Abstract
Immunotherapy is a promising approach for the treatment of cancers. Modified Adenovirus 5 (Ad5) vectors have been used as a platform to deliver genes encoding TAA. A major obstacle to Ad5 vector immunotherapy has been the induction of vector immunity following administration or the presence of pre-existing Ad5 immunity, which results in vector mitigation. It has been reported by us that the Ad5 [E1-, E2b-] platform with unique deletions in the E1, E2b and E3 regions can induce potent cell mediated immunity (CMI) against delivered transgene products in the presence of pre-existing Ad5 immunity. Here we report the use of an Ad5 [E1-, E2b-] vector platform expressing the TAA HER2/neu as a breast cancer immunotherapeutic agent. Ad5 [E1-, E2b-]-HER2/neu induced potent CMI against HER2/neu in Ad5 naïve and Ad5 immune mice. Humoral responses were also induced and antibodies could lyse HER2/neu expressing tumor cells in the presence of complement in vitro. Ad5 [E1-, E2b-]-HER2/neu prevented establishment of HER2/neu-expressing tumors and significantly inhibited progression of established tumors in Ad5 naïve and Ad5 immune murine models. These data demonstrate that in vivo delivery of Ad5 [E1-, E2b-]-HER2/neu can induce anti-TAA immunity and inhibit progression of HER2/neu expressing cancers.
Keywords: Immunotherapy, breast cancer, HER2/neu, Adenovirus vector, Ad5
1. Introduction
Tumor associated antigens (TAA) are typically weak immunogens; however, anti-tumor effects in many immunotherapeutic studies have been correlated with the induction of T-cell responses against TAA. Therapeutic administration of a patient’s autologous dendritic cells (DC) extracorporeally loaded with a TAA has exhibited survival benefit (1). Non-viral and viral vector platforms encoding TAA epitopes have been investigated as therapeutic vaccines (2). Importantly, viral vectors effectively transfect DC leading to antigen processing and immune presentation (3–5). Of the viral vectors investigated, Ad5 has been reported to induce robust T-cell and antibody responses against the delivered transgene product (6, 7). DC infection with Ad5 vectors encoding a variety of antigens, including the tumor antigens MART-1, MAGE-A4, DF3/MUC1, p53, hugp100 melanoma antigen, and polyoma virus middle–T antigen, have been reported to induce antigen specific cytotoxic T lymphocyte (CTL) responses, have an enhanced antigen presentation capacity, and initiate T-cell proliferation in mixed lymphocyte reactions (8–12). Arthur et al. demonstrated that Ad5 vectors encoding a variety of antigens could efficiently transduce 95% of in vivo exposed DCs to high titers of the vector (13). Increasing levels of foreign gene expression were noted in DC during increasing multiplicities of infection (MOI) (10). Ad5 infection has been reported to result in direct induction of DC maturation (14,15) and triggers IL-12 production by DC, which is a marker of DC maturation (15). These events may possibly be the result of NF-kB pathway interaction due to Ad5 viral infection (15–17). Immunization of animals with DC that have been previously transduced with Ad5 vectors encoding TAA has been reported to result in significant levels of protection when animals were challenged with tumor cells expressing the immunizing TAA (18). Studies of immature bone marrow derived DC from mice suggest that Ad5 vector infection can result in up regulation of cell surface markers, MHC I and II, CD40, CD80, CD86, and ICAM-1, normally associated with DC maturation as well as down-regulation of CD11c, an integrin known to be down regulated upon myeloid DC maturation.
The major limitation of immunotherapy using Ad5 vectored TAA is rapid neutralization of the vector due to host anti-vector immunity. The preponderance of humans harbor Ad5 immunity due to natural infection and this immunity has been reported to be a barrier to immunization in multiple animal models and in human clinical trials (19–23). The use of different Ad serotypes or even non-human forms of Ad have been evaluated in an effort to overcome pre-existing Ad5 immunity (19). Another strategy to overcome Ad5 immunity is to delete Ad5 genes necessary for producing viral proteins against which naturally arising CMI and antibodies react. Current recombinant Ad5 vectors are deleted in the E1/E3 regions ([Ad5 [E1-]). We and others have reported on immunization protocols using an improved Ad5 vector deleted in the early 1 (E1), early 2b (E2b), and early 3 (E3) gene regions (Ad5 [E1-, E2b-]) (22, 24–27). The deletion of the Ad5 polymerase (pol) and preterminal protein (pTP) within the E2b region has been reported to reduce Ad5 downstream gene expression which includes Ad5 late genes that encode highly immunogenic and potentially toxic proteins (24,28). As a result, in vivo use of the Ad5 [E1-, E2b-] vector platform has been reported to extend transgene expression, reduce inflammatory responses and exhibit fewer hepatic adverse effects (26, 28). This novel vector platform has also been reported to induce potent CMI responses in the presence of Ad5 immunity (22, 27,29).
We recently reported on the use of the Ad5 [E1-, E2b-] platform expressing the TAA carcnioembryoninc antigen (CEA) (Ad5 [E1-, E2b-]-CEA) as an immunization and immunotherapeutic modality to induce TAA targeted CMI (29). Ad5 immune mice immunized multiple times with Ad5 [E1-, E2b-]-CEA induced significantly increased CEA-specific CMI responses as compared to those detected in Ad5 immune mice immunized multiple times with an earlier generation Ad5 [E1-]-CEA. Both immunotherapy modalities resulted in significant inhibition of CEA expressing tumor progression in an Ad5 immune murine tumor model. However, Ad5 immune mice bearing CEA expressing tumors that were treated with Ad5 [E1-, E2b-]-CEA had a significantly increased anti-tumor response as compared to mice treated with Ad5 [E1-]-CEA. (29).
Here we investigated the use of an Ad5 [E1-, E2b-] platform expressing the TAA HER2/neu to induce immune responses in an animal model. HER2/neu protein expression has been indentified in up to 10–34% of invasive breast cancers and is associated with aggressive tumor progression, shorter relapse time following treatment, and reduced survival (30, 31). The immunogenicity of HER2/neu has been well demonstrated in phase I and phase II clinical trials (32,33). In one study, thirty-one patients with stage III or IV HER2/neu positive breast cancer received monthly immunization with a HER2/neu derived T helper epitope administered with granulocyte colony stimulating factor (GM-CSF) for 6 months (32). Following the treatments, 92% of patients demonstrated HER2/neu immunity as measured by T-cell proliferation and this immunity lasted for at least one year in 38% of responding patients (32). In another vaccine trial, patients were administered an immunogenic peptide from the HER2/neu protein (E75) in combination with GM-CSF to prevent recurrence in resected node-positive (NP) and node-negative (NN) breast cancer patients (33). After vaccination all patients demonstrated delayed type hypersensitivity (DTH) response and immunologic responses in an in vitro assay. A recurrence rate of 5.6% was reported in immunized patients compared with 14.2% in the controls (P = 0.04) at a median of 20 months follow-up (33). The demonstrated immunogenicity, high expression, and association with poor prognosis makes HER2/neu a promising target for CMI induced immunotherapy or prophylactic vaccination. To test the hypotheses that Ad5 [E1-, E2b-] vectored TAA can induce immunity in the Ad5 host immune state; we investigated the potential of the Ad5 [E1-, E2b-]-HER2/neu vector to induce CMI and antibody responses in Ad5 naïve and Ad5 immune mice. Furthermore, we evaluated the propensity of the Ad5 [E1-, E2b-]-HER2/neu vector to inhibit the progression of HER2/neu expressing tumors in vivo.
2. Materials and Methods
2.1. Animals
Specific pathogen-free, BALB/c mice (Charles River, Wilmington, MA) ages 6 to 8 weeks old were housed in animal facilities at the Infectious Disease Research Institute (IDRI) (Seattle, Washington). All procedures were conducted according to Institutional Animal Care and Usage Committee (IACUC) approved protocols. All mice were availed food and water ad libitum. In Ad5 immunized studies, mice were made Ad5 immune by immunizing Ad5 naïve BALB/c mice twice at a two-week interval with 1010 VP of Ad5 [E1-]-null (no transgene). This protocol has been reported by us to induce Ad5 neutralizing antibody (NAb) and CMI against Ad5 (22,34). The presence of pre-existing Ad5 immunity in Ad5 [E1-]-null immunized mice was confirmed using an Ad5 NAb assay (see section 2.6).
2.2. Vector Platforms
Ad5 [E1-, E2b-]-HER2/neu was constructed and produced as previously described (22) using a human modified HER2/neu as the insert (35). Briefly, the HER2/neu cDNA was sub-cloned into the E1 region of the Ad5 [E1-, E2b-] vector using a previously described homologous recombination based procedure (24, 25). HER2/neu production was placed under the control of a cytomegalovirus (CMV) enhancer/promoter element. The replication deficient virus was then propagated in the necessary and sufficient E.C7 packaging cell line, CsCl2 purified, dialyzed and then titered as previously described (22, 24). The plaque forming units (PFU) and virus particle (VP) numbers of Ad5 [E1-, E2b-]-HER2/neu were determined. The infectious titer as determined on an E.C7 cell monolayer was 1.0 × 1010 PFU/ml. The VP concentration was determined by SDS disruption and spectrophotometry at 260 and 280nm and was 0.9 × 1012 VP/ml. Ad5 [E1-, E2b-]-null (an Ad5 [E1-, E2b-] vector having no inserted transgene) and Ad5 [E1-]-null (an E1/E3 deleted vector with no inserted transgene) were produced by ViraQuest (North Liberty, Iowa). ViraQuest also amplified the Ad5 [E1-, E2b-]-HER2/neu vector.
2.3 Tumor cell line
The CT-26-HER2/neu expressing cell line was kindly provided by Dr. Manuel L. Penichet, U.C.L.A. This BALB/c murine cell line is a carcinoma that has been transfected to stably express human HER2/neu (36). Cells were maintained and grown in tissue culture employing Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS) (Gemini Bio-Products).
2.4. Immunization Studies
Mice were subcutaneously (SQ) injected with Ad5 [E1-, E2b-]-HER2/neu, Ad5 [E1-, E2b-]-null or Ad5 [E1-]-null at a dose of 1010 VP/dose (unless otherwise specified). Vectors were administered in 25 μL of injection buffer (20 mM HEPES with 3% sucrose). Control mice received 25 μL of injection buffer alone per dose.
2.5. Western Blot Analysis
Western Blot analysis to determine HER2/neu expression by the Ad5 [E1-, E2b-]-HER2/neu and the lack therein of Ad5 [E1-, E2b-]-null and Ad5 [E1-]-null was performed as previously described (22). Briefly, 106 human lung carcinoma cells (A-549) (ATCC number CCL-185) were infected at a MOI of 100 VP/cell, incubated for 24 hours, followed by lysis of the cells. A-549 cell lysates were separated on a 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane (GE Heathcare, Piscataway, NJ). The membrane was then blocked with TBS containing 5% (w/v) blocking reagent (GE Healthcare, Piscataway, NJ) for 2 hours at room temperature and sequentially incubated with mouse HER2/neu antibody (1:250) (Genway, San Diego, CA) and goat anti-mouse-HRP conjugated antibody (1:1000) (Kirkegaard & Perry Laboratories, Gaithersburg, MD) for one hour at room temperature. Reactivity was determined by chemilluminescence using an ECL Western Blotting analysis system (GE Healthcare, Piscataway, NJ) according to the manufacturer’s specifications.
2.6. Adenovirus Neutralization Assay
Dilutions of heat-inactivated mouse sera in 100 μL DMEM containing 10% fetal calf serum (FCS) were mixed with 4 × 107 VP Ad5 [E1-]-null and incubated for 60 minutes. The samples were then added to microwells of a 96-well plate containing HEK-293 cells at 2 × 103 cells/well and incubated for 72 hours. An MTS bioreduction assay was used to quantify cell death, and endpoint Ad5 NAb titers were determined (22).
2.7. Enzyme linked immunosorbent assay (ELISA) for HER2/neu antibody
Mouse sera were collected and frozen at −20°C until assayed. Microtiter plates for ELISA (Nunc, Rochester, NY) were coated with 150 ng of purified HER2/neu protein (GenWay Biotech, Inc., San Diego, CA). Plates were incubated at room temperature overnight, washed with PBS and blocked with 1% BSA in PBS for 30 minutes. Thawed sera were diluted 1:100 with 1% BSA in PBS and 200 μL was added to each well. Samples were incubated for 1 h followed by incubation with 200 μL of a 1:12500 dilution of goat peroxidase-conjugated anti-mouse IgG (gamma chain specific) (Sigma Chemicals, St. Louis, MO) for 1 h at room temperature. The 96-well plates were washed three times and 200 μL per well of 1,2-phenylenediamine substrate solution was added to each well. The reaction was stopped by adding 50 μL per well of a 5N HCl solution. Absorbance was measured at 492 nm. A standard IgG reference curve was concomitantly generated using purified mouse IgG (Sigma Chemicals St. Louis, MO). The O.D. reading of unknown samples were correlated against the standard IgG curve to obtain nanogram equivalents of IgG bound per well utilizing an INSTAT statistical program as previously described (37).
2.8. In vitro tumor binding antibody assay
CT26-HER2/neu tumor cells were harvested by physical scraping from T75 flasks (Corning, Corning, NY) then washed three times with PBS+5%FCS. Cells stained with mouse sera were left intact. Cells stained with a commercial antibody were first permeabilized by a 30 minute incubation with 1× BD perm/wash (Becton Dickinson, Franklin Lakes, NJ) to allow for detection of antibody directed at the intracellular region of HER2/neu and all subsequent procedures for these cells were performed using this buffer. Permeabilized cells were incubated with 0.25 mg/mL of mouse HER2/neu antibody (Invitrogen, Carlsbad, CA) for 30 minutes at 4°C. Intact cells were incubated with pooled mouse sera diluted 1:5 for 30 minutes at 4°C. Cells were then washed 3 times, stained with PE-conjugated rat anti-mouse IgG (Becton Dickinson, Franklin Lakes, NJ) at 2 mg/mL for 30 minutes at 4°C. After incubation, cells were washed 3 times and analyzed on an LSR II flow cytometer (Becton Dickinson, Franklin, NJ) for fluorescence.
2.9. Complement-Dependent Cellular Cytotoxicity (CDCC) assay
CT26-HER2/neu tumor cells were cultured overnight at a density of 2×104 cells/well in 96 well tissue culture microplates. Pooled mouse sera was added at a 1:100 dilution and incubated at 37°C for one hour. Rabbit serum was then added at a 1:100 dilution as a source of complement and cells were incubated an additional 2.5 hours at 37°C. For control studies, rabbit serum was heat inactivated by incubation for 1 hour at 56°C before use. Cell culture supernatants were assayed using Promega Cytotox 96 Non-radioactive Cytotoxicity Assay (Promega, Madison, WI), according to the manufacture’s instructions. Percent lysis of CT26-HER2/neu cells was calculated by the formula: %lysis=(experimental-target spontaneous)/(target maximum-target spontaneous) × 100%.
2.10. ELISpot Assay
ELISpotassays were used to determine the number of HER2/neu specific interferon gamma (IFN-γ) or interleukin-2 (IL-2) secreting T-cells from freshly collected mouse splenocytes as previously described (22). The ELISpot assay was run according to the manufacture’s specifications (eBioscience, San Diego, CA). Briefly, 2 × 105 mouse splenocytes were seeded per well in a 96-well microtiter plate and cytokine secreting T-cells were reported as the number of spots per 106 spleocytes. HER2/neu peptides (kindly provided by Dr. Timothy Clay, Duke University), β-galactosidase protein (Rockland, Gilbertsville, PA), CMV (Cytomegalovirus) pp65 (495–503) peptides (Beckman Coulter, San Diego, CA), and HIV-1 Gag (p24) protein (IDRI, Seattle, WA) were used as stimulants at a final concentration of 1 μg/well for protein and 0.1 μg/well for peptides. Cells stimulated with concanavalin A (ConA) at a concentration of 0.0625 μg/well served as a positive control. Colored spot-forming cells (SFC) were counted using an Immunospot ELISpot plate reader (Cellular Technology, Shaker Heights, OH) and responses were considered to be positive if: 1) ≥50 SFC/106 cells were detected after subtraction of the number of spots in the negativecontrol wells and 2) SFCwere ≥2-fold greater than thosedetected in the negative controlwells.
2.11. Prevention of tumor establishment
Studies were performed to determine if vaccination with Ad5 [E1-, E2b-]-HER2/neu could inhibit establishment of CT-26 HER2/neu expressing tumors in mice. Ad5 naïve or Ad5 immune BALB/c mice (n=7/group) were immunized with injection buffer (tumor controls), 1010 VP of Ad5 [E1-, E2b-]-null (vector control) or 1010 VP of Ad5 [E1-, E2b-]-HER2/neu (vaccine) in the left flank on days −28, −21 and −14. On day 0 mice were inoculated with 1 × 106 viable CT26-HER2/neu cells subcutaneously in the left subcostal region. Tumor growth was measured by two opposing dimensions (a, b) and volume was calculated according to the formula V=(axb)2/2 as previously described (29,38). Tumor studies were terminated when a pre-determined IACUC defined maximum tumor size was achieved in mice.
2.12. Tumor Treatment Studies
To determine if a reduction of tumor progression could be achieved in established HER2/neu expressing tumors, mice were implanted with 106 CT-26-HER2/neu cells SQ in the left subcostal region on day 0. Once tumors were palpable, mice were treated by administration of injection buffer, 1010 VP of Ad5 [E1-, E2b-]-null or 1010 VP of Ad5 [E1-, E2b-]-HER2/neu in the right flank on days 7 and 14. Tumor growth was determined, volumes calculated, and studies were terminated as described above (2.11).
2.13. Statistical analysis
Statistical analysis was performed using GraphPad Prism. Statistically significant differences were determined by comparative Student’s t-tests with a p-value of ≤0.05 being considered significant.
Results
3.1. Characterization of Ad5 HER2/neu vector
As determined by plaque assay and spectrophotometry, the ratio of Ad5 [E1-, E2b-]-HER2/neu VP to PFU was 1:90 PFU/VP in the lot utilized in these studies. Initial studies were performed to confirm that the Ad5 [E1-, E2b-]-HER2/neu construct expressed HER2/neu. A549 cells were infected at an MOI of 100 for 24 hours and total cellular protein was harvested. Western blot analysis employing a monoclonal antibody directed against HER2/neu revealed that cells transfected with Ad5 [E1-, E2b-]-HER2/neu expressed HER2/neu (data not shown).
3.2. Dose Response of Ad5 [E1-, E2b-]-HER2/neu
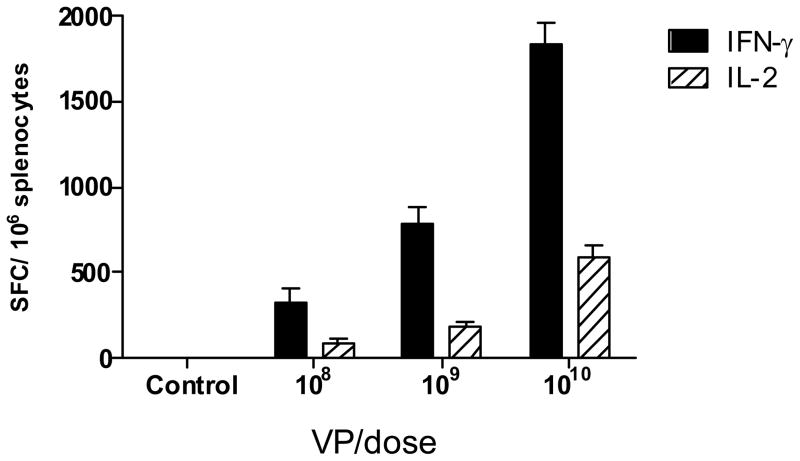
A study was performed to determine the effect of immunizations with increasing doses of Ad5 [E1-, E2b-]-HER2/neu on the induction of CMI responses in Ad5 naïve mice. Groups of Ad5 naïve BALB/c mice (n=5/group) were immunized SQ three times at weekly intervals with 108, 109, or 1010 VP of Ad5 [E1-, E2b-]-HER2/neu, respectively. Control mice were injected with buffer solution only. Two weeks after the last immunization, T-cell CMI responses were assessed by IFN-γ and IL-2 ELISpot analysis. As shown in Figure 1, a dose response effect was observed on the CMI response with the highest CMI response level being obtained after immunizations with 1010 VP of Ad5 [E1-, E2b-]-HER2/neu. Based on these results, the dose of 1010 VP was chosen for further studies below.
Figure 1. CMI dose response titration of Ad5 [E1-, E2b-]-HER2/neu.
Ad5 naïve BALB/c mice (n=5/group) were immunized three times using a dose of 108, 109 or 1010 VP Ad5 [E1-, E2b-]-HER2/neu or buffer alone (controls). Fourteen days after the final immunization, splenocytes from the mice were assessed by ELISpot analysis. The greatest induction of CMI was achieved using 1010 VP of the vector. For positive controls, splenocytes were exposed to concanavalin A in all ELISpot assays as described (data not shown). The error bars depict the standard error of the mean (SEM).
3.3. Induction of CMI responses by Ad5 [E1-, E2b-]-HER2/neu vaccination
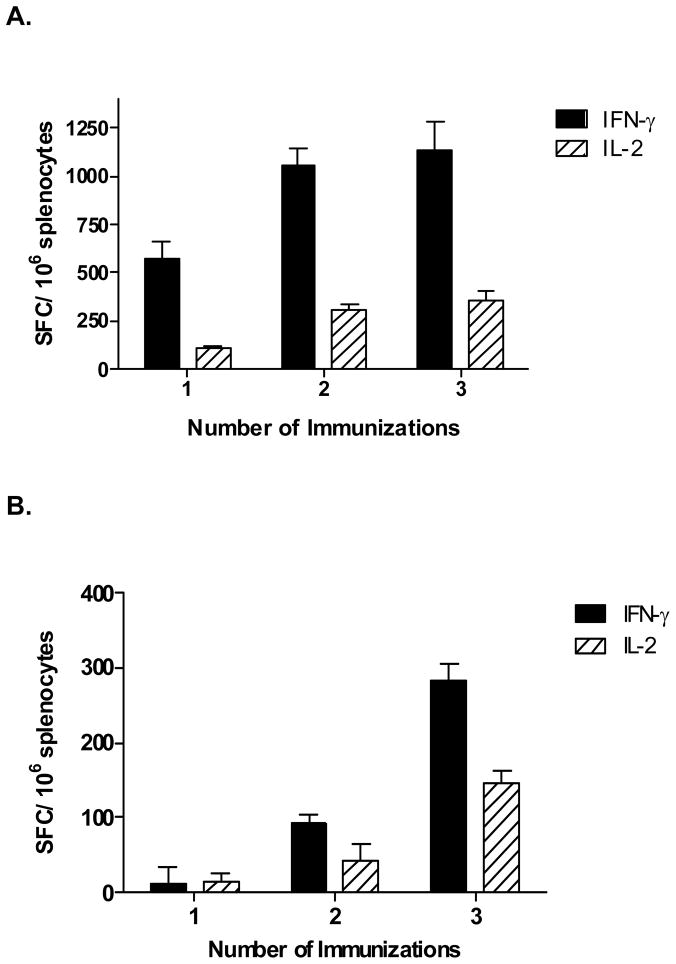
To assess CMI induction following a single or multiple homologous immunizations with Ad5 [E1-, E2b-]-HER2/neu, groups of Ad5 naïve BALB/c mice (n=5/group) were immunized once, twice, or three times at weekly intervals with 1010 VP of Ad5 [E1-, E2b-]-HER2/neu. Two weeks following the last immunization, splenocytes were exposed to HER2/neu peptides or irrelevant antigens and analyzed by ELISpot for the number of IFN-γ and IL-2 secreting T-cells. As shown in Figure 2A, significantly elevated numbers of IFN-γ and IL-2 secreting cells were observed in Ad5 naïve mice after one, two, or three immunizations with Ad5 [E1-, E2b-]-HER2/neu. The numbers of IFN-γ and IL-2 secreting cells observed after two or three immunizations were significantly higher than those observed after one immunization (p<0.02 and p<0.002, respectively). However, the differences were not statistically significant between two and three immunizations. Specificity studies revealed that CMI responses were specific to HER2/neu and there were no responses against irrelevant antigens such as the cytomegalovirus (CMV) antigen, β-galactosidase, or HIV-1 Gag (data not shown).
Figure 2. HER2/neu transgene specific CMI after immunization with Ad5 [E1-, E2b-]-HER2.
Ad5 naïve (A) or Ad5 immune (B) BALB/c mice (n=5/group) were immunized one, two or three times at a seven day intervals with 1010 VP Ad5 [E1-, E2b-]-HER2/neu. Splenocytes were assessed 14 days following the final immunization for IFN-γ and IL-2 secretion by ELISpot assay. No significant CMI responses were observed when splenocytes were exposed to β-galactosidase protein, CMV peptides, or HIV-1 Gag protein (data not shown). Error bars indicate the standard error from the mean.
Experiments were performed to assess immune responses in Ad5 immune mice following a single or multiple immunizations. To induce Ad5 immunity, Ad5 naïve BALB/c mice (n=5/group) were injected subcutaneously twice at two week intervals with 1010 VP of Ad5-null. Two weeks following the final Ad5-null administration, serum samples were collected and assessed for endpoint neutralizing Ad5 antibody titers (NAb). Assessment of sera two weeks after the final Ad5-null immunization revealed that the average endpoint Ad5 NAb was 1:100. Mice were then immunized once, twice, or three times at weekly intervals with 1010 VP of Ad5 [E1-, E2b-]-HER2/neu. Two weeks following the final immunization, splenocytes were assessed for the number of IFN-γ and IL-2 secreting cells by ELISpot analysis. Vaccine induced HER2/neu specific IFN-γ and IL-2 secretion was significantly reduced in the Ad5 immune mice as compared to the Ad5 naïve mice. Again, as observed in the Ad5 naïve mice above, the highest average numbers of IFN-γ and IL-2 secreting splenocytes in Ad5 immune mice were obtained two weeks after a third immunization (Figure 2B). IFN-γ secretion increased significantly (p<0.005) after two and three vaccine administrations but not after one. Significant increases (p<0.03) in IL-2 secretion over control values were also noted after each immunization. Although significantly higher than control values, the increases in IL-2 secretion between one and two immunizations were not significantly different from each other (p=0.0504). The levels of IL-2 secretion observed after the third immunization were significantly elevated as compared with the levels observed after one or two immunizations (p<0.0001 and p=0.0073, respectively). Sera collected from mice after the final immunization revealed that the average endpoint Ad5 NAb titer was 1:500, indicating hyper immune levels to Ad5. These results demonstrate that immunization(s) of Ad5 naïve or Ad5 immune mice with Ad5 [E1-, E2b-]-HER2/neu induced HER2/neu specific CMI responses with the highest responses occurring two weeks after three immunizations.
3.4. Induction of humoral immune responses by Ad5 [E1-, E2b-]-HER2/neu
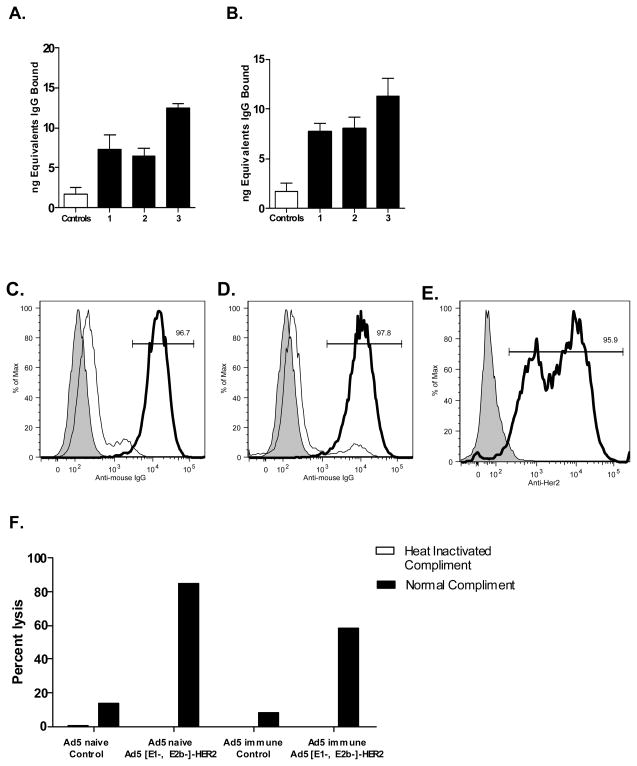
To determine if humoral immune responses could be induced by Ad5 [E1-, E2b-]-HER2/neu, an ELISA assay for circulating IgG to HER2/neu was performed on serum from Ad5 naïve and Ad5 immune Ad5 [E1-, E2b-]-HER2/neu immunized mice. As shown in Figure 3A and 3B, increasing quantities of HER2/neu antibody was detected after one, two, and three immunizations with Ad5 [E1-, E2b-]-HER2/neu in Ad5 naïve and Ad5 immune mice, with the greatest quantities of antibody observed after the third immunization. These results show that, in addition to CMI, antibody responses directed toward the TAA HER2/neu could also be induced in immunized Ad5 naïve and Ad5 immune mice.
Figure 3. HER2/neu antibody activity from sera from immunized mice.
HER2/neu IgG levels in Ad5 naïve (A) or Ad5 immune (B) mice immunized one (1), two (2), or three (3) times with Ad5 [E1-, E2b-]-HER2/neu. Ad5 naïve or Ad5 immune mice BALB/C mice (n=5) were immunized 3 times at 7-day intervals with 1010 VP Ad5 [E1-E2b]-HER2/neu. Two weeks after the final immunization, serum from the mice was tested by flow cytometry for binding to HER2/neu-expressing cell line CT26-HER2/neu compared to controls. Ad5 naïve sera (C) showed 96.7% reactivity and Ad-immune sera (D) showed 97.8% reactivity (right side peaks). A commercially available antibody to HER2/neu (E) showed reactivity to 95.9% of CT26-HER2/neu cells (right side peaks). Left side peaks represent control serum binding and secondary detection antibody only (shaded). CDCC against CT26-HER2/neu cells was performed (F). Complement mediated lysis was detected in sera from both Ad5 naïve (84.9%) and Ad5 immune (58.7%). No lysis was detected when sera were heat-inactivated. Values represent Mean ± SEM.
To determine if the HER2/neu antibody from Ad5 [E1-, E2b-]-HER2/neu immunized mice could bind to HER2/neu expressing cells, we performed a modified flow cytometry assay. Serum from Ad5 naïve and Ad5 immune mice immunized by three weekly injections of 1010 VP of Ad5 [E1-, E2b-]-HER2/neu was incubated with CT-26-HER2/neu murine tumor cells. A commercial HER2/neu antibody was also incubated with CT-26-HER2/neu cells to serve as a positive control. Serum from Ad5 naïve mice immunized with Ad5 [E1-, E2b-]-HER2/neu bound to 96.7% of the HER2/neu expressing cells and serum from Ad5 immune mice immunized with Ad5 [E1-, E2b-]-HER2/neu bound to 97.8% of the CT-26-HER2/neu cells (Figure 3C, 3D). Tumor cells incubated with a commercial HER2/neu monoclonal antibody bound to 95.9% of HER2/neu expressing cells (Figure 3E). We also evaluated the functionality of vaccine induced antibodies against HER2/neu in a complement dependent cellular cytotoxicity assay (CDCC). HER2/neu-vaccine induced antibodies in Ad5 naïve and Ad5 immune mice exhibited robust CDCC by lysis of CT26-HER2/neu tumor cells (Figure 3F). Tumor cell lysis was not observed in samples incubated with heat-inactivated complement indicating that the observed cytotoxicity was complement dependent.
3.5. Prevention of HER2/neu expressing tumor establishment in vivo
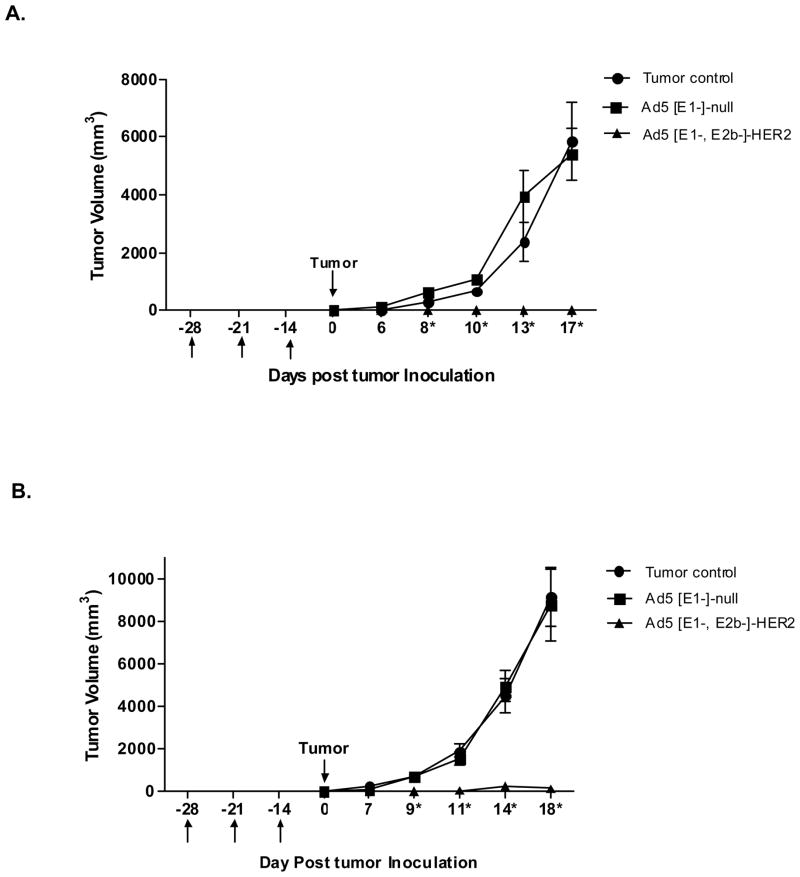
To determine if immunization with Ad5 [E1-, E2b-]-HER2/neu could prevent implantation and progression of HER2/neu expressing tumor cells, Ad5 naïve mice (n=7/group) were immunized three times at weekly intervals with Ad5 [E1-, E2b-]-HER2/neu. Vector control mice were injected with Ad5 [E1-, E2b-]-null (no inserted transgene) and tumor control mice were injected with buffer alone. Two weeks following the final immunization, mice were inoculated with 106 CT-26-HER2/neu tumor cells in the right subcostal region. Tumors were monitored for growth and volumes were calculated. Mice immunized with the Ad5 [E1-, E2b-]-HER2/neu vector had significant reduction in tumor progression on study day 8 (p<0.001), 10 (p<0.001), 13 (p<0.001) and 17 (p<0.001) as compared to rapidly progressing tumors observed in both the vector alone injected and tumor control groups (Figure 4A).
Figure 4. Prevention of HER2/neu expressing tumor establishment.
Ad5 naïve (A) or Ad5 immune (B) BALB/c mice (n=7/group) were immunized with injection buffer (tumor controls), 1010 VP Ad5 [E1-, E2b-]-null (vector control) or 1010 VP Ad5 [E1-, E2b-]-HER2/neu (treatment) in the left flank on days −28, −21 and −14. On day 0 mice were inoculated with 106 CT26-HER2/neu cells subcutaneously in the left subcostal region. Tumor growth was monitored and volumes calculated. * Indicates days when Ad5 [E1-, E2b-]-HER2/neu treated mice had significantly smaller (p<0.01) tumors than both controls. Error bars represent the standard error of the means.
This study was then repeated in Ad5 immune mice (n=7/group). These mice were made Ad5 immune by two immunizations with Ad5 [E1-]-null at a two-week interval. Two weeks later the mice were immunized three times at weekly intervals with Ad5 [E1-, E2b-]-HER2/neu. Two weeks following the final immunization, mice were inoculated with 106 CT-26-HER2/neu cells in the right subcostal region. As was observed in Ad5 naïve mice, Ad5 immune mice had significant reduction of tumor progression on day 9 (p<0.01), 11 (p<0.001) 14 (p<0.001) and 18 (p<0.001) as compared to Ad5 [E1-, E2b-]-null injected and control groups (Figure 4B).
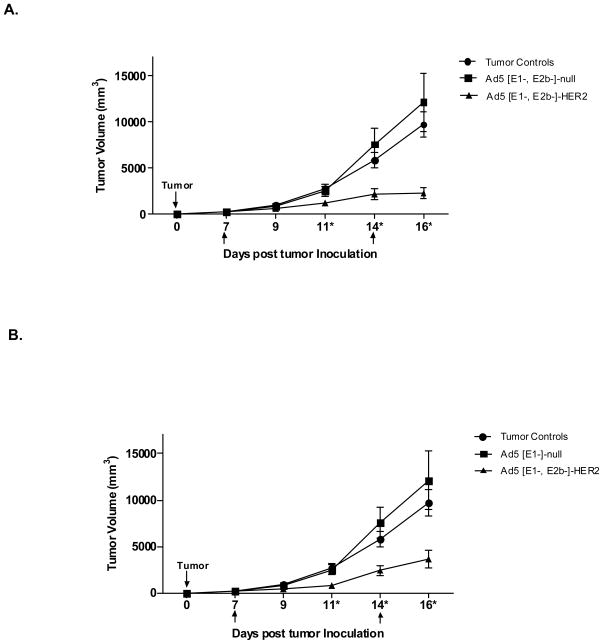
3.6. Treatment of established HER2/neu expressing tumors
The effectiveness of treating established HER2/neu expressing CT-26-HER2/neu tumors in vivo with Ad5 [E1-, E2b-]-HER2/neu was evaluated. Ad5 naïve or Ad5 immune BALB/c mice (n=7/group) were implanted SQ into the right subcostal with 106 CT-26-HER2/neu cells on day 0. Tumors were palpable by days 4–6. On days 7 and 14, mice were treated by SQ injections of injection buffer alone, 1010 VP of Ad5 [E1-, E2b-]-null or 1010 VP of Ad5 [E1-, E2b-]-HER2/neu. All mice were monitored for tumor growth and tumor volumes were calculated. Ad5 naïve mice immunized with Ad5 [E1-, E2b-]-HER2/neu had significantly smaller tumors than control mice on days 11, 14 and 16 (p<0.05) (Figure 5A). Of particular importance is the observation that Ad5 immune mice treated with Ad5 [E1-, E2b-]-HER2/neu had significantly smaller tumors than control mice measured on days 11, 14 and 16 (p<0.05) (Figure 5B). These results demonstrate that the Ad5 [E1-, E2b-]-HER2/neu vector platform has the potential to be utilized as an immunotherapeutic agent to treat HER2/neu expressing tumors in a clinically relevant Ad5 immune setting.
Figure 5. Treatment of established HER2/neu expressing tumors in Ad5 naïve and Ad5 immune mice.
Ad5 naïve (A) or Ad5 immune (B) BALB/c mice (n=7/group) were inoculated with 106 CT26-HER2/neu cells subcutaneously in the left subcostal region (Day 0). Mice were administered injection buffer (tumor controls), 1010 VP Ad5 [E1-, E2b-]-null (vector control) or 1010 VP Ad5 [E1-, E2b-]-HER2/neu (treatment) in the right flank on days 7 and 14 as indicated in the figures by arrows. Tumor growth was monitored and volumes calculated. * Indicates days when Ad5 [E1-, E2b-]-HER2/neu treated mice had significantly smaller (p<0.05) tumors than both controls. Error bars represent the standard error of the means.
4. Discussion
In the present study, we report that dose dependent immune responses against the TAA HER2/neu were induced by Ad5 [E1-, E2b-]-HER2 in Ad5 naïve and Ad5 immune mouse models. These responses were demonstrated by increases in the levels of IFN-γ and IL-2 secreting splenocytes and HER2/neu antibody induction after immunization(s). The CMI and antibody responses induced by vaccination trended to be the most robust two weeks after three immunizations as compared to two weeks after one or two immunizations. We have yet to determine if the highest levels of CMI and antibody responses observed in mice receiving three immunizations was the result of multiple administrations or was due to the longer vaccination time in mice that received three dosages as compared to the shorter vaccination times after one or two doses. It is possible that one or two immunizations with Ad5 [E1-, E2b-]-HER2/neu could have achieved similar levels of immune responses as observed with three immunizations if followed over a longer period of time. This parameter needs further investigation.
In another TAA model, Osada, et al, reported on CEA specific immune responses induced by Ad5 [E1+]-CEA (an Ad5 vector retaining the E1 genes but deleted for the E3 gene), Ad5 [E1-]-CEA and Ad5 [E1-, E2b-]-CEA (27). They reported that mice made Ad5 immune by one exposure to a replication competent Ad5 (Ad5 [RC]) and then immunized with Ad5 [E1+]-CEA or Ad5 [E1-]-CEA, had a significant reduction of CEA specific T-cell responses (36 and 37%, respectively) as compared to Ad5 naïve mice immunized with these same vaccines. They reported a further reduction of CEA specific T-cells (48 and 46%, respectively) in mice that were exposed to the Ad5 [RC] vector three times and then immunized with the vaccines. In contrast, the Ad5 [E1-, E2b-]-CEA vaccine induced robust CEA-specific T-cell responses in mice exposed once or three times to Ad5 [RC]. In mice exposed three times to Ad5 [RC] (anti-Ad5 titer of 1:20,000) and then immunized with Ad5 [E1-, E2b-]-CEA, only a 10% reduction of CEA specific T-cells was observed as compared to Ad5 [E1-, E2b-]-CEA immunized Ad5 naïve mice. In the present study, Ad5 immune mice immunized multiple times with Ad5 [E1-, E2b-]-HER2/neu exhibited a significant reduction of HER2/neu specific immune responses compared to Ad5 naïve animals. The transgene specific immune response due to pre-existing Ad5 immunity may depend on multiple factors including the dose, the transgene and/or the number of administrations. As demonstrated in the current study, the immune response induced by the Ad5 [E1-, E2b-]-HER2 vector was dose dependant (Figure 1). Osada, et al used an immunizing dose more than 2 times higher than the dose used in the current study (2.6 × 1010 verses 1.0 ×1010 VP, respectively), which may have contributed to the contrast in immune induction results. Also, the ratio of PFU to VP of the Ad5 [E1-, E2b-]-HER2/neu used in the current study was 1:90 (PFU/VP) which is high for Ad5 preparations that ideally have a ratio of 1:30 or less as stipulated by the Food and Drug Administration for Ad5-based vectors. We expect that optimization of manufacturing conditions will improve this ratio, which, in turn, may improve the vaccine efficiency of this new vector platform resulting in greater immune responses. Even though reductions in HER2/neu specific responses occurred in Ad5 immune mice and the PFU/VP ratio of the Ad5 [E1-, E2b-]-HER2 viral prep was high relative to other studies, the levels of induced immune responses resulted in significant therapeutic and prophylactic benefit in tumor treatment and tumor challenge studies. Thus, the influence of Ad5 immunity on induced immune responses is apparent, yet, despite this influence, the improved Ad5 [E1-, E2b-] vector platform can still induce anti-tumor immunity.
In order to induce effective anti-tumor immunity, vectored immunotherapeutic cancer agents targeting TAA must be potent even in the presence of anti-vector immunity, independent of whether pre-existing vector immunity has been established though natural infection or newly acquired immunity is developed following primary immunizations using vectored vaccines. Studies in humans and animals using Ad5 vectors, the most widely used subtype for human vector vaccines, have demonstrated that pre-existing Ad5 immunity can mitigate immunization, especially re-immunization. This Ad5 immunity may preclude the repeated administration of current generation Ad5 [E1-]-based vaccines (19, 22, 23). A preponderance of humans has neutralizing antibodies to Ad5, with two-thirds of humans studied having lympho-proliferative responses against Ad5 (21). Overcoming the barrier of pre-existing Ad5 anti-vector immunity has been a subject of intense investigation. The use of different Ad serotypes or even non-human forms of Ad have been evaluated in efforts to overcome pre-existing Ad5 immunity (19). Even if these approaches succeed in an initial immunization, subsequent vaccination using that serotype would be mitigated due to the immune response induced against that subtype. These observations, coupled with the altered biodistribution and potentially increased toxicity of alternative serotype vectors along with serotype cross-reactivity renders these diverse approaches problematic, especially relative to the well-characterized Ad5 [E1-] platform (39). In order to retain the positive attributes of Ad5 vectors while moving toward overcoming said limitations, next generation Ad5 vectors have been constructed using further deletion of Ad5 viral genes. Our Ad5 [E1-, E2b-] platform has additional deletions in the Ad5 DNA polymerase and the preterminal protein (pTP) genes that are contained in the E2b genome region. As a result, it has an expanded cloning capacity with up to a 12 kb gene-carrying capacity as compared to the 7 kb capacity of Ad5 [E1-] vectors. A key aspect of the novel Ad5 [E1-, E2b-] vector is that expression of Ad5 late genes is greatly reduced (24,25). Infection of human cells reveals that despite the deletion of the E1 gene, Ad5 [E1-] vectors still express low-levels of Ad5 viral genes in human cells (24). This is contrasted with the significantly diminished Ad5 late gene expression in cells by the modified Ad5 [E1-, E2b-] vector platform (24). Low-level transcriptional activity of the downstream Ad5 encoded gene promoters is thought to be the cause for the limited blockade afforded by sole deletion of the E1 genes in Ad5 [E1-] vectors. More importantly, once Ad genome replication commences, a series of other Ad encoded genes, the structural and highly toxic “late” capsid proteins are also expressed as a direct result of the physical act of Ad genome replication. Additionally, fiber protein (the spike emanating from the 12 vertices of the Ad capsid) expression is also detected after infection of human cells with the current generation Ad5 [E1-] vector. This is in contrast to extremely low expression of fiber protein with the Ad5 [E1-, E2b-] vector (24,25). These findings may account, in part, for the reason that Ad5 [E1-, E2b-] vectors exhibit less adverse effects and can express a transgene more proficiently than current Ad5 [E1-] vectors, especially in the Ad5 immune state (40).
Deletion in the early gene 2b (E2b) region has been reported to allow for induction of potent immune responses to transgene specific antigens in the presence of Ad5 immunity. We previously reported on the development of an Ad5 [E1-, E2b-]-HIV-1 vector vaccine that generated significantly higher CMI responses following multiple immunizations as compared with the current generation Ad5 [E1-]-HIV-1 vector (22,34). Moreover, significant antigen specific CMI responses were induced in mice and non-human primates despite the presence of pre-existing Ad5 immunity (22, 34). In addition, we have recently extended studies of Osada et al (27) and have demonstrated that immunotherapy of Ad5 immune mice with Ad5 [E1-, E2b-]-CEA can significantly decrease progression of CEA expressing tumors in comparison to a current generation Ad5 [E1-]-CEA (29). Supporting evidence for the present study has also been put forth by Hartman et al, employing a current generation Ad5 [E1-]-HER2/neu vector in a HER2/neu tolerant transgenic mouse model (35). They reported that immunization with the vector platform induced HER2/neu specific immune responses in Ad5 naïve HER2/neu transgenic mice (35). Additionally, immunotherapy of HER2/neu expressing tumors in their tolerant transgenic model resulted in significant inhibition of tumor progression. Thus, not only could an Ad5-HER2/neu vector break tolerance in transgenic mice, it could induce robust immune responses resulting in anti-tumor activity. The above findings and the observations in the present study have important implications in the immunotherapy of Ad5 immune HER2/neu positive cancer patients with the Ad5 [E1-, E2b-]-HER2/neu vector.
The results of the studies employing Ad5 [E1-, E2b-]-CEA and Ad5 [E1-, E2b-]-HER2/neu collectively demonstrate the immunotherapeutic potential of this new vector platform approach for the treatment of certain cancers. Of particular importance is the observation that the Ad5 [E1-, E2b-] vector can induce immune responses resulting in significant anti-tumor responses in a clinically relevant Ad5 immune setting. Given the results reported herein, we believe that Ad5 [E1-, E2b-]-HER2/neu warrants further investigation and clinical trial testing in patients with HER2/neu expressing cancers. In addition, further studies need to be performed to elucidate the mechanism of action that induces the anti-TAA responses in both Ad5 naïve and Ad5 immune hosts. The levels and breath of the CMI responses needs clarification as well as the implications of the TAA antibody responses with respect to tumor cell killing and inhibition of progression.
Acknowledgments
This study was funded in part by NIH-NCI grant 1R43CA139663-01. We acknowledge ViraQuest, Inc, North Liberty, IA for amplification of the vaccines. The authors thank Dr. Winston Witcomb for management and care of the animals. We also thank Ms. Carol Jones for management of the grant activities and Susan Nguyen for assistance in preparation of the manuscript.
Footnotes
Conflict of Interest Statement
Frank R. Jones work has been funded in part by the National Cancer Institutes and Etubics Corporation. All authors are employees of Etubics Corporation and are funded in part by grants from the National Cancer Institute.
References
- 1.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 2.Romano G, Pacilio C, Giordano A. Gene transfer technology in therapy: current applications and future goals. Stem Cells. 1999;17:191–202. doi: 10.1002/stem.170191. [DOI] [PubMed] [Google Scholar]
- 3.Kirk CJ, Hartigan-O’Connor D, Mule JJ. The dynamics of the T-cell anitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001a;61:8794–8802. [PubMed] [Google Scholar]
- 4.Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, et al. T cell-dependant antitumor immunity mediated by secondary lympoid tissue chemokine augmentation of dendrtitic cell-based immunotherapy. Cancer Res. 2001b;61:2062–2070. [PubMed] [Google Scholar]
- 5.Polo JM, Dubensky TW., Jr Virus-based vectors for human vaccine applications. Drug Discov Today. 2002;1:53–100. doi: 10.1016/s1359-6446(02)02324-3. [DOI] [PubMed] [Google Scholar]
- 6.Liniger M, Zuniga A, Naim HY. Use of viral vectors for the development of vaccines. Expert Rev Vaccines. 2007;6:255–266. doi: 10.1586/14760584.6.2.255. [DOI] [PubMed] [Google Scholar]
- 7.Gaydos CA, Gaydos JC. Adenovirus vaccine. In: Orenstein WA, editor. Vaccines. 4. Sauders; Philadelphia: 2004. pp. 863–885. [Google Scholar]
- 8.Butterfield LH, Jilani SM, Chakraborty NG, Bui LA, Ribas A, Dissette VB, et al. Generation of Melanoma-Specific Cytotoxic T Lymphocytes by Dendritic Cells Transduced with a MART-1 Adenovirus. Journal of Immunology. 1998;161:5607–5613. [PubMed] [Google Scholar]
- 9.Diao J, Smythe JA, Smyth C, Rowe PB, Alexander IE. Human PBMC-Derived Dendritic Cells Transduced With An Adenovirus Vector Induce Cytotoxic T-Lymphocyte Responses Against A Vector-Encoded Antigen In Vitro. Gene Therapy. 1999;6:845–853. doi: 10.1038/sj.gt.3300899. [DOI] [PubMed] [Google Scholar]
- 10.Dietz AB, Vuk-Pavlovic S. High Efficiency Adenovirus-Mediated Gene Transfer To Human Dendritic Cells. Blood. 1998;91:392–398. [PubMed] [Google Scholar]
- 11.Ishida T, Chada S, Stipanov M, Nadaf S, Ciernik FI, Gabrilovich DI, et al. Dendritic Cells Transduced with Wild-Type P53 Gene Elicit Potent Anti-Tumour Immune Responses. Clinical and Experimental Immunology. 1999;117:244–251. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribas A, Butterfield LH, McBride WH, Jilani SM, Bui LA, Vollmer CM, et al. Genetic Immunization for the Melanoma Antigen Mart-1/Melan-A Using Recombinant Adenovirus-Transduced Murine Dendritic Cells. Cancer Research. 1997;57:2865–2869. [PubMed] [Google Scholar]
- 13.Arthur JF, Butterfield LH, Roth MD, Bui LA, Kiertscher SM, Lau R, Dubinett S, et al. A Comparison of Gene Transfer Methods In Human Dendritic Cells. Cancer Gene Therapy. 1997;4:17–25. [PubMed] [Google Scholar]
- 14.Rea D, Schagen FH, Hoeben RC, Mehtali M, Havenga MJ, Toes RE, et al. Adenoviruses Activate Human Dendritic Cells Without Polarization Toward a T-Helper Type 1-Inducing Subset. Journal of Virology. 1999;73:10245–10253. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschowitz EA, Weaver JD, Hidalgo GE, Doherty DE. Murine Dendritic Cells Infected With Adenovirus Vectors Show Signs Of Activation. Gene Therapy. 2000;7:1112–1120. doi: 10.1038/sj.gt.3301210. [DOI] [PubMed] [Google Scholar]
- 16.Loser P, Jennings GS, Strauss M, Sandig V. Reactivation Of The Previously Silenced Cytomegalovirus Major Immediate-Early Promoter In The Mouse Liver Involvement Of Nf-K-B. Journal of Virology. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, et al. Recombinant Adenovirus Induces Maturation of Dendritic Cells Via An Nf-KappaB-Dependent Pathway. Journal of Virology. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan Y, Emtage P, Foley R, Carter R, Gauldie J. Murine Dendritic Cells Transduced With An Adenoviral Vector Expressing A Defined Tumor Antigen Can Overcome Anti-Adenovirus Neutralizing Immunity And Induce Effective Tumor Regression. International Journal Of Oncology. 1999;14:771–776. doi: 10.3892/ijo.14.4.771. [DOI] [PubMed] [Google Scholar]
- 19.McCoy K, Tatsis N, Korioth-Schmitz, Lasaro MO, Hensley SE, Lin SW, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the Immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007;81:6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kresge KJ. Clinical trials yield promising results form two adenovirus-based vaccine. IAVI Rep. 2005;9:24. [PubMed] [Google Scholar]
- 21.Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in adult populations of The Gambia, South Africa and the United States. Clin Diagn Lab Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabitzsch ES, Xu Y, Yosida L, Balint J, Amalfitano A, Jones FR, et al. A Preliminary and Comparative Evaluation of a Novel Ad5 [E1-, E2b-] Recombinant Based Vaccine Used to Induce Cell Mediated Immune Responses. Immuno Lett. 2009;122:44–51. doi: 10.1016/j.imlet.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papp Z, Babiuk LA, Baca-Estrada ME. The effect of pre-existing adenovirus-specific immunity on immune responses induced by recombinant adenovirus expressing glycoprotein D of bovine herpesvirus type 1. Vaccine. 1999;17:933–943. doi: 10.1016/s0264-410x(98)00279-5. [DOI] [PubMed] [Google Scholar]
- 24.Amalfitano A, Hauser MA, Hu H, et al. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodges BL, Serra D, Hu H, et al. Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Medicine. 2000;2:250–259. doi: 10.1002/1521-2254(200007/08)2:4<250::AID-JGM113>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Serra D, Amalfitano A. Persistence of an [E1-, polymerase-] adenovirus vector despite transduction of a neoantigen into immune-competent mice. Hum Gene Ther. 1999;10:355–364. doi: 10.1089/10430349950018805. [DOI] [PubMed] [Google Scholar]
- 27.Osada T, Yang XY, Hartman ZC, Glass O, Hodges BL, Niedzwiecki D, et al. Optimization of vaccine responses with an E1, E2b, E3 deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer Gene Ther. 2009;16:673–682. doi: 10.1038/cgt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everett RS, Hodges BL, Ding EY, Xu F, Serra D, Amalfitano A. Liver toxicities typically induced by first- generation adenoviral vectors can be reduced by use of E1, E2b-deleted adenoviral vectors. Hum Gene Ther. 2003;14:1715–1726. doi: 10.1089/104303403322611737. [DOI] [PubMed] [Google Scholar]
- 29.Gabitzsch ES, Xu Younong, Balint Joseph P, Jr, Hartman Zachary C, Lyerly H Kim, Jones Frank R. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral [E1-, E2b-]-CEA. Cancer Immunology Immunotherapy. 2010;59:1131–1135. doi: 10.1007/s00262-010-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 31.Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 32.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 33.Peoples GE, Khoo S, Dehqanzada ZA, Mittendorf EA, Hueman MT, Gurney JM, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for prevention of recurrence in high-risk breast cancer patients U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2008;14:797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 34.Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Amalfitano A, Jones FR. Novel adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine. 2009;27:6394–6398. doi: 10.1016/j.vaccine.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartman ZC, Wei J, Osada T, Glass O, Lei G, Yang XY, et al. An adenoviral vaccine encoding full-length inactivated human Her2 exhibits potent immunogenicity and enhanced therapeutic efficacy without oncogenicity. Clin Cancer Research. 2010;16:1466–1477. doi: 10.1158/1078-0432.CCR-09-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helguera G, Rodriguez JA, Penichet ML. Cytokines fused to antibodies and their combinations as therapeutic agents against different peritoneal HER2/neu expressing tumors. Mol Cancer Ther. 2006;5:1029–1040. doi: 10.1158/1535-7163.MCT-05-0488. [DOI] [PubMed] [Google Scholar]
- 37.Balint JP, Jr, Jones FR. Detection of elevated levels of antiidiotypic antibody levels in immune thrombocytopenic patients expressing antiplatelet antibody. Blood. 1994;84:664–665. [PubMed] [Google Scholar]
- 38.Carlsson G, Gullberg B, Hafstrom L. Estimation of Tumor Volume Using Different Formulas - An Experimental Study in Rats. J Cancer Res Clin Oncol. 1983;105:20–23. doi: 10.1007/BF00391826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appledorn DM, Kiang A, McBride A, Jiang H, Seregin S, Scott JM, et al. Wild-Type Adenoviruses from groups A-F elicit unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Therapy. 2008;15:885–901. doi: 10.1038/gt.2008.18. [DOI] [PubMed] [Google Scholar]
- 40.Seregin SS, Amafitano A. Overcoming pre-existing Adenovirus immunity by genetic engineering of adenovirus-based vaccine vectors. Expert Opin Biol Ther. 2009;9:1–11. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]