Abstract
Methylmercury (MeHg) preferentially accumulates in glia of the central nervous system (CNS), but its toxic mechanisms have yet to be fully recognized. In the present study, we tested the hypothesis that MeHg induces neurotoxicity via oxidative stress mechanisms, and that these effects are attenuated by the antioxidant, ebselen. Rat neonatal primary cortical astrocytes were pretreated with or without 10 μM ebselen for 2 hours followed by MeHg (0, 1, 5, and 10 μM) treatments. MeHg-induced changes in astrocytic [3H]-glutamine uptake were assessed along with changes in mitochondrial membrane potential (ΔΨm), using the potentiometric dye tetramethylrhodamine ethyl ester (TMRE). Western blot analysis was used to detect MeHg-induced ERK (extracellular-signal related kinase) phosphorylation and caspase-3 activation. MeHg treatment significantly decreased (p<0.05) astrocytic [3H]-glutamine uptake at all time points and concentrations. Ebselen fully reversed MeHg's (1 μM) effect on [3H]-glutamine uptake at 1 min. At higher MeHg concentrations, ebselen partially reversed the MeHg-induced astrocytic inhibition of [3H]-glutamine uptake [at 1 min (5 and 10 μM) (p<0.05); 5 min (1, 5 and 10 μM) (p<0.05)]. MeHg treatment (1 hour) significantly (p<0.05) dissipated the ΔΨm in astrocytes as evidenced by a decrease in mitochondrial TMRE fluorescence. Ebselen fully reversed the effect of 1 μM MeHg treatment for 1 hour on astrocytic ΔΨm and partially reversed the effect of 5 and 10 μM MeHg treatments for 1 hour on ΔΨm. In addition, ebselen inhibited MeHg-induced phosphorylation of ERK (p<0.05) and blocked MeHg-induced activation of caspase-3 (p<0.05 to 0.01). These results are consistent with the hypothesis that MeHg exerts its toxic effects via oxidative stress and that the phosphorylation of ERK and the dissipation of the astrocytic mitochondrial membrane potential are involved in MeHg toxicity. In addition, the protective effects elicited by ebselen reinforce the idea that organic selenocompounds represent promising strategies to counteract MeHg-induced neurotoxicity.
Keywords: ebselen, methylmercury neurotoxicity, mitochondrial membrane potential, oxidative stress
Introduction
Methylmercury (MeHg) is an organic form of mercury (Hg) with toxic effects in multiple organs, and is one of the most poisonous environmental pollutants (Bakir et al., 1973; Takeuchi, 1989; Castoldi et al., 2008). It is a highly and selectively neurotoxic compound, leading to neurological and developmental deficits in the central nervous system (CNS), both in humans and experimental animals (Choi 1989; Clarkson et al., 2003; Pinheiro et al., 2008). MeHg preferentially accumulates in astrocytes and inhibits glutamate uptake in these cells. The toxic mechanism(s) of MeHg has yet to be fully understood (Aschner 2000).
Mitochondria, which are the main sites for the glutamate/GABA-glutamine cycle, represent a major target of MeHg (Allen et al., 2001). Earlier studies reported that cultured astrocytes ceased respiration at ~30 min after MeHg treatment, reflecting inhibition of the mitochondrial electron transport chain (Yee and Choi 1996; Allen et al., 2001; Shanker et al., 2004). MeHg-induced decrease of mitochondrial membrane potential has also been reported in neurons (Limke and Atchison 2002) and other cell types (InSug et al., 1997; Shenker et al., 1998). In the liver, MeHg has been shown to inhibit mitochondrial function, leading to K+ influx and membrane depolarization (Sone et al., 1977).
Glutamine is an important precursor for the synthesis of the primary excitatory neurotransmitter glutamate and inhibitory neurotransmitter γ-aminobutyric acid (GABA) (Boulland et al., 2002). Astrocytes-derived glutamine is taken up by neurons, where it is metabolized to glutamate, which, in turn, upon neuronal activity is released into the synaptic cleft and taken up by astrocytes via a Na+-dependent mechanism. Subsequently, glutamate is converted to glutamine by a highly active glutamine synthetase (Sidoryk-Wegrzynowicz et al., 2009).
Ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one) is an organic selenium compound. Selenium is a structural component of several enzymes with physiological antioxidant properties, including glutathione (GSH) peroxidases (Flohé, et al., 1973), and it is known to possess anti-oxidant and anti-inflammatory properties (Cotgreave et al., 1989; Yang et al., 1999; Mugesh and Singh, 2000; Parnham and Sies, 2000). Of particular importance, the organoselenium compound, ebselen, has been demonstrated to be neuroprotective in preclinical studies (Saito et al., 1998; Davalos, 1999; Porciuncula et al., 2001; Satoh, et al., 2004; Centuriao, et al., 2005; Yamagata K et al., 2008). The antioxidant activity of organoselenides has been attributed to their GSH peroxidase-like activity (Muller et al., 1984; Wendel et al., 1984). More recently it has been demonstrated that ebselen is reduced by mammalian thioredoxin reductase (TrxR) forming ebselen selenol/selenolate (Zhao and Holmegren, 2002; De Freitas et al. 2010). Selenolate intermediates are potent nucleophiles and can readily react with electrophilic species, including reactive oxygen species (ROS) (Masumoto et al. 1996; Zhao and Holmegren 2002)
The present study was carried out to examine the effects of MeHg on glutamine metabolism and mitochondrial inner membrane potential (ΔΨm) in cultured astrocytes and to test the hypothesis that ebselen can effectively attenuate the toxicity of this metal. Additional studies addressed the efficacy of ebselen in attenuating MeHg-induced ERK phosphorylation and apoptosis via the activation of caspase-3.
2. Materials and methods
2.1. Materials
L-[G-3H]glutamine (specific activity: 49.0 Ci/mmol) was purchased from Amersham Biosciences (Piscataway, NJ). Methylmercuric chloride (MeHgCl) was purchased from ICN Biomedicals (Costa Mesa, CA). Minimal essential medium (MEM) with Earle's salts, heat-inactivated horse serum, penicillin, streptomycin and tetramethylrhodamine ethyl ester (TMRE) were purchased from Invitrogen (Carlsbad, CA).
2.2. Primary astrocyte cultures
Methodologies for the isolation and culturing of cerebral cortical astrocytes derived from newborn (1-day-old) Sprague–Dawley rats were previously described (Yin at al 2007). In brief, rat pups were decapitated and the cerebral cortices removed. After carefully removing the meninges, the cerebral cortices were digested with bacterial neutral protease (Dispase, Invitrogen, Carlsbad, CA) and astrocytes recovered by repeated removal of dissociated cells from brain tissues. Twenty-four hours after the initial plating in BD Falcon 6- and 12-well plates, the medium was changed to preserve the adhering astrocytes and remove the neurons, microglia and oligodendrocytes. The cultures were maintained at 37°C in a 95% air / 5% CO2 incubator for 3 weeks in MEM with Earle's salts supplemented with 10% heat-inactivated horse serum, 100 U/ml penicillin and 100 μg/ml streptomycin. The medium was changed twice per week. These monolayer, surface-adhering cultures were >95% positive for the astrocytic marker, glial fibrillary acidic protein (GFAP).
2.3. Measurement of changes in mitochondrial membrane potential (ΔΨm)
The ΔΨm was measured with the potentiometric dye tetramethylrhodamine ethyl ester (TMRE). TMRE accumulates in mitochondria as a function of the ΔΨm. At the end of treatments, the culture medium was removed (duplicate plates per experiment; repeated three times using different batches of astrocytes) and the cells were loaded for 20 min at 37°C in a 5% CO2 incubator with TMRE at a final concentration of 50 nM in sodium-HEPES buffer. Cells were rinsed with phosphate buffered saline (PBS) and examined with a Zeiss inverted fluorescent microscope (Zeiss Axiovert S100, Carl Zeiss MicroImaging, Inc.) equipped with a cooled digital camera (Photometrics CoolSNAP, Roper Scientific Photometrics, Tucson, AZ) controlled by computer software (Image Pro Laboratories, Stamford, CT). Images of various fields in each plate were captured at 10× magnification. Fluorescent intensities were obtained from 8 randomly selected fields per experiment and were analyzed with NIH software (Scion Incorporation, Frederick, MD). In each image field, the total number of pixels was quantified on a gray scale (0–255 counts) and the mean pixel value in was expressed as mean ± S.E.M. of the total number of mean pixel values in each group. The fluorescent intensities were expressed as percent fluorescence change over control.
2.4. Determination of the neuroprotective effects of ebselen on ΔΨm
Immediately after 2 hour pretreatment with or without ebselen (10 μM) in Na-HEPES buffer, MeHg was added for 1 hour to confluent astrocyte cultures (3 weeks post isolation) at 0, 1, 5, or 10 μM. Next, the cells were washed twice with 2 ml of Na-HEPES buffer and TMRE was loaded at a final concentration of 50 nM in Na-HEPES buffer for 20 min. Next astrocytes were washed with PBS and fluorescence was monitored as described above.
2.5. Western blot analysis
Astrocytes were treated with or without ebselen (10 μM) for 2 hours before exposure to MeHg (1, 5 or 10 μM) for various time periods. The cells were then lysed with lysis buffer [Tris-HCl, pH 7.4, 20 mM, EDTA 2.5 mM, Triton X-100 1%, sodium deoxycholate 1%, SDS 0.1%, NaCl 100 mM, PMSF 1.0 mM, leupeptin 10 μg/ml, pepstatin 10 μg/ml] and collected for protein concentration determination by BCA assay (Pierce, Rockford, IL). An equal amount of protein (30 μg) was loaded and run on a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane (PerkinElmer Life Sciences, Boston, MA). The primary antibodies used were polyclonal anti-ERK1/2, monoclonal anti-phospho-ERK, polyclonal anti-caspase-3, and monoclonal anti-β-actin. The secondary antibodies were peroxidase conjugated (HRP) goat anti-rabbit IgG or goat anti-mouse IgG (Pierce, Rockford, IL). Supersignal West Pico (Pierce, Rockford, IL) was used for horseradish peroxidase (HRP) detection on a Hyperfilm ECL system (Nikon, Melville, NY). Stripping of the membrane was performed in Restore Western Blot Stripping Buffer (Pierce, Rockford, IL) as required. The levels of phosphorylated ERK (p–ERK) were expressed as arbitrary units of optical density, following the correction for content of total ERK (ERK1/2). Band intensities of caspase-3 were corrected for loading with β-actin. Densitometry measurement of band intensities was quantified and expressed as arbitrary units (AlphaEaseFC Imaging System software, Alpha Innotech, San Leandro, CA).
2.6. 3H-Glutamine uptake in astrocytes
Astrocytes were studied 3 weeks post isolation, when the cell monolayer became fully confluent. Cells in 6-well plates were washed three times with 2 ml of fresh sodium-HEPES buffer consisting of: 122 mM NaCl, 3.3 mM KCl, 0.4 mM MgSO4, 1.3 mM CaCl2, 1.2 mM KH2PO4, 10 mM glucose, and 25 mM HEPES (N-2-hydroxy-ethylpiperazine N'-2-ethansulfonic acid), adjusted to pH 7.4 with 10 M NaOH. Immediately after 2 hour treatment with or without ebselen (10 μM) in sodium-HEPES buffer, astrocytes were treated in sodium-HEPES buffer only, or with sodium-HEPES buffer containing MeHg (1, 5, or 10 μM) for 30 min in a 37°C, 95% air / 5% CO2 incubator. Cells were then washed three times with 2 ml of Na-HEPES buffer; thereafter, 1 ml of pre-warmed buffer containing 1 μCi/ml L-[G-3H]glutamine was added to each well and glutamine uptake was measured at 1 min and 5 min at room temperature. At each time point, the reactions were stopped by aspirating the buffer from the well, followed by 5 washes with 2 ml of ice-cold mannitol buffer [290 mM mannitol, 10 mM Tris-nitrate, 0.5 mM Ca(NO3)2, pH adjusted to 7.4 with KOH]. At the end of the experiments, cells were lysed in 2 ml of 1 M NaOH. An aliquot of 25 μl was used for protein determination with the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). An aliquot of 750 μl was used for radioactivity measurement by liquid scintillation and the values were expressed as cpm/mg protein (Tri-Carb 2900TR, Perkin Elmer Life Science).
2.7. Statistical analysis
3H-glutamine uptake and release experiments were conducted in duplicate wells/experiment, and the mean from three to four independent experiments was used for statistical analysis. 3H-Glutamine uptake and release were corrected for cellular protein levels and expressed as cpm/mg protein. TMRE staining was conducted in triplicate wells and the mean from three independent experiments was used for statistical analysis. Western blotting was performed three to four times. All data were expressed as percentage of control (100%) ± S.E.M. The data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni multiple test with statistical significance set at p<0.05. When the overall significance resulted in the rejection of the null hypothesis (p<0.05), the source of the variance was determined with the Tukey-Kramer test (also known as the Tukey range test). All analyses were carried out with GraphPad software for Microsoft Windows (Graph Pad Software, San Diego, CA).
3. Results
3.1. Ebselen abolishes MeHg-induced astrocytic ΔΨm
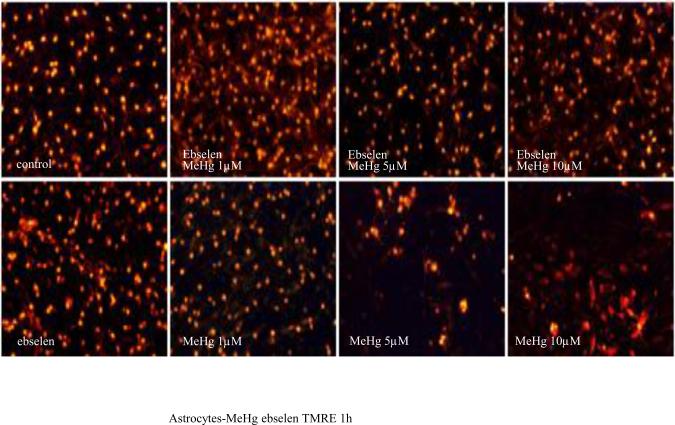
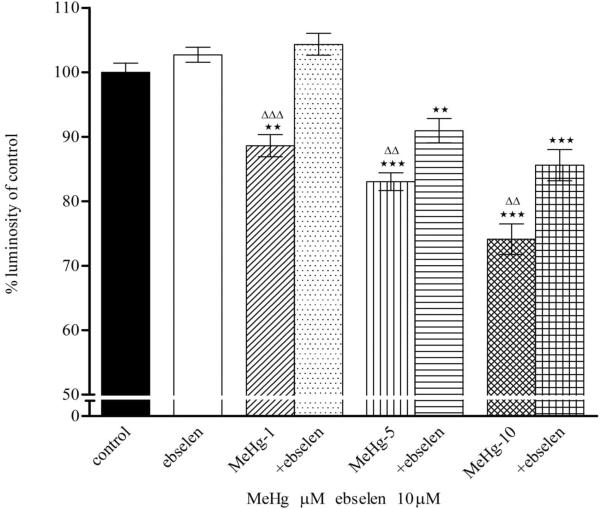
Reactive oxygen species (ROS) generation has been linked to MeHg-induced neurotoxicity. To investigate the mechanisms by which MeHg induces ΔΨm, astrocytes were treated with various concentrations of MeHg for 1 hour, and ΔΨm was measured by TMRE fluorescence. As shown in Fig. 1A, treatments with MeHg alone for 1 hour resulted in qualitative dissipation of the ΔΨm in cultured astrocytes, as demonstrated by decrease in mitochondrial TMRE fluorescence. Quantification of TMRE fluorescent intensities (Fig. 1B) revealed that 1-hour treatment with MeHg at all tested concentrations (1, 5, and 10 μM) caused significant dissipation of ΔΨm. Ebselen alone (2 hour treatment) did not affect astrocytic ΔΨm (p>0.05 compared with controls). Pre-treatment with ebselen (2 hours) followed by treatment with MeHg fully abolished the 1 μM MeHg-induced decrease in TMRE fluorescence (p>0.05 compared with control), and partially prevented the dissipation of the mitochondrial membrane potential in astrocytes treated with 5 or 10 μM MeHg.
Figure 1.
Effects of MeHg/ebselen on mitochondrial ΔΨ. Cultured astrocytes were treated with or without ebselen (10 μM) for 2 hours and then were exposed to MeHg at various concentrations (0, 1, 5, or 10 μM) for 1 hour. (A) Fluorescent microscopy shows the mitochondrial ΔΨ after TMRE staining. (B) Quantitative analyses (see Materials and Methods Section) of TMRE fluorescence (ΔΨ). Values are expressed as mean ± SEM of 24 random fields in each group. Experiments were performed in three independently isolated sets of cultures. * p<0.05, ** p<0.01, *** p<0.001 versus control; ΔΔΔ p<0.001 versus MeHg plus ebselen treatments in the paired groups by one-way ANOVA followed by Bonferroni multiple comparison tests.
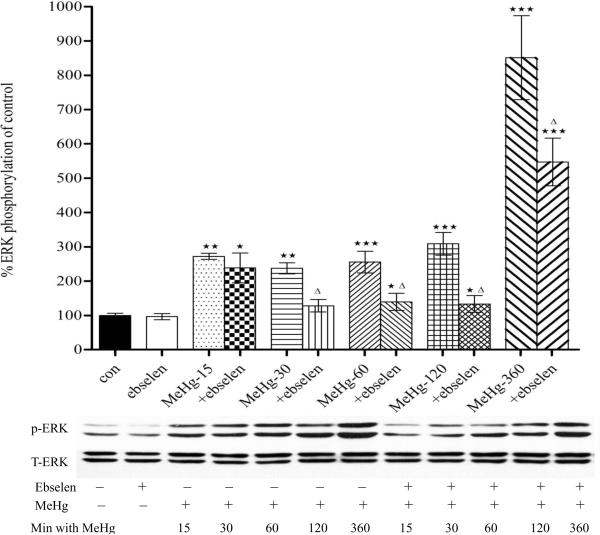
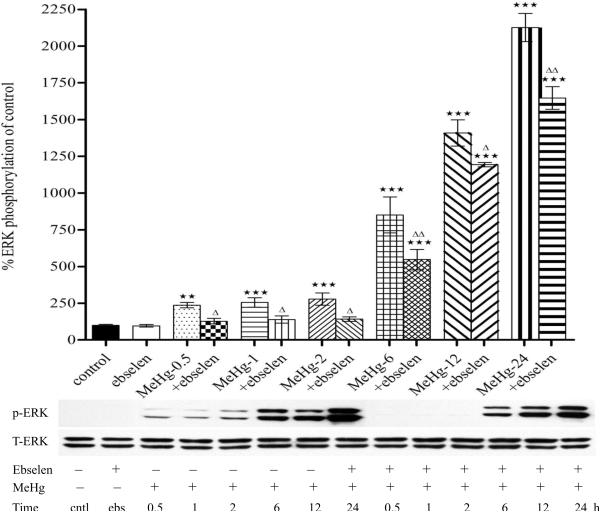
3.2. Ebselen attenuates MeHg-induced ERK phosphorylation in astrocytes
The possible involvement of the mitogen-activated protein kinase (MAPK) subfamily in MeHg-induced neuronal injury was studied by measuring the phosphorylation levels of extracellular-signal regulated kinase (ERK). As shown in Fig. 3A, MeHg alone induced a robust increase in the levels of phosphorylated ERK in astrocytes as early at 15 min post exposure (p<0.01, compared with control) and the phosphorylation status remained elevated for at least 24 hours post MeHg treatment (Figs. 2A and 2B). Ebselen alone (2 hour treatment) did not increase the basal levels of phosphorylated ERK (p>0.05, compared with controls). Pretreatment with ebselen (2 hours) followed by MeHg exposure significantly (p<0.05 vs. MeHg alone) attenuated the effect of MeHg-induced ERK phosphorylation from 30 min to 24 hours of treatment (Figs. 2A and 2B).
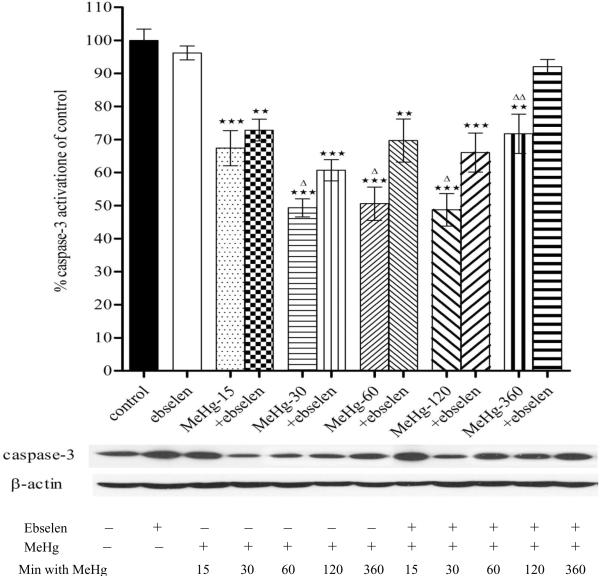
Figure 3.
Effects of MeHg/ebselen on activation of caspase-3 precursor in cultured astrocytes. Prior to exposure to MeHg, astrocytes were pretreated with or without ebselen (10 μM) for 2 hours. Values are mean ± SEM of 4–6 independent experiments in each group. Statistical analysis was carried out by one-way ANOVA followed by Bonferroni multiple comparison tests; ** p<0.01, *** p<0.001 versus control; Δ p<0.05; ΔΔ p<0.01 versus MeHg plus ebselen treatments in the paired groups.
Figure 2.
Effects of MeHg/ebselen on ERK phosphorylation in cultured astrocytes as determined by immunoblotting (Figure 3A, 5 to 360 min; Figure 3B, 30 min to 24 hours). Prior to exposure to MeHg, astrocytes were pretreated with or without ebselen (10 μM) for 2 hours. Values are mean ± SEM of 4–6 independent experiments in each group. Statistical analysis was carried out by one-way ANOVA followed by Bonferroni multiple comparison tests; * p<0.05, ** p<0.01, *** p<0.001 versus control; Δ p<0.05; ΔΔ p<0.01 versus MeHg plus ebselen treatments in the paired groups.
3.43. Ebselen diminishes MeHg-induced caspase-3 cleavage in astrocytes
Next we investigated whether MeHg promotes caspase-3 cleavage in astrocytes. As shown in Fig. 3, MeHg treatment significantly promoted caspase-3 cleavage in astrocytes from 15 min to 6 hours. Ebselen alone (2 hour treatment) did not change caspase-3 cleavage values (p>0.05, compared with control). Combination treatments of ebselen (2 hour pretreatment) followed by MeHg exposure significantly prevented the MeHg alone-induced promoting effect on caspase-3 cleavage.
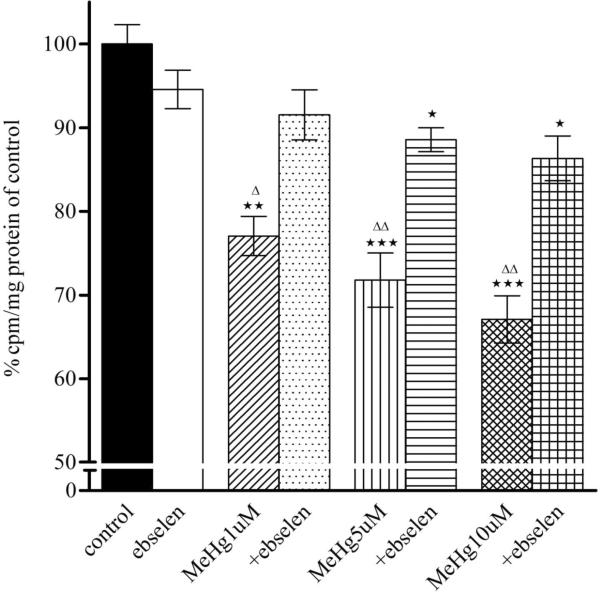
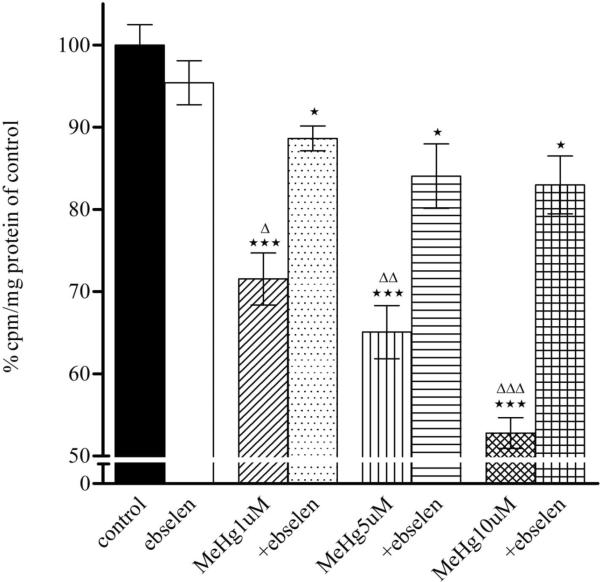
3.4. Ebselen reverses astrocytic MeHg-induced glutamine uptake inhibition
As shown in Fig. 4, MeHg (1, 5, and 10 μM) treatment alone for 30 min significantly inhibited in a concentration-dependent manner the astrocytic uptake of glutamine at 1 min (p<0.01 or p<0.001) and 5 min (p<0.001). Ebselen (10 μM) treatment alone for 2 hours did not affect astrocytic glutamine uptake (p>0.05 compared with control). Pre-treatment with ebselen displayed a significant protective effect against MeHg-induced glutamine uptake inhibition and this protection was observed at either 1 or 5 min.
Figure 4.
Effects of MeHg/ebselen on glutamine uptake in astrocytes. Rat primary astrocytes cultures were pretreated with/without ebselen for 2 hours and then incubated for 30 min at 37°C in the absence or presence of MeHg (1, 5, or 10 μM), and the net uptake of glutamine (3H-glutamine) was quantified at 1 min (1A) and 5 min (1B), respectively. Values are expressed as, mean ± SEM (n=4–6). Experiments were performed in three independently isolated sets of cultures. * p<0.05, ** p<0.01, *** p<0.001 versus control; Δ p<0.05; ΔΔ p<0.01, ΔΔΔ p<0.001 versus MeHg plus ebselen treatments in the paired groups by one-way ANOVA, followed by Bonferroni multiple comparison tests.
4. Discussion
The present study demonstrates for the first time, that the antioxidant ebselen pretreatment stabilizes the mitochondrial membrane potential (Fig. 1), inhibits MeHg-induced ERK phosphorylation (Fig. 2) and attenuating the MeHg-induced promoting effect on caspase-3 cleavage (Fig. 3) and protects astrocytes from MeHg by restoring glutamine uptake (Fig 4),. Phosphorylation of ERK, caspase-3 cleavage and the collapse of the mitochondrial inner membrane potential (ΔΨm) represent early events in MeHg-induced neurotoxicity (Milatovic et al., 2007), which are linked to the demise in cellular homeostasis and ROS generation. Oxidative stress has been implicated in various neurodegenerative conditions as well as in metal-induced neurotoxicity (Bush, 2000). Studies in neuronal cultures, neuronal and glial co-cultures and recent studies in primary astrocytic cultures have all demonstrated increased ROS formation upon MeHg exposure (Ali et al., 1992; Gasso et al., 2001; Mundy and Freudenrich, 2000; Shanker et al., 2003; 2005; Yin et al., 2007).
Ebselen is an organic selenium compound. Selenium is a structural component of several enzymes with physiologically antioxidant properties (Muller et al. 1984; Mugesh and Singh 2000). A number of selenium compounds possess chemical and biological antioxidant properties (Mugesh and Singh, 2000; Parnham and Sies, 2000; Imai et al., 2001; Nakamura et al., 2002; Herrera et al., 2003; Kalayci et al., 2005; Gabryel and Małecki, 2006; Johnsen-Soriano et al., 2007; Tripathi and Jena, 2008; Tak and Park, 2009). Ebselen was demonstrated to be neuroprotective in preclinical and clinical studies (Saito et al., 1998; Yamaguchi et al., 1998; Davalos, 1999) and in a variety of in vitro and in vivo animal models of neuropathological conditions, including ischemia (Dawson et al., 1995; Imai et al. 2003; Porciuncula et al., 2003), quinolinic acid- or glutamate-induced excitotoxicity (Porciuncula et al., 2001; Rossato et al., 2002a,b) and exposure to MeHg (Farina et al., 2003; Moretto et al., 2005; Funchal et al., 2006; Roos et al., 2009). The antioxidant activity of ebselen has been tentatively attributed to its GSH peroxidase-like activity (Muller et al., 1984; Wendel et al., 1984) and to its ability to serve as a substrate for mammalian thioredoxin reductase (TrxR), which metabolizes ebselen to its selenol/selenolate intermediate (Zhao and Holmegren, 2002; De Freitas et al. 2010). In vivo treatment with ebselen and diphenyl diselenide can reduce MeHg neurotoxicity in rodents (Farina et al. 2003a,b; de Freitas et al. 2009). Accordingly, ebselen could have decreased MeHg toxicity in astrocytes as reported herein via its selenol by decreasing MeHg-induced oxidative stress and by a direct interaction of its selenol with MeHg, forming a non-toxic stable intermediate.
Our experiment also showed that MeHg, in a concentration-dependent manner, led to significant dissipation of ΔΨm (Figure 1). These changes were fully or partially prevented by pretreatment with ebselen, attesting to the effectiveness of ebselen in reducing MeHg-induced ROS generation. Furthermore, as discussed above, the selenol intermediated of ebselen could interact directly with MeHg, thus decreasing its toxicity. Loss of the ΔΨm leads to colloid osmotic swelling of the mitochondrial matrix (Gunter and Pfeiffer, 1990), redistribution of metabolites (Ca2+, Mg2+, Glutathione, NADPH) across the inner membrane, defective oxidative phosphorylation, cessation of ATP synthesis, and the generation of ROS. These mitochondrial changes may initiate a cascade of events culminating in cell death (apoptosis or necrosis) (Berbardi et al., 1998; Kroemer and Reed, 2000). The ΔΨm is a sensitive indicator for the energetic state of the mitochondria and the cell and can be used to assess the activity of the mitochondrial respiratory chain, electrogenic transport systems and the activation of the mitochondrial permeability transition (Ly et al., 2003). Thus, evaluation of ΔΨm depolarization is of critical importance for the assessment of cellular metabolism, viability and apoptosis.
Extracellular signal-regulated kinases (ERK1/2), one of the members of mitogen-activated protein kinase (MAPK), respond to several extracellular stimuli and are activated by MAPK/ERK kinase1/2 (MEK1/2) by phosphorylating threonine and tyrosine residues (Seger and Krebs 1995). It is known that oxidative stress activates MAPK cascades (Herrlich and Böhmer, 1999; Allen and Tresini 2000). ERK activation is generally considered a pro-survival pathway (Baines et al., 2002; de Bernardo et al., 2003), but increasing evidence suggests that phosphorylation of ERK also contributes to cell death (Chu et al., 2004; Zhuang and Schnellmann, 2006; Ren et al., 2009). The level of ERK phosphorylation or its kinetics may play a role, as inhibiting basal ERK signaling has different effects than inhibiting toxin-induced ERK activation (Gomez-Santos et al., 2002). Furthermore, the time course of ERK activation is tightly correlated with mitochondrial ROS production and antioxidants inhibit ERK phosphorylation and rescue from neuronal injury (Chu et al., 2004; Kulich et al., 2007). ERK1/2 stimulation by ROS has been described in neurons (Samanta et al., 1998) and neuroprotection by MEK inhibition against oxidative stress in both neurons and in astrocytes (Satoh et al., 2000; Rosenberger et al., 2001). A rapid or transient activation of ERK promotes neuronal survival (Weng et al., 2007; Lin et al., 2008), while sustained or delayed ERK activation promotes cell death (Kulich and Chu, 2001; Gomez-Santos et al., 2002; Zhu et al., 2007). ERK can modulate mitochondrial functions (particularly those associated with cell death) and promote oxidative injuries (Alonso et al., 2004; Chu et al., 2004; Kulich et al., 2007; Dagda et al., 2008). In addition, activation of ERK is involved in the induction of apoptosis in cortical astrocytes (Blazquez et al., 2000; Oh et al., 2006). Our results showed that MeHg activates ERK phosphorylation in a time-dependent manner (Fig. 2A and 2B). While early ERK phosphorylation may be protective, sustained activation (for at least 24 hours) (Fig. 2B) is likely deleterious. Accordingly, the ability of ebselen to attenuate the time-dependent phosphorylation of ERK likely reflects its ability to protect astrocytes from the sequalae of sustained MeHg-induced ERK phosphorylation (Fig. 2A and 2B).
Caspase-3 plays a central role in mediating apoptosis, chromatin condensation and DNA fragmentation (Riedl and Shi, 2004). Therefore, caspase-3 is considered one of the major executioners of apoptosis and has classically been viewed as a terminal event in the process of programmed cell death. Accordingly, caspase-3 activation has been described in neuronal cells following specific types of central nervous system (CNS) insults, including traumatic brain injury and ischemic/excitotoxic damage (Beer et al., 2000; Nath et al., 2000; Brecht et al., 2001; Manabat et al., 2003). In vitro studies have also suggested that caspase-3 proteolytic activity plays a crucial role in excitotoxin-induced neuronal apoptosis (Allen et al., 1999; Tenneti and Lipton, 2000). In addition, expression of caspase-3 has been described in oligodendrocytes (Beer et al., 2000; Nottingham and Springer, 2003) and astrocytes following CNS damage (Beer et al., 2000; Benjelloun et al., 2003; Mouser et al., 2006). The present study also demonstrated that MeHg promotes caspase-3 cleavage and that pretreatment with ebselen partially prevents this effect (Fig. 3).
Glutamine is an energy substrate for most cells (Fox et al., 1996) and an important precursor for neurotransmitters glutamate, GABA, and in particular for GSH synthesis (Albrecht et al., 2007). Our present study indicates that MeHg inhibits the astrocytic uptake of glutamine in a concentration-dependent manner (Fig. 4), corroborating our previous reports (Allen et al. 2000; Aschner, et al. 1990; 1994; 2000; 2007; and Mutkus et al., 2005). Ebselen pretreatment (2 hours) effectively abolished the MeHg-induced reduction in glutamine uptake (Fig. 1), suggesting that it may restore the efficient cycling of glutamine between astrocytes and neurons, assuring optimal glutamine homeostasis. Mechanisms associated with decreased astrocytic glutamine uptake are related to inhibition of mRNA coding of the glutamine transporters, SNAT3/SN1 and ASCT2 (Yin et al., 2007; Sidoryk-Wegrzynowicz et al., 2009) and potentially direct inhibition of the transporter by ROS, as has been previously ascribed to glutamate uptake inhibition by MeHg (Shanker et al., 2004).
In summary, the present study demonstrates that MeHg exerts its toxic effects, at least in part, by inhibiting astrocytic glutamine uptake, collapsing the mitochondrial inner membrane potential, and triggering phosphorylation of ERK and activation of caspase-3. The selenium-containing compound, ebselen, can markedly attenuate these MeHg-induced effects. From a molecular point of view, both the thiol-peroxidase activity of ebselen and the chemical interaction of MeHg with selenol ebselen intermediate appear to be responsible for the observed protective effects. These results indicate that organic selenocompounds represent promising strategies to counteract MeHg-induced toxicity, shedding light on new pharmacological modalities for treatment of MeHg poisonings.
Acknowledgement
This study was supported by NIH Public Health Service Grant ES07331 to M.A.
Abbreviations
- ΔΨm
mitochondrial membrane potential
- CNS
central nervous system
- ERK
extracellular-signal related kinase
- MeHg
methylmercury
- ROS
reactive oxygen species
- TMRE
tetramethylrhodamine ethyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–43. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- Ali SF, LeBel CP, Bondy SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13:637–48. [PubMed] [Google Scholar]
- Allen JW, Mutkus LA, Aschner M. Isolation of neonatal rat cortical astrocytes for primary cultures. In: Maines M, Costa LG, Sipes GI, editors. Current Protocols in Toxicology. John Wiley and Sons; New York: 2000. pp. 12.4.1–12.4.15. [DOI] [PubMed] [Google Scholar]
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001;902(1):92–100. doi: 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- Allen JW, El-Oqayli H, Aschner M, Syversen T, Sonnewald U. Methylmercury has a selective effect on mitochondria in cultured astrocytes in the presence of [U-13C]glutamate. Brain Res. 2001;908:149–54. doi: 10.1016/s0006-8993(01)02628-2. [DOI] [PubMed] [Google Scholar]
- Allen JW, Knoblach SM, Faden AI. Combined mechanical trauma and metabolic impairment in vitro induces NMDA receptor-dependent neuronal cell death and caspase-3-dependent apoptosis. FASEB J. 1999;13:1875–82. doi: 10.1096/fasebj.13.13.1875. [DOI] [PubMed] [Google Scholar]
- Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–99. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Alonso M, Melani M, Converso D, Jaitovich A, Paz C, Carreras MC, Medina JH, Poderoso JJ. Mitochondrial extracellular signal-regulated kinases 1/2 (ERK1/2) are modulated during brain development. J Neurochem. 2004;89:248–56. doi: 10.1111/j.1471-4159.2004.02323.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Eberle NB, Miller K, Kimelberg HK. Interactions of methylmercury with rat primary astrocyte cultures: inhibition of rubidium and glutamate uptake and induction of swelling. Brain Res. 1990;530:245–50. doi: 10.1016/0006-8993(90)91290-w. [DOI] [PubMed] [Google Scholar]
- Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocyte cultures. Brain Res. 1994;664:133–40. doi: 10.1016/0006-8993(94)91963-1. [DOI] [PubMed] [Google Scholar]
- Aschner M. Astrocytic swelling, phospholipase A2, glutathione and glutamate: interactions in methylmercury induced neurotoxicity. Cell Mol Biol. 2000;46:843–54. 2000. [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int. 2000;37:199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40:285–91. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Atchison WD, Narahashi T. Methylmercury-induced depression of neuromuscular transmission in the rat. Neurotoxicology. 1982;3:37–50. [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–97. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–41. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Beer R, Franz G, Srinivasan A, Hayes RL, Pike BR, Newcomb JK, Zhao X, Schmutzhard E, Poewe W, Kampfl A. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J Neurochem. 2000;75:1264–73. doi: 10.1046/j.1471-4159.2000.0751264.x. [DOI] [PubMed] [Google Scholar]
- Benjelloun N, Joly LM, Palmier B, Plotkine M, Charriaut-Marlangue C. Apoptotic mitochondrial pathway in neurones and astrocytes after neonatal hypoxia-ischaemia in the rat brain. Neuropathol Appl Neurobiol. 2003;29:350–60. doi: 10.1046/j.1365-2990.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Berbardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. BioFactors. 1998;8:273–281. doi: 10.1002/biof.5520080315. [DOI] [PubMed] [Google Scholar]
- de Bernardo S, Canals S, Casarejos MJ, Solano RM, Menendez J, Mena MA. Role of extracellular signal-regulated protein kinase in neuronal cell death induced by glutathione depletion in neuron/glia mesencephalic cultures. J Neurochem. 2004;91:667–82. doi: 10.1111/j.1471-4159.2004.02744.x. [DOI] [PubMed] [Google Scholar]
- Blázquez C, Galve-Roperh I, Guzmán M. De novo-synthesized ceramide signals apoptosis in astrocytes via extracellular signal-regulated kinase. FASEB J. 2000;14:2315–22. doi: 10.1096/fj.00-0122com. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Osen KK, Levy LM, Danbolt NC, Edwards RH, Storm-Mathisen J, Chaudhry FA. Cell-specific expression of the glutamine transporter SN1 suggests differences in dependence on the glutamine cycle. Eur J Neurosci. 2002;15:1615–31. doi: 10.1046/j.1460-9568.2002.01995.x. [DOI] [PubMed] [Google Scholar]
- Brecht S, Gelderblom M, Srinivasan A, Mielke K, Dityateva G, Herdegen T. Caspase-3 activation and DNA fragmentation in primary hippocampal neurons following glutamate excitotoxicity. Brain Res Mol Brain Res. 2001;94:25–34. doi: 10.1016/s0006-8993(01)02767-6. [DOI] [PubMed] [Google Scholar]
- Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–91. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regul Toxicol Pharmacol. 2008;51:215–29. doi: 10.1016/j.yrtph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Centurião FB, Corte CL, Paixão MW, Braga AL, Zeni G, manuelli T, Rocha JB. Effect of ebselen and organochalcogenides on excitotoxicity induced by glutamate in isolated chick retina. Brain Res. 2005;1039:146–52. doi: 10.1016/j.brainres.2005.01.062. [DOI] [PubMed] [Google Scholar]
- Choi BH. The effects of methylmercury on the developing brain. Prog Neurobiol. 1989;32:447–70. doi: 10.1016/0301-0082(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–66. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury-current exposures and clinical manifestation. N Engl J Med. 2003;349:1731–37. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA, Duddy SK, Kass GE, Thompson D, Moldéus P. Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C? Biochem Pharmacol. 1989;38:649–656. doi: 10.1016/0006-2952(89)90211-6. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson's disease. Autophagy. 2008;4:770–82. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos A. New treatments in cerebrovascular diseases. Neurologia. 1999;14:77–83. [PubMed] [Google Scholar]
- Dawson DA, Masayasu H, Graham DI, Macrae IM. The neuroprotective efficacy of ebselen (a glutathione peroxidase mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci Lett. 1995;185:65–69. doi: 10.1016/0304-3940(94)11226-9. [DOI] [PubMed] [Google Scholar]
- de Freitas AS, Funck VR, Rotta MD, Bohrer D, Morschbacher V, Puntel RL, Nogueira CW, Farina M, Aschner M, Rocha JBT. Brain Res Bull. 2009;79:77–84. doi: 10.1016/j.brainresbull.2008.11.001. [DOI] [PubMed] [Google Scholar]
- de Freitas AS, Prestes AS, Wagner C, Sudati JH, Alves D, Porciúncula LO, Kade IJ, Rocha JBT. Reduction of diphenyl diselenide and analogs by mammalian Thioredoxin Reductase is independent of their gluthathione peroxidase-like activity: A possible novel pathway for their antioxidant activity. Molecules. 2010;15:7699–714. doi: 10.3390/molecules15117699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, Rocha JB, Souza DO. Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicol Lett. 2003a;144:351–57. doi: 10.1016/s0378-4274(03)00242-x. [DOI] [PubMed] [Google Scholar]
- Farina M, Dahm KCS, Schwalm FD, Brusque AM, Frizzo MES, Zeni G, Souza DO, Rocha JBT. Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: Modulatory effect of ebselen. Tox Sci. 2003b;73:135–40. doi: 10.1093/toxsci/kfg058. [DOI] [PubMed] [Google Scholar]
- Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, Suñol C. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci. 2009;112:416–26. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- Fox RE, Hopkins IB, Cabacungan EB, Tildon JT. The role of glutamine as an energy source in the developing rat lung. J Nutr. 1996;126:1131S–36S. doi: 10.1093/jn/126.suppl_4.1131S. [DOI] [PubMed] [Google Scholar]
- Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol. 2007;20(12):1919–26. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med. 2009;47(4):449–57. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Funchal C, Moretto MB, Vivian L, Zeni G, Rocha JBT, Pessoa-Pureur R. Diphenyl ditelluride- and methylmercury-induced hyperphosphorilation of the high molecular weight neurofilament subunit is prevented by organoselenium compounds in cerebral cortex of young rats. Toxicology. 2006;222:143–53. doi: 10.1016/j.tox.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Gabryel B, Małecki A. Ebselen attenuates oxidative stress in ischemic astrocytes depleted of glutathione. Comparison with glutathione precursors. Pharmacol Rep. 2006;58:381–92. [PubMed] [Google Scholar]
- Gasso S, Cristofol RM, Selema G, Rosa R, Rodriguez-Farre E, Sanfeliu C. Antioxidant compounds and Ca2+ pathway blockers differentially protect against methylmercury and mercuric chloride neurotoxicity. J Neurosci Res. 2001;66:135–45. doi: 10.1002/jnr.1205. [DOI] [PubMed] [Google Scholar]
- Ghisleni G, Porciúncula LO, Mioranzza S, Boeck CR, Rocha JB, Souza DO. Selenium compounds counteract the stimulation of ecto-nucleotidase activities in rat cultured cerebellar granule cells: putative correlation with neuroprotective effects. Brain Res. 2008;1221:134–40. doi: 10.1016/j.brainres.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Gómez-Santos C, Ferrer I, Reiriz J, Viñals F, Barrachina M, Ambrosio S. MPP+ increases alpha-synuclein expression and ERK/MAP-kinase phosphorylation in human neuroblastoma SH-SY5Y cells. Brain Res. 2002;935:32–9. doi: 10.1016/s0006-8993(02)02422-8. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–86. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA. 2003;100:7919–24. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P, Böhmer FD. Redox regulation of signal transduction in mammalian cells. Biochem Pharmacol. 2000;59:35–41. doi: 10.1016/s0006-2952(99)00298-1. [DOI] [PubMed] [Google Scholar]
- Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–54. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- Imai H, Graham DI, Masayasu H, Macrae IM. Antioxidant ebselen reduces oxidative damage in focal cerebral ischemia. Free Radic Biol Med. 2003;34:56–63. doi: 10.1016/s0891-5849(02)01180-2. [DOI] [PubMed] [Google Scholar]
- InSug O, Datar S, Koch CJ, Shapiro IM, Shenker BJ. Mercuric compounds inhibit human monocyte function by inducing apoptosis: evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transition and loss of reductive reserve. Toxicology. 1997;124:211–24. doi: 10.1016/s0300-483x(97)00153-4. [DOI] [PubMed] [Google Scholar]
- Johnsen-Soriano S, Bosch-Morell F, Miranda M, Asensio S, Barcia JM, Romá J, Monfort P, Felipo V, Romero FJ. Ebselen prevents chronic alcohol-induced rat hippocampal stress and functional impairment. Alcohol Clin Exp Res. 2007;31:486–92. doi: 10.1111/j.1530-0277.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- Kalayci M, Coskun O, Cagavi F, Kanter M, Armutcu F, Gul S, Acikgoz B. Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem Res. 2005;30:403–10. doi: 10.1007/s11064-005-2615-2. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–19. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J Neurochem. 2001;77:1058–66. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med. 2007;43:372–83. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limke TL, Atchison WD. Acute exposure to methylmercury opens the mitochondrial permeability transition pore in rat cerebellar granule cells. Toxicol. Appl. Pharmacol. 2002;178:52–61. doi: 10.1006/taap.2001.9327. [DOI] [PubMed] [Google Scholar]
- Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J Neurosci Res. 2008;86:108–17. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8:115–28. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 2003;34:207–13. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto K, Kissner R, Koppenol WH, Sies H. Kinetic study of the reaction of ebselen with peroxynitrite. FEBS Let. 1996;398:179–82. doi: 10.1016/s0014-5793(96)01237-9. [DOI] [PubMed] [Google Scholar]
- Moretto MB, Funchal C, Santos AQ, Gottfried C, Boff B, Zeni G, Pureur RP, Souza DO, Wofchuk S, Rocha JB. Ebselen protects glutamate uptake inhibition caused by methyl mercury but does not by Hg2+ Toxicology. 2005;214:57–66. doi: 10.1016/j.tox.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Mouser PE, Head E, Ha KH, Rohn TT. Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer's disease brain. Am J Pathol. 2006;168:936–46. doi: 10.2353/ajpath.2006.050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugesh G, Singh HB. Synthetic organoselenium compounds as antioxidants: glutathione peroxidase activity. Chem Soc Rev. 2000;29:347–57. [Google Scholar]
- Muller A, Cadenas E, Graf P, Sies H. A novel biologically active sleno-organic compound: Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen) Biochem Pharmacol. 1984;15:3235–39. doi: 10.1016/0006-2952(84)90083-2. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM. Sensitivity of immature neurons in culture to metal-induced changes in reactive oxygen species and intracellular free calcium. Neurotoxicology. 2000;21:1135–44. [PubMed] [Google Scholar]
- Mutkus L, Aschner JL, Syversen T, Aschner M. Methylmercury alters the in vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells. Biol Trace Elem Res. 2005;107:231–45. doi: 10.1385/BTER:107:3:231. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Feng Q, Kumagai T, Torikai K, Ohigashi H, Osawa T, Noguchi N, Niki E, Uchida K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J Biol Chem. 2002;277:2687–94. doi: 10.1074/jbc.M109641200. [DOI] [PubMed] [Google Scholar]
- Nath R, Scott M, Nadimpalli R, Gupta R, Wang KK. Activation of apoptosis-linked caspase(s) in NMDA-injured brains in neonatal rats. Neurochem Int. 2000;36:119–26. doi: 10.1016/s0197-0186(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Nottingham SA, Springer JE. Temporal and spatial distribution of activated caspase-3 after subdural kainic acid infusions in rat spinal cord. J Comp Neurol. 2003;464:463–71. doi: 10.1002/cne.10806. [DOI] [PubMed] [Google Scholar]
- Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology. 2006;27:31–8. doi: 10.1016/j.neuro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Parnham M, Sies H. Ebselen: prospective therapy for cerebral ischemia. Exp Opin Investig Drugs. 2000;9:607–19. doi: 10.1517/13543784.9.3.607. [DOI] [PubMed] [Google Scholar]
- Pinheiro MCN, Crespo-López ME, Vieira JLF, Oikawa T, Guimarães GA, Araújo CC, Amoras WW, Ribeiro DR, Herculano AM, Nascimento JLM, Silveira LCL. Mercury pollution and childhood in Amazon riverside villages. Environment International. 2008;133:56–61. doi: 10.1016/j.envint.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Porciúncula LO, Rocha JB, Boeck CR, Vendite D, Souza DO. Ebselen prevents excitotoxicity provoked by glutamate in rat cerebellar granule neurons. Neurosci Lett. 2001;299:217–20. doi: 10.1016/s0304-3940(01)01519-1. [DOI] [PubMed] [Google Scholar]
- Porciúncula LO, Rocha JB, Cimarosti H, Vinadé L, Ghisleni G, Salbego CG, Souza DO. Neuroprotective effect of ebselen on rat hippocampal slices submitted to oxygen-glucose deprivation: correlation with immunocontent of inducible nitric oxide synthase. Neurosci Lett. 2003;346:101–4. doi: 10.1016/s0304-3940(03)00580-9. [DOI] [PubMed] [Google Scholar]
- Ren Y, Jiang H, Yang F, Nakaso K, Feng J. Parkin protects dopaminergic neurons against microtubule-depolymerizing toxins by attenuating microtubule-associated protein kinase activation. J Biol Chem. 2009;284:4009–17. doi: 10.1074/jbc.M806245200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Santos MM, Souza DO, Farina M, Nogueira CW, Aschner M, Burger ME, Barbosa NB, Rocha JB. Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: involvement of oxidative stress and glutamatergic system. Toxicol In Vitro. 2009;23:302–7. doi: 10.1016/j.tiv.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Rosenberger J, Petrovics G, Buzas B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J Neurochem. 2001;79:35–44. doi: 10.1046/j.1471-4159.2001.00520.x. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Ketzer LA, Centurião FB, Silva SJ, Lüdtke DS, Zeni G, Braga AL, Rubin MA, Rocha JB. Antioxidant properties of new chalcogenides against lipid peroxidation in rat brain. Neurochem Res. 2002a;27:297–303. doi: 10.1023/a:1014907228580. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Zeni G, Mello CF, Rubin MA, Rocha JB. Ebselen blocks the quinolinic acid-induced production of thiobarbituric acid reactive species but does not prevent the behavioral alterations produced by intra-striatal quinolinic acid administration in the rat. Neurosci Lett. 2002b;318:137–40. doi: 10.1016/s0304-3940(01)02504-6. [DOI] [PubMed] [Google Scholar]
- Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, Kikuchi H, Ohta T, Ishibashi S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurismal subarachoid hemorrhage. Neurosurgery. 1998;42:269–78. doi: 10.1097/00006123-199802000-00038. [DOI] [PubMed] [Google Scholar]
- Samanta S, Perkinton MS, Morgan M, Williams RJ. Hydrogen peroxide enhances signal-responsive arachidonic acid release from neurons: role of mitogen-activated protein kinase. J Neurochem. 1998;70:2082–90. doi: 10.1046/j.1471-4159.1998.70052082.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Nakatsuka D, Watanabe Y, Nagata I, Kikuchi H, Namura S. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci Lett. 2000;288:163–6. doi: 10.1016/s0304-3940(00)01229-5. [DOI] [PubMed] [Google Scholar]
- Satoh T, Ishige K, Sagara Y. Protective effects on neuronal cells of mouse afforded by ebselen against oxidative stress at multiple steps. Neurosci Lett. 2004;371:1–5. doi: 10.1016/j.neulet.2004.04.055. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- Schousboe A, Sonnewald U, Civenni G, Gegelashvili G. Role of astrocytes in glutamate homeostasis. Implications for excitotoxicity. Adv Exp Med Biol. 1997;429:195–206. doi: 10.1007/978-1-4757-9551-6_14. [DOI] [PubMed] [Google Scholar]
- Shanker G, Syversen T, Aschner M. Astrocyte-mediated methylmercury neurotoxicity. Biol Trace Elem Res. 2003;95:1–10. doi: 10.1385/BTER:95:1:1. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner JL, Syversen T, Aschner M. Free radical formation in cerebral cortical astrocytes in culture induced by methylmercury. Molecular Brain Research. 2004;128:48–57. doi: 10.1016/j.molbrainres.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Shanker G, Syversen T, Aschner JL, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Brain Res Mol Brain Res. 2005;137:11–22. doi: 10.1016/j.molbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Guo TL, Shapiro IM. Low-level methylmercury exposure causes human T-cells to undergo apoptosis: evidence of mitochondrial dysfunction. Environ Res. 1998;77:149–59. doi: 10.1006/enrs.1997.3816. [DOI] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee E, Albrecht J, Aschner M. Manganese disrupts astrocyte glutamine transporter expression and function. J Neurochem. 2009;110:822–30. doi: 10.1111/j.1471-4159.2009.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N, Larsstuvold MK, Kagawa Y. Effect of methyl mercury on phosphorylation, transport, and oxidation in mammalian mitochondria. J Biochem. 1977;82:859–68. doi: 10.1093/oxfordjournals.jbchem.a131762. [DOI] [PubMed] [Google Scholar]
- Tak JK, Park JW. The use of ebselen for radioprotection in cultured cells and mice. Free Radic Biol Med. 2009;46:1177–85. doi: 10.1016/j.freeradbiomed.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Eto K, Tokunaga H. Mercury level and histochemical distribution in a human brain with Minamata disease following a long-term clinical course of twenty-six years. Neurotoxicology. 1989;10:651–7. [PubMed] [Google Scholar]
- Takeuchi T. Biological reactions and pathological changes in human beings and animals caused by organic mercury contamination. In: Hartung R, Dinman BD, editors. Environmental mercury contamination. Ann Arbor Science; Ann Arbor: 1972. pp. 247–89. [Google Scholar]
- Tenneti L, Lipton SA. Involvement of activated caspase-3-like proteases in N-methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 2000;74:134–42. doi: 10.1046/j.1471-4159.2000.0740134.x. [DOI] [PubMed] [Google Scholar]
- Tripathi DN, Jena GB. Ebselen attenuates cyclophosphamide-induced oxidative stress and DNA damage in mice. Free Radic Res. 2008;42:966–77. doi: 10.1080/10715760802566558. [DOI] [PubMed] [Google Scholar]
- Wendel A, Fausel M, Safayhi H, Tiegs G, Otter R. A novel biologically active seleno-organic compound-II. Activity of PZ 51 in relation to glutathione peroxidase. Biochem Pharmacol. 1984;15:3241–45. doi: 10.1016/0006-2952(84)90084-4. [DOI] [PubMed] [Google Scholar]
- Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:34479–91. doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Stroke. 1998;29:12–7. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Ichinose S, Miyashita A, Tagami M. Protective effects of ebselen, a seleno-organic antioxidant on neurodegeneration induced by hypoxia and reperfusion in stroke-prone spontaneously hypertensive rat. Neuroscience. 2008;153:428–35. doi: 10.1016/j.neuroscience.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Yang CF, Shen HM, Ong CN. Protective effect of ebselen against hydrogen peroxide-induced cytotoxicity and DNA damage in HepG2 cells. Biochem Pharmacol. 1999;57:273–9. doi: 10.1016/s0006-2952(98)00299-8. [DOI] [PubMed] [Google Scholar]
- Yee S, Choi H. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology. 1996;17:17–26. [PubMed] [Google Scholar]
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JBT, Souza DO, Sidoryk M, Albrecht J, Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–7. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]