoptosis
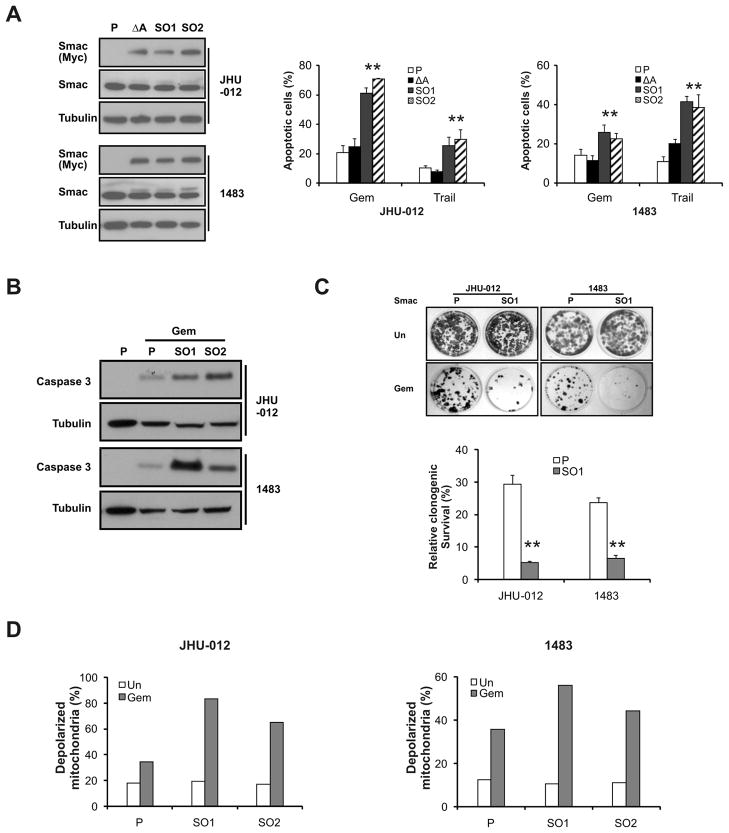
The N-terminal AVPI residues of cytosolic Smac mediate caspase activation (36). To determine whether this function of Smac is important for apoptosis induced by chemotherapeutics in HNSCC cells, we stably expressed either a wild-type (AVPI) or mutant Smac, containing a deletion of alanine in the AVPI domain (ΔA) that abolishes the interactions between Smac and IAPs (19, 32), in JHU-012 and 1483 cells. Expression of exogenous, mature Smac was lower than that of the endogenous Smac in the stable lines (Fig. 3A, Smac panel). Nonetheless, gemcitabine-induced apoptosis and caspase activation were potentiated by the expression of the wild-type, but not the mutant Smac (Fig. 3A and 3B). Further, wild-type, but not mutant Smac, enhanced growth inhibition (Fig. 3C) and mitochondrial membrane depolarization induced by gemcitabine (Fig. 3D). These results suggest that elevated levels of Smac sensitize HNSCC cells to anticancer drug-induced apoptosis and growth inhibition by promoting caspase activation and mitochondrial damage.
Figure 3. SMAC over-expression potentiates gemcitabine-induced apoptosis.
(A) Generation of stable WT SMAC over-expression (SO) cells and mutant (ΔA) cells. P-parental cells; ΔA -mutant SMAC over-expression cells; SO1 and SO2- independent SMAC over-expression clones. Left, Smac expression was analyzed by Western blotting in indicated cell lines. Right, apoptosis in JHU-012 or 1483 cells following indicated treatment for 48 h was analyzed by nuclear fragmentation assay. **, P < 0.01, SO vs. P. Gem, 50 μM and Trail, 100 ng/ml. (B) The indicated cell lines were treated with gemcitabine (50 μM) for 48 h. Activation of caspase 3 was analyzed by Western blotting. (C) Long-term cell growth was assessed by colony formation assay as in 1C. Cells were treated with 10 μM gemcitabine for 6 h before plating. Upper, representative pictures of colonies. Lower, quantitation of colony numbers with untreated cells set at 100%. **, P < 0.01, SO vs. P. (D) Mitochondrial membrane depolarization was analyzed by flow cytometry 48 h after 50 μM gemcitabine treatment in indicated cells.
A Smac mimetic potentiates gemcitabine-induced apoptosis through the mitochondrial pathway
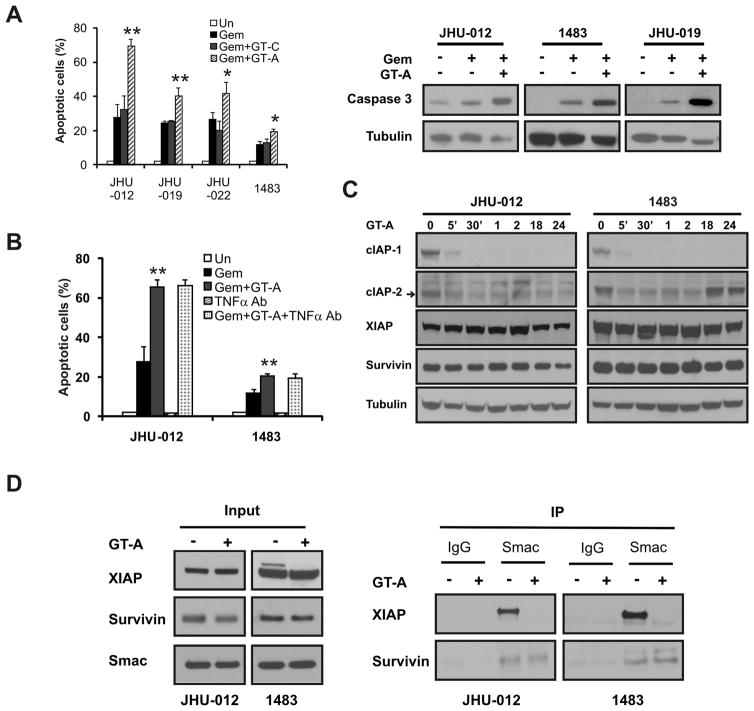
The requirement of the AVPI domain for Smac function prompted us to test whether pharmacologic agents that mimic this domain can enhance gemcitabine-induced apoptosis. An active Smac mimetic compound GT-A at nanomolar concentrations, but not the control compound GT-C, sensitized HNSCC cells to gemcitabine-induced apoptosis (Fig. 4A). GT-A at 100nM markedly enhanced gemcitabine-induced apoptosis in JHU-012 cells, increasing from 32% to 69% at 48 h with 50 μM gembitabine (Fig. 4A), which is associated with markedly enhanced caspase 3 activation and cytochrome c release (Figs. 4A and S3A). Combinations of gemcitabine with GT-A, but not GT-C, inhibited long-term survival and growth of HNSCC cells more effectively compared with gemcitabine alone (Fig. S3B and S3C). The GT-A compound also sensitized HNSCC cells to cisplatin-induced apoptosis and long-term growth suppression (Fig. S4). GT-A or the control compound alone up to 1 μM did not have detectable growth inhibitory or apoptotic effects on HNSCC cells, or on caspase activation or cytochrome c release (Fig. 4A, 4B and data not shown).
Figure 4. The Smac mimetic potentiates gemcitabine-induced apoptosis.
Cells were treated with 50 μM gemcitabine with or without 100 nM control (GT-C) or active (GT-A) Smac mimetic compound for 48 h or as indicated. (A) Left, apoptosis induction was determined by nuclear staining in 4 HNSCC lines at 48 h. **, P < 0.01, *, P < 0.05, GT-A+ Gem vs. GT-C+Gem. Right, caspase 3 activation was analyzed at 48 h by Western blotting. (B) JHU-012 and 1483 cells were incubated with TNFα Ab (5 μg/ml) for 1 h, then treated with gemcitabine alone or combined with GT-A for 48 h. Apoptosis was determined by nuclear fragmentation assay. **, P < 0.01, GT-A+Gem vs. GT-C+Gem. (C) The indicated cells were treated with 100 nM GT-A. The expression of cIAP-1, cIAP-2, XIAP and survivin was analyzed by Western blotting at indicated time points. (D) The indicated cells were treated with 150 nM GT-A for 24 h. Left, the whole cell extracts (5% input) were analyzed for the expression of XIAP, Survivin and Smac. Right, the cell extracts were immunnoprecipitated with IgG control or Smac antibody, and blotted for XIAP or Survivin.
Smac mimetics were recently reported to induce rapid degradation of cIAP-1/2, leading to nuclear factor-κB activation, TNF-α secretion and apoptosis in some cancer cells (23–26). To probe this potential mechanism in Smac mimetic-induced chemosensitization of HNSCC cells, we treated the cells with TNF-α neutralizing antibody prior to exposing them to gemcitabine and GT-A. However, the TNF-α antibody did not block apoptosis induced by the gemcitabine and GT-A combination in JHU-012, JHU-019, JHU-022 or 1483 cells (Fig. 4B and data not shown). In contrast, the TNF-α neutralizing antibody effectively blocked apoptosis induced by TNF-α alone or by the TNF-α and GT-A combination in both JHU-012 and JHU-019 cells (Fig. S6A), as well as apoptosis induced by GT-A in HT-29 cells as reported before (Fig, S6B). We further determined the levels of cIAP-1/2 at several time points following GT-A treatment. The GT-A compound induced a rapid down-regulation of cIAP-1/2, but cIAP-2 levels restored within 18 h, long before significant levels of apoptosis (Fig. 4C). Reduced levels in cIAP-1/2 proteins were not due to decreased mRNA levels (Fig. S5).
Other IAP proteins such as XIAP (36) or Survivin (37, 38) can bind to Smac either in the cytosol or mitochondria to suppress apoptosis. Therefore, the Smac mimetic might promote dissociation of endogenous Smac from XIAP or survivin via competitive binding. The treatment of Smac mimetic did not affect the levels of XIAP, survivin, or Smac, but induced a complete dissociation of Smac and XIAP within 24 h in HNSCC cells (Fig. 4D). The interactions between Smac and survivin were unaffected by GT-A (Fig. 4D). These studies established that Smac mimetic-induced chemosensitization in HNSCC cells is mediated through enhanced caspase activation and mitochondrial damage, but not the TNF-α signaling. Nonetheless, the TNF-α signaling is intact and synergizes with the Smac mimetic to induced apoptosis in HNSCC cells.
A Smac mimetic restores gemcitabine sensitivity in the SMAC-KD cells
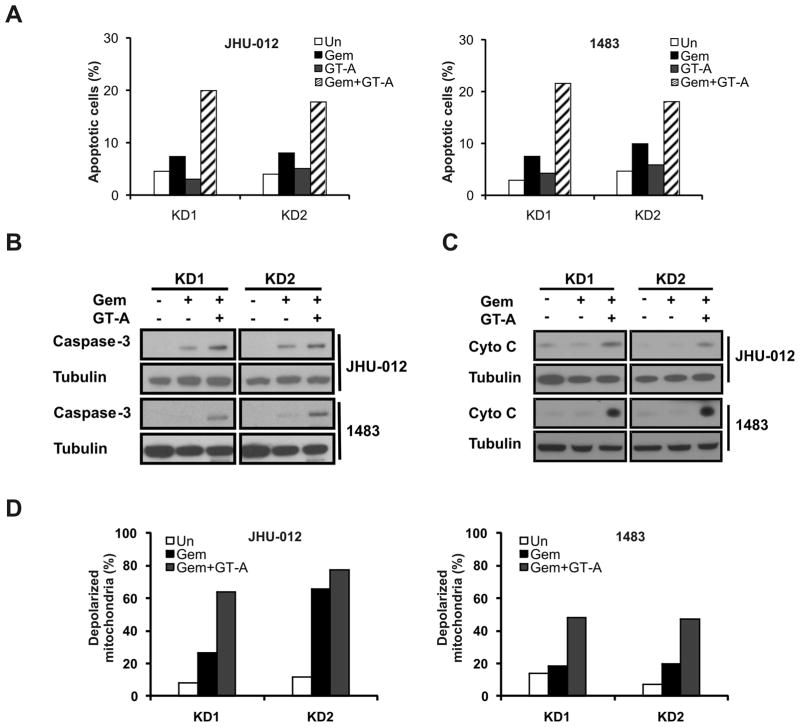
SMAC-KD cells are resistant to gemcitabine-induced caspase activation and apoptosis compared to parental cells (Figs. 4A and 5A). We expected the GT-A compound to restore these events, bypassing the need for Smac protein. Indeed, the active compound GT-A, but not the control compound GT-C, restored apoptosis induced by gemcitabine in SMAC-KD cells (Fig. 5A). The GT-A compound markedly enhanced caspase activation and cytochrome c release (Fig. 5B and 5C), and fully restored mitochondrial membrane depolarization in SMAC-KD cells (Fig. 5D). These data suggest that activation of the mitochondrial apoptotic pathway by Smac or Smac mimetics can potentially overcome gemcitabine resistance.
Figure 5. The Smac mimetic restores gemcitabine sensitivity in SMAC-KD cells.
SMAC-KD cells were treated with 50 μM gemcitabine with or without 100 nM control (GT-C) or active (GT-A) Smac mimetic compound for 48 h. (A) Apoptosis was analyzed by flow cytometry. (B) Caspase 3 activation was analyzed by Western blotting. (C) Cytochrome c release was analyzed by Western blotting. (D) Mitochondrial membrane depolarization was analyzed by flow cytometry.
Smac modulates gemcitabine sensitivity of HNSCC in vivo
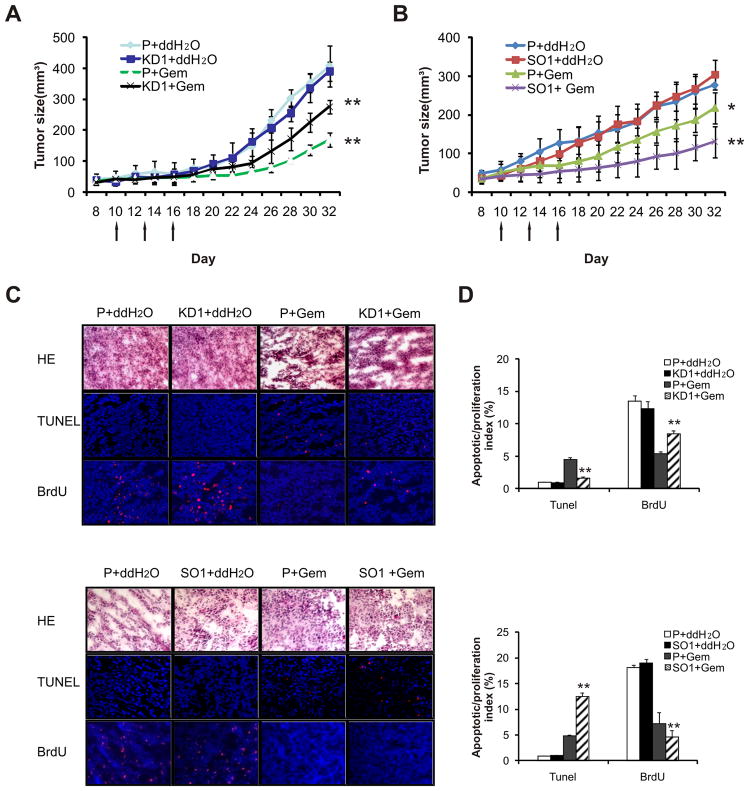
To assess whether Smac modulates therapeutic responses in vivo, parental cells, SMAC over-expression (SMAC-SO) or knockdown (SMAC-KD) HNSCC cells were injected subcutaneously into the flanks of BALB/c (nu/nu) nude mice to establish xenografts. Gemcitabine was administered i.p. into tumor-bearing mice on three occasions. Comparing with water control, gemcitabine treatment resulted in 70.5% (P < 0.01) and 41.4 % (P < 0.01) growth inhibition in 1483 parental and SMAC-KD tumors, respectively (Fig. 6A). Similarly, gemcitabine administration inhibited JHU-012 parental and SMAC-KD tumors by 71.8 % (P < 0.01) and 33.8% (P < 0.01), respectively (Supplementary Fig. S4A). Meanwhile, gemcitabine treatment resulted in 21.2% (P < 0.01) and 57.2% (P < 0.01) growth inhibition in parental and SMAC-SO tumors, respectively (Fig. 6B). The differences between the responses of the tumors with different SMAC genotypes were statistically significant, while little or no difference was found in the efficiency or growth rate in tumor establishment in the absence of treatment (Figs. 6A, 6B and S7A).
Figure 6. Smac modulates gemcitabine sensitivity of HNSCC cells in vivo.
The responses of SMAC knockdown (KD) or overexpression (SO) xenograft tumors to gemcitabine were compared that to 1483parental (P) xenograft tumors. Gemcitabine (80 mg/kg/day) was administrated to tumor bearing mice on day 10, 13 and 16 as indicated by arrows. (A) Growth curves of 1483 parental (P) and SMAC KD1 xenograft tumors (n = 7 per group) subjected to gemcitabine or control treatments. **, P < 0.01. KD1+Gem vs. P+Gem, and KD1+Gem vs. KD1+ddH2O. (B) Growth curve of 1483 parental and SMAC over-expression (SO1) tumors (n = 7 per group) subjected to gemcitabine or control treatment. *, P < 0.05, SO1+Gem vs. P+Gem, **, P < 0.01, SO1+Gem vs. SO1+ddH2O. (C) Frozen sections of indicated 1483 tumors 48 h after the second injection were analyzed by H&E staining. Apoptosis and proliferation were analyzed by TUNEL staining (red) and BrdU incorporation (red), respectively. The nuclei were counterstained by 4′,6-diamidino-2-phenylindole (blue). Magnification, ×400. (D) Index of TUNEL-positive or BrdU-labeled cells in 1483 tumors with indicated genotypes 24 h after the second gemcitabine injection. **, P < 0.01, KD1 (or SO1)+Gem vs. P+Gem.
Analysis of tumor sections after the second gemcitabine injection (day 15) revealed significantly lower apoptosis and higher cell proliferation in SMAC-KD tumors compared with parental tumors. In contrast, SMAC-SO tumors showed more extensive apoptosis (13% vs. 5%) and lower proliferation than parental tumors (Figs. 6D and S7B). These results demonstrate that the levels of Smac modulate the therapeutic responses of HNSCC cells to gemcitabine in vivo through apoptosis.
Discussion
Smac in anticancer agents-induced apoptosis in HNSCC cells
IAP family members are frequently over-expressed in many solid tumors including HNSCC. Over-expression of IAPs was reported to be associated with worse prognosis in HNSCC (13, 14). Biochemical studies indicate that IAP proteins are antagonized by Smac in mammals, which promotes caspase activation and apoptosis through its N-terminal AVPI motif (21). The structural basis of such interactions led to the development of several small molecule Smac mimetics, also called IAP antagonists, which are believed to compete with caspases for IAP binding and consequently release caspases to promote cell death (20–22). Despite extensive biochemical data, SMAC-KO mice or mouse fibroblasts show limited if any alteration in apoptosis (18). Our study showed, for the first time in HNSCC cells, that Smac plays an important role in apoptosis induced by several classes of anticancer agents, and elevated Smac levels or a Smac mimetic compound potentiates therapeutic responses of HNSCC cells by promoting apoptosis.
Our observations are consistent with the notion that a requirement of Smac in apoptosis appears to be cell type- and agent-dependent (30, 35). Over-expression of Smac or Smac mimetics can potentiate anticancer effects of chemotherapeutic agents and irradiation in glioma, hepatoma, neuroblastoma, glioblastoma, or pancreatic carcinoma cells (25, 39–41). It is of interest to note that the killing or sensitizing effects of Smac or Smac mimetics appear somewhat selective towards cancer cells compared with normal or untransformed cells (16). The precise mechanisms of this differential sensitivity remain unclear, which might be explained partly by the addiction of cancer cells to over-expression of IAPs or perhaps alterations in other upstream regulators such as the Bcl-2 family of proteins and the death receptors (16).
Signaling events in Smac-mediated apoptosis and the mitochondria
Emerging evidence suggests that induction of BH3-only proteins by therapeutic agents might be a universal mechanism underlying favorable and apoptotic responses of cancer cells (28, 31, 42–45). In HNSCC cells, the BH3-only sub family plays a critical role in regulating their survival, whose expression is suppressed by an oncogenic form of p63 over-expressed in majority of squamous cancers (31, 46, 47). Our data demonstrate that BH3-only proteins PUMA, Bim and Noxa are induced by gemcitabine mostly likely through a p53-independent mechanism, and SMAC knockdown blocked apoptosis induced by PUMA or Noxa. Therefore, multiple BH3-only proteins might be involved in promoting mitochondrial damage and Smac release during gemcitabine-induced apoptosis. In addition, SMAC knockdown abrogated apoptotic responses to several anticancer agents, which is rescued by the Smac mimetic. Despite a clear role of Smac in activating capases following its release from the mitochondria, our data suggest that the release of apoptogenic proteins might not be independent, and a complex and positive feedback mechanism might exist to regulate mitochondrial outer membrane permeability (MOMP) and caspase activation (30, 34, 35, 48).
Mechanisms of Smac mimetic-induced chemosensitization in HNSCC cells
Smac mimetics can induce rapid degradation of cIAP-1/2, leading to TNF-α-dependent apoptosis through the nuclearfactor-κB signaling in some cells (23–26, 49). In this study, the GT-A compound was also found to induce rapid down-regulation of cIAP-1/2, with cIAP-2 levels restored long before apoptosis. These observations coupled with failure of the TNF-α neutralizing antibody to block apoptosis induced by the gemcitabine and Smac mimetic combination would suggest that the TNF-α signaling is unlikely to be responsible for the chemosensitization effects of Smac mimetics. Rather, a more direct mechanism engaging caspase activation and mitochondrial damage is mediated, at least in part, via the dissociation of endogenous Smac and XIAP. Since Smac is found predominantly in the mitochondria cells such as HNSCC cells, it is reasonable to predict that this dissociation occurs at the mitochondria. It is also possible that Smac mimetics active additional proapoptic proteins (50) by displacing them from IAPs either in the cytosol or mitochondria. The selective involvement of TNF-α signaling (23–26, 49) or the mechanism described here might reflect the cell type specific role of endogenous Smac and/or the structural and functional differences of the small molecule Smac mimetics.
Implications on novel combination therapies in HNSCC
In Phase II studies, gemcitabine in combination with other chemotherapeutics or radiation has shown improved response rates in HNSCC patients with advanced diseases, compared to single-agent regimes (2–4). However, severe mucocitis is a common complication in combination settings. Our data suggest that this side effect might be reduced without compromising therapeutic efficacies, potentially by using lower doses of gemcitabine with Smac mimetics. Smac mimetics might be useful as sensitizers for other anticancer agents to boost apoptosis in otherwise resistant tumor cells. Several apoptotic blocks exist in cancer cells, and the Bcl-2 family of proteins have become promising targets with the development of a class of so called BH3 mimetics, or Bcl-2 antagonists (51, 52). In HNSCC cells, induction of BH3-only proteins in response to 5-FU or cisplatin is often blocked by defective p53 signaling (28), while their induction by gemcitabine appears largely p53-independent (this study). Even modest over-expression of BH3-only proteins such as PUMA or Noxa can sensitize HNSCC cells to these agents, independent of p53 status. Smac and BH3-only proteins act at different steps of apoptosis and participate in a positive feedback loop to activate caspase and mitochondrial damage. Therefore, targeting either or both steps with Smac and BH3 mimetics depending on the genetic background of the tumors might bring us one step closer to individualized HNSCC treatment.
Supplementary Material
Acknowledgments
We thank other members of Yu and Zhang laboratories for helpful discussions. This work is supported by NIH grant CA129829, American Cancer Society grant RGS-10-124-01-CCE and FAMRI (J Yu), and by NIH grants CA106348, CA121105 and American Cancer Society grant RSG-07-156-01-CNE (L Zhang).
Abbreviations
- IAPs
Inhibitors of apoptosis proteins (IAPs)
- TNF-α
Tumor Necrosis Factor Alpha
- Trail
Tumor Necrosis Factor-related Apoptosis-inducing Ligand
- 5-FU
5-Fluorouracil
- NF-κB
nuclear factor kappa B
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Airoldi M, Cattel L, Cortesina G, et al. Gemcitabine and vinorelbine in recurrent head and neck cancer: pharmacokinetic and clinical results. Anticancer Res. 2003;23:2845–52. [PubMed] [Google Scholar]
- 3.Jiang Y, Wei YQ, Luo F, et al. Gemcitabine and cisplatin in advanced nasopharyngeal carcinoma: a pilot study. Cancer Invest. 2005;23:123–8. [PubMed] [Google Scholar]
- 4.Aguilar-Ponce J, Granados-Garcia M, Villavicencio V, et al. Phase II trial of gemcitabine concurrent with radiation for locally advanced squamous cell carcinoma of the head and neck. Ann Oncol. 2004;15:301–6. doi: 10.1093/annonc/mdh071. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Zhang L. Apoptosis in human cancer cells. Curr Opin Oncol. 2004;16:19–24. doi: 10.1097/00001622-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86:104–14. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 10.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trask DK, Wolf GT, Bradford CR, et al. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002;112:638–44. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 13.Lippert BM, Knauer SK, Fetz V, Mann W, Stauber RH. Dynamic survivin in head and neck cancer: molecular mechanism and therapeutic potential. Int J Cancer. 2007;121:1169–74. doi: 10.1002/ijc.22941. [DOI] [PubMed] [Google Scholar]
- 14.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 15.Velculescu VE, Madden SL, Zhang L, et al. Analysis of human transcriptomes. Nat Genet. 1999;23:387–8. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 16.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–75. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 17.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Okada H, Suh WK, Jin J, et al. Generation and characterization of Smac/DIABLO-deficient mice. Mol Cell Biol. 2002;22:3509–17. doi: 10.1128/MCB.22.10.3509-3517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bank A, Wang P, Du C, Yu J, Zhang L. SMAC mimetics sensitize nonsteroidal anti-inflammatory drug-induced apoptosis by promoting caspase-3-mediated cytochrome c release. Cancer Res. 2008;68:276–84. doi: 10.1158/0008-5472.CAN-07-5242. [DOI] [PubMed] [Google Scholar]
- 20.Oost TK, Sun C, Armstrong RC, et al. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem. 2004;47:4417–26. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design of potent, conformationally constrained Smac mimetics. J Am Chem Soc. 2004;126:16686–7. doi: 10.1021/ja047438+. [DOI] [PubMed] [Google Scholar]
- 23.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Vince JE, Wong WW, Khan N, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Petersen SL, Wang L, Yalcin-Chin A, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q, Sakaida T, Yue W, Gollin SM, Yu J. Chemosensitization of head and neck cancer cells by PUMA. Mol Cancer Ther. 2007;6:3180–8. doi: 10.1158/1535-7163.MCT-07-0265. [DOI] [PubMed] [Google Scholar]
- 29.Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–42. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- 30.Kohli M, Yu J, Seaman C, et al. SMAC/Diablo-dependent apoptosis induced by nonsteroidal antiinflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl Acad Sci U S A. 2004;101:16897–902. doi: 10.1073/pnas.0403405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Q, Ming L, Thomas SM, et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009;18:2348–57. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang QH, Du C. Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem. 2004;279:16963–70. doi: 10.1074/jbc.M401253200. [DOI] [PubMed] [Google Scholar]
- 33.Rolff J, Dorn C, Merk J, Fichtner I. Response of Patient-Derived Non-Small Cell Lung Cancer Xenografts to Classical and Targeted Therapies Is Not Related to Multidrug Resistance Markers. J Oncol. 2009;2009:814140. doi: 10.1155/2009/814140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7:227–33. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Wang P, Ming L, Wood MA, Zhang L. SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189–98. doi: 10.1038/sj.onc.1210196. [DOI] [PubMed] [Google Scholar]
- 36.Wu G, Chai J, Suber TL, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–12. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 37.Song Z, Yao X, Wu M. Direct interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosis. J Biol Chem. 2003;278:23130–40. doi: 10.1074/jbc.M300957200. [DOI] [PubMed] [Google Scholar]
- 38.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–27. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–15. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, Jin J, Zhang X, et al. Transfection of Smac sensitizes tumor cells to etoposide-induced apoptosis and eradicates established human hepatoma in vivo. Cancer Gene Ther. 2006;13:420–7. doi: 10.1038/sj.cgt.7700910. [DOI] [PubMed] [Google Scholar]
- 41.Giagkousiklidis S, Vogler M, Westhoff MA, Kasperczyk H, Debatin KM, Fulda S. Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res. 2005;65:10502–13. doi: 10.1158/0008-5472.CAN-05-0866. [DOI] [PubMed] [Google Scholar]
- 42.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–79. doi: 10.1371/journal.pmed.0040315. discussion 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–89. doi: 10.1371/journal.pmed.0040316. discussion 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J, Yue W, Wu B, Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin Cancer Res. 2006;12:2928–36. doi: 10.1158/1078-0432.CCR-05-2429. [DOI] [PubMed] [Google Scholar]
- 46.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Barbieri CE, Barton CE, Pietenpol JA. Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem. 2003;278:51408–14. doi: 10.1074/jbc.M309943200. [DOI] [PubMed] [Google Scholar]
- 48.Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297:1352–4. doi: 10.1126/science.1074721. [DOI] [PubMed] [Google Scholar]
- 49.Probst BL, Liu L, Ramesh V, et al. Smac mimetics increase cancer cell response to chemotherapeutics in a TNF-alpha-dependent manner. Cell Death Differ. 2010;17:1645–54. doi: 10.1038/cdd.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8:613–21. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 51.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–17. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.