Abstract
Pro-opiomelanocortin (POMC) neurons are identified in two brain sites, the arcuate nucleus (ARC) of the hypothalamus and nucleus of the solitary tract (NTS) in brainstem. Earlier pharmacological and POMC gene transfer studies demonstrate melanocortin activation in either site alone improves insulin sensitivity and reduces obesity. The present study, for the first time, investigated the long-term efficacy of POMC gene transfer concurrently into both sites in the regulation of energy metabolism in aged F344xBN rats bearing adult-onset obesity. Pair feeding was included to reveal food-independent POMC impact on energy expenditure. We introduced adeno-associated virus encoding either POMC or green fluorescence protein to the two brain areas in 22-month-old rats, then recorded food intake and body weight, assessed oxygen consumption, serum leptin, insulin and glucose, tested voluntary wheel running, analyzed POMC expression, and examined fat metabolism in brown and white adipose tissues. POMC mRNA was significantly increased in both the hypothalamus and NTS region at termination. Relative to pair feeding, POMC caused sustained weight reduction and additional fat loss, lowered fasting insulin and glucose, and augmented white fat hormone-sensitive lipase activity and brown fat uncoupling protein 1 level. By wheel running assessment, the POMC-animals ran twice the distance as the control or pair-fed rats. Thus, the dual-site POMC treatment ameliorated adult-onset obesity effectively, involving a moderate hypophagia lasting ~ 60 days, enhanced lipolysis and thermogenesis, and increased physical activity in the form of voluntary wheel running. The latter finding provides a clue for countering age-related decline in physical activity.
Keywords: Melanocortins, voluntary wheel running, energy expenditure
Introduction
The brain melanocortin (MC) system is an important anorexic pathway in the central nervous system (CNS) that tempers food intake, augments energy expenditure and induces weight loss (Schwartz et al., 1997; Cone, 2005). The MCs are peptides cleaved from a common precursor, pro-opiomelanocortin (POMC) (Schwartz et al., 1997; Cone, 2005). Up to date, POMC neurons are only found in the arcuate nucleus (ARC) of the hypothalamus (Schwartz et al., 1997) and within the commissural region of nucleus of the solitary tract (NTS) in the brainstem (Mountjoy et al., 1994; Kishi et al., 2003; Cone, 2005). A key central melanocortin involved in homeostatic regulation of body weight is POMC-derived alpha-melanocyte-stimulating hormone (α-MSH) (Schwartz et al., 1997; Cone, 2005). This neuropeptide or its synthetic analog, melanotan II (MTII), inhibits food intake and enhances energy expenditure mainly through activation of central melanocortin 3 (MC3R) and 4 (MC4R) receptors in the hypothalamus, and they are especially effective against adult-onset or diet-induced obesity (Hansen et al., 2001; Hwa et al., 2001; Pierroz et al., 2002; Li et al., 2004; Zhang et al., 2004). Unfortunately, desensitization to these agents occurs within days in animal studies (McMinn et al., 2000; Pierroz et al., 2002; Zhang et al., 2004), presenting one challenge for clinical application of these compounds in treating chronic obesity.
Acute pharmacological administration of MC4R agonists and antagonists into the fourth ventricle or the caudal brainstem affects food consumption as potently as that in the hypothalamus (Grill et al., 1998; Williams et al., 2000; Zheng et al., 2005), and central infusion of MTII into either the third or fourth ventricle also augments brown adipose tissue (BAT) thermogenesis (Cettour-Rose & Rohner-Jeanrenaud, 2002; Williams et al., 2003; Li et al., 2004). We demonstrated earlier that overexpression of POMC in either the hypothalamus or brainstem NTS region alone caused a significant and sustained weight loss for 42 days in aged-obese rats (Li et al., 2005; Li et al., 2007). Such observations underscore the importance of the melanocortin pathway in either brain region in the control of energy balance. Although these earlier studies demonstrated obvious metabolic improvements, the influence of POMC gene transfer on physical activity was not examined. The current study assessed the combined long-term contributions of the MC systems from both brain areas to metabolic and physical activity regulation in rats with adult-onset obesity. The nature of the long-term outcomes, as to whether they may be related to energy intake or food independent but POMC-specific, was specifically addressed by including the pair feeding paradigm.
Materials and methods
Experimental animals
Male F344xBN rats of 22-month of age were obtained from Harlan Sprague–Dawley (Indianapolis, IN) under contract with the National Institute on Aging. Animals were cared for in accordance with the principles of the NIH Guide to the Care and Use of Experimental Animals and adhere to the protocol approved by the local VA IACUC. Rats were housed individually with a 12:12 h light:dark cycle (lights on 07:00 to 19:00 h). Free access was provided to standard Purina 5001 rodent diet and water.
Preparation and administration of rAAV-POMC
pTR-POMC encodes the full-length 935 base pair murine POMC cDNA (Uhler & Herbert, 1983) under the control of the hybrid cytomegalovirus immediate early enhancer/chicken β-actin promoter. Construction of this vector, designated as rAAV-POMC, was described previously (Li et al., 2003). The control vector is similar to rAAV-POMC except for the incorporation of the cDNA encoding an enhanced form of green fluorescent protein (GFP) in place of the POMC, thus, designated as rAAV-GFP. Both vectors were packaged as Serotype 5 AAV, then purified, concentrated and tittered (Zolotukhin et al., 1999). The titers for both vectors used in this study were 2.51×1013 physical particles/ml.
Rats, under isoflurane anaesthesia (5% induction, 2-2.5% for maintenance), were administered rAAV-POMC or rAAV-GFP. Viral particles (containing 2.51×1010 in 1μl) were introduced unilaterally by separate injections targeting the NTS and basomedial hypothalamus using coordinates established previously (Li et al., 2005; Li et al., 2007). A 10μl-capacity Hamilton syringe was used to deliver 1μl virus over 5 min to the target site. The needle remained in place at the injection site for a further 2 min before withdrawal. The analgesic, Buprenex (buprenorphine hydrochloride, 0.05 mg/kg; Reckitt and Colman, Richmond, VA), was administered to the animals subcutaneously before and after the surgery.
Experimental time line
Eight rats per group were used, and one rat from each of the three experimental groups (Control, POMC and PF) displayed abnormal food intake and/or body weight change that strayed beyond two times the standard deviation from the group mean post gene delivery and was excluded from data analysis. Both the control and pair-fed (PF) animals received rAAV-GFP control vector. The PF rats were fed the average amount of food consumed daily by the POMC-treated rats (lagging one day behind). Oxygen consumption was recorded at day 10 (7 rats/group), grip strength measured at day 65 (7 rats/group), tail blood collected at day 71 (7 rats/group) and running wheel test performed from days 75 to 78 (6 rats/group).
O2 consumption
O2 consumption was assessed in up to seven rats simultaneously with an Oxygen analyzer (Model S-3A; AEI Technologies, Naperville, IL) on day 10 post-viral gene delivery. Flow rates were 150 ml/min with a 1-min sampling time at 8 min intervals. The rats were placed into the chamber until readings were stable and then sampled for 60 min. Food was not available during assessment. Data were expressed as lean body mass adjusted consumption (ml · min -1 · kg0.67) and as oxygen consumption per rat (ml · min -1).
Wheel Running
Rats were housed in cages equipped with Nalgene Activity Wheels (1.081 meters circumference) obtained from Fisher Scientific (Pittsburgh, PA) and allowed free access to the wheel from days 75 to 78 after virus injections. Each wheel was equipped with a magnetic switch and a counter with liquid crystal display (LCD) that records the number of wheel revolutions. The wheel revolutions were recorded during both the day (8am to 5pm) and night (5pm to 8am) times each day for 3 consecutive days, and the sum of the two recordings represents the daily WR activity. The majority of the running happened at night. The cumulative WR activity over the entire 3-day period, expressed in meters as the distance ran, was reported.
Grip strength
Forelimb grip strength was measured at day 65 post-viral vector delivery using an automated grip strength meter (Columbus Instruments, Columbus, OH) as described previously (Carter et al., 2004). Data were expressed as kilograms of force/kilograms of body weight.
Tissue harvesting and preparation
Rats were killed at day 86 by thoracotomy under 5% isoflurane deep anesthesia. Blood samples were collected by heart puncture, and serum collected in serum separator tubes after a 10-min centrifugation. The circulatory system was perfused with 200 ml cold saline. BAT, perirenal, retroperitoneal, epididymal white adipose tissue (PWAT, RTWAT, EWAT) and soleus muscle were excised. The hypothalamus was removed by making an incision medial to piriform lobes, caudal to the optic chiasm and anterior to the cerebral crus to a depth of 2–3 mm. For dissection of the caudal brainstem NTS, excised brains were chilled on ice-cold saline and sliced using a Stoelting tissue slicer (Stoelting Co, Wood Dale, IL). Brains were sectioned at 0, -2 mm (rostral) relative to the level of obex in the caudal medulla oblongata (Paxinos G, 1997). The NTS-containing tissue was removed from the 2 mm-thick slice by a tissue puncher and frozen quickly in liquid nitrogen and stored at – 80°C until analysis.
Western analysis
The protein homogenate (20 - 60 μg) was separated on a SDS-PAGE gel and electro-transfered to PVDF membranes. BAT UCP1 was identified by a human anti-UCP1 antibody (Linco Research, St. Charles, MO) (Li et al., 2002). Antibodies specific to the phosphorylated hormone-sensitive lipase (P-HSL), AMP-activated kinase (P-AMPK) (Cell Signaling Technology, Inc., Beverly, MA), acetyl-CoA carboxylase (P-ACC, Upstate Cell Signalling Solutions, Lake Placid, NY) and total AMPK (Cell Signalling Technology, Inc., Beverly, MA), were used all at 1:1000 dilution. Signals were visualized by enhanced chemiluminescent detection (ECL-plus, Amersham Pharmacia Biotech, Piscataway, NJ) using a STORM fluorescent scanner (Molecular Dynamics, Sunnyvale, CA), and data analyzed by ImageQuant software (Molecular Dynamics).
Serum leptin, insulin and glucose
Radio-immunoassay (RIA) kits from Linco Research were used to measure serum leptin and insulin (rat RIA). Fasting glucose was measured using Trinder agent (Sigma Diagnostics).
Immunohistochemistry analysis of GFP overexpression
Separate rats receiving rAAV-GFP injection to both the ARC and NTS were used for immunohistological verification of GFP expression, which was a marker representing the potential POMC transgene overexpression and distribution pattern. One month after rAAV-GFP gene delivery (a duration of time to allow GFP protein expression to accumulate to maximal), the rats were killed with sodium pentobarbital (100 mg/kg i.p.) and perfused at 65 ml/min through the left ventricle with 100 ml 0.1 M phosphate-buffered saline (PBS) pH 7.2-7.4 at ambient temperature, followed by 400 ml PBS containing 10% formalin. The intact rat bodies were refrigerated for 2 hours at 4°C prior to brain extraction and then equilibrated in 30% sucrose PBS cryoprotectant. Coronal blocks were frozen-sectioned at 50 um from the excised whole brain, and sections collected in 300μl PBS/well in 24-well polystyrene culture plates (Phoenix Pharmaceutical Inc, MO). Plates were incubated overnight on a rotating shaker in 200 μl/well PBS plus 0.02% sodium azide, 0.1% Triton X-100 with 1 μg/ml primary anti-GFP antibody (Clontech Inc, DE). Sections were then incubated overnight in biotinylated goat anti-rabbit IgG at 1:1000, then in extravidin peroxidase at 1:1000 in PBS overnight. After washing, sections were reacted 5 min in 0.5 mg/ml diaminobenzidine in 0.1 M sodium acetate with 1.2 to 5% hydrogen peroxide, then mounted on glass slides from 0.1 M saline and coverslipped with glycerol gelatin. All chemicals were from Sigma (MO) unless noted otherwise. Bright-field images were collected using an Olympus BH-2 microscope equipped with Hitachi KP-D581 color digital video camera and ImagePro Plus software (Media Cybernetics).
RT-PCR
Expression levels of POMC and AgRP in the hypothalamus and NTS were identified by relative quantitative RT-PCR using the QuantumRNA 18S Internal Standards kit (Ambion, Austin, TX, USA) as described previously (Li et al., 2002; Li et al., 2003; Li et al., 2004).
Statistical analysis
Data are expressed as means ± SE. The α-level was set at 0.05 for all analyses. Comparisons of mean daily food intake and body weight change (Fig 1A and 1B) were made by a repeated-measures two-way ANOVA with POMC treatment and time as factors, followed by Tukey's post-hoc analysis for pairwise comparisons. Two tailed, unpaired Student's t-test was used in place of Tukey test when there were only two group means to compare (in Fig 1A for mean daily food intake between only the control and POMC rats). All other data were analyzed by one-way ANOVA followed by Tukey's post-hoc test. GraphPad Prism software version 4·0 (San Diego, CA, USA) was used for all statistical analysis and graphing.
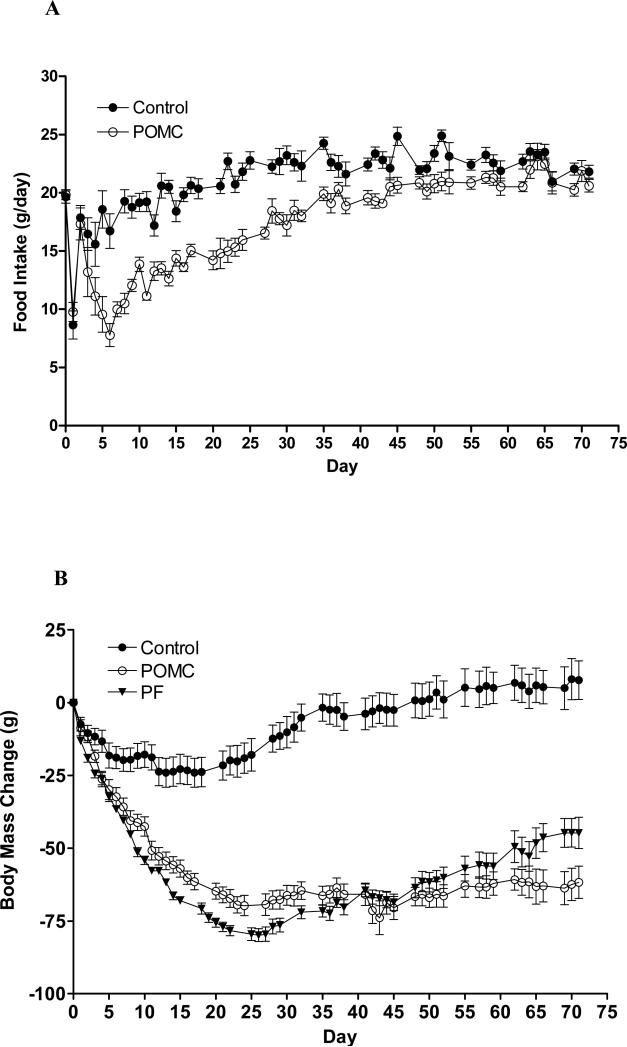
Fig 1.
Food consumption (A) and body weight change (B) after rAAV-POMC (open circles) or rAAV-GFP (closed circles) gene delivery in aged obese rats. Closed triangles represent body weight change in rats administered control virus and pair-fed to the amount of food consumed by the POMC-treated rats. The vectors were injected at day 0. All three groups of rats had an average of body weight around 600g at day 0. Data are means ± SE of 7 rats per group. Significance was found for food consumption (A) by repeated-measures two-way ANOVA for both the POMC treatment (F1,720 = 677.15, P < 0·0001) and time (F71,720 = 24.68, P < 0·0001) main effects. The interaction between POMC and time was also significant (F71,720 = 4.41, P < 0·0001). Body mass change (B) was significant with respect to both the POMC (F2,1080 = 3827, P < 0·0001) and time (F71,1080 = 21.02, P < 0·0001) main effects. The interaction between the two was also significant (F142,1080 = 7.95, P < 0·0001). Comparison of the mean daily food intake between only the POMC and the control groups (A) was made by Student's t-test, and significant difference in the mean values were found on days from 5 to 57 with the exceptions on day 37, 44, 48 and day 52. By Tukey post hoc analysis, body weight change (B) was different between the Control and either the POMC or PF group from day 4 till day 72, and between the POMC and PF rats between days 8 to 35 and 51 to 72.
Results
Food consumption and body weight
The POMC gene transfer resulted in a rapid decrease in food consumption that became significantly different from control rats by day 5 (Fig. 1A; Repeated-measures two-way ANOVA: F1,720 = 677.15, P < 0·0001 with POMC, F71,720 = 24.68, P < 0·0001 with time, and interaction between the two variables, F71,720 = 4.41, P < 0·0001). By day 6, food intake reached a nadir of less than 8 g/day, amounting to a 58% reduction compared with the control rats. After day 6, the anorectic response began to wane, finally attenuating around day 60 (Fig. 1A).
The 22-month-old POMC, control, and PF rats weighed similarly at about 600g prior to vector delivery. They were relatively obese because a 6-month-old young rat with the same naso-anal length has only an average weight of 375 g (Li et al., 1997). The surgical procedure for the gene delivery caused a nearly 20g weight loss in control rats, similar to what we normally observe after surgeries in aged-obese rats (Scarpace et al., 2002; Li et al., 2005). The control rats regained the lost weight in nearly a month, and thereafter, their body weights remained steady (Fig. 1B). A repeated-measures two-way ANOVA demonstrated statistical significance in body mass with POMC (F2,1080 = 3827, P < 0·0001) and time (F71,1080 = 21.02, P < 0·0001) as variables, and also a significant interaction between the two factors (F142,1080 = 7.95, P < 0·0001).
Both the POMC and PF rats exhibited steady and parallel decreases in body weight until 45 days after gene delivery (Fig. 1B). Between days 8 to 35, however, the PF animals displayed more body mass loss than the POMC rats. This observation is elaborated on later in the Discussion session. After day 51, body weight began to diverge between the POMC and PF rats despite similar food consumptions (Fig. 1B). Between days 45 and 71, the PF group gained 23.0 ± 4.0 g in body weight relative to only 7.0 ± 3.1 g in the POMC rats (two tailed Student's t-test, P < 0.01).
Energy expenditure
We measured whole body oxygen consumption at day 10 after vector delivery and UCP1 protein levels in BAT at the termination of the experiment (Table 1). Oxygen consumption was reduced in both the POMC and PF compared with the control rats whether expressed as consumption per rat (F2,18 = 28.60, P < 0.0001 with one-way ANOVA; P < 0.001 for POMC or PF vs. Control by Tukey post-hoc analysis) or normalized to lean body weight (F2,18 = 27.47, P < 0.0001 with one-way ANOVA; P < 0.01 for POMC vs. Control and P < 0.001 for PF vs Control by Tukey). Pair feeding decreased the oxygen consumption further relative to the POMC treatment only when the measurement was normalized to lean body weight (P < 0.01 for POMC vs. PF by Tukey) but not when expressed as consumption per rat (Table 1).
Table 1.
Whole body oxygen consumption at day 10 post rAAV-POMC delivery and BAT UCP1 at termination.
| Treatment | |||
|---|---|---|---|
| Control | POMC | Pair-Fed | |
| Oxygen Consumption (mL/min/kg-.67) | 6.29±0.15 | 6.05±0.11*† | 5.85±0.05* |
| Oxygen Consumption (mL/min/rat) | 4.35±0.12 | 4.08±0.10* | 3.98±0.05* |
| BAT weight (mg) | 531 ± 17 | 404 ± 21* | 403 ± 26* |
| UCP1 protein (arbitrary units/total BAT) | 100 ± 17 | 177 ± 22*† | 124 ± 11 |
Data represent the mean ± SE of 7 rats per group. BAT: brown adipose tissue; UCP1: uncoupling protein 1. Oxygen consumption was determined at day 10 after rAAV-POMC gene delivery. For UCP1 protein, the levels in control rats were set to 100 and SE adjusted proportionally. P < 0.0001 (oxygen consumption), P = 0.023 (BAT) and P = 0.024 (UCP1 protein) indicate significant difference with the POMC treatment by one-way ANOVA.
represents significant difference (P <0.05 or 0.01 or 0.001) between the POMC or Pair-fed and Control rats
indicates significance (P < 0.05 or 0.01) between the POMC and Pair-fed groups by Tukey's post-hoc analysis.
Induction of UCP1 in BAT is one indicator of enhanced thermogenesis and constitutes one component of energy expenditure in rodents (Cannon & Nedergaard, 2004). We demonstrated earlier that either leptin or MTII treatment elevates BAT UCP1 protein level and reduces BAT mass (Scarpace et al., 2002; Li et al., 2004). In the present study, total BAT weight declined by 25% in both the PF and POMC animals (F2,18 = 11.56, P = 0.0006 by one-way ANOVA; P < 0.01 for PF or POMC vs Control by Tukey). UCP1 protein was significantly elevated only by the POMC treatment compared with either the control or PF group (F2,18 = 5.19, P = 0.017 by one-way ANOVA; P < 0.05 for POMC vs. Control or vs. PF by Tukey), whereas pair feeding did not alter UCP1 level relative to that of the control (Table 1).
White adipose tissues and serum parameters
The control rats had greatest amounts of three selected fat depot mass, PWAT, RTWAT and EWAT (Table 2) (F2,18 = 31.45, P < 0.0001 for PWAT; F2,18 = 19.90, P < 0.0001 for RTWAT, and F2,18 = 12.95, P = 0.0003 for EWAT by one-way ANOVA). The PF animals had lower adiposity relative to the control (P < 0.001 for PWAT; P < 0.01 for RTWAT and P < 0.05 for EWAT by Tukey), and the adiposity was further decreased in the POMC rats (POMC vs. PF: P < 0.01 for PWAT; P < 0.05 for RTWAT or EWAT by Tukey). When serum leptin, a common indicator of adiposity, was measured (Table 2), it was 41% and 32% lower in the POMC animals than that of the control and PF rats, respectively (F2,18 = 12.34, P = 0.0004 by one-way ANOVA; P < 0.001 for POMC vs. Control and P < 0.01 for POMC vs. PF by Tukey).
Table 2.
White adipose tissue weights and serum parameters.
| Treatment | |||
|---|---|---|---|
| Control | POMC | Pair-fed | |
| PWAT (g) | 4.38 ± 0.19 | 2.65 ± 0.22*† | 3.27 ± 0.17* |
| RTWAT | 16.09 ± 0.85 | 10.23 ± 0.54*† | 13.04 ± 0.51* |
| EWAT (g) | 14.84 ± 0.99 | 9.64 ± 0.97*† | 12.29 ± 0.60* |
| Leptin (ng/ml) | 19.1 ± 1.5 | 11.2 ± 0.8*† | 17.1 ± 1.1 |
| Glucose (ng/dL) | 117 ± 4 | 69 ± 4*† | 105 ± 6 |
| Insulin (ng/ml) | 0.35 ± 0.03 | 0.20 ± 0.03*† | 0.31 ± 0.03 |
Data are means ± SE of 7 rats per group. PWAT, RTWAT and EWAT: perirenal, retroperitoneal, epididymal white adipose tissue. P < 0.0001 with PWAT, RTWAT or EWAT, P = 0.0004 (leptin), P < 0.0001 (glucose) and P = 0.034 (insulin) indicates difference in regards to the POMC treatment by one-way ANOVA.
represents significant difference (P <0.05 or 0.01 or 0.001) between the POMC or Pair-fed and Control rats
indicates significance (P < 0.05 or 0.01) between the POMC and Pair-fed groups by Tukey's post-hoc analysis.
On day 71 after the POMC gene delivery, at the time when body weights in all three groups were distinctly different, the overnight fasting glucose level (Table 2) was unchanged with pair feeding but reduced by 45% with the POMC treatment (F2,18 = 27.53, P < 0.0001 by one-way ANOVA; P < 0.001 for POMC vs. Control or vs. PF by Tukey). Likewise, fasting insulin levels (Table 2) remained similar between the control and PF animals while decreased by nearly 60% in the POMC group (F2,18 = 6.70, P = 0.0067 by one-way ANOVA; P < 0.01 for POMC vs. Control and P < 0.05 for POMC vs. PF by Tukey). The quantitative insulin sensitivity check index (QUICKI) (Katz et al., 2000) revealed significantly enhanced insulin sensitivity in the POMC rats (0.87 ± 0.05 vs. Control: 0.67 ± 0.03 or PF: 0.67 ± 0.02; F2,15 = 10.53, P = 0.0014 by one-way ANOVA; P < 0.01 for POMC vs. Control or vs. PF by Tukey).
Fat metabolism
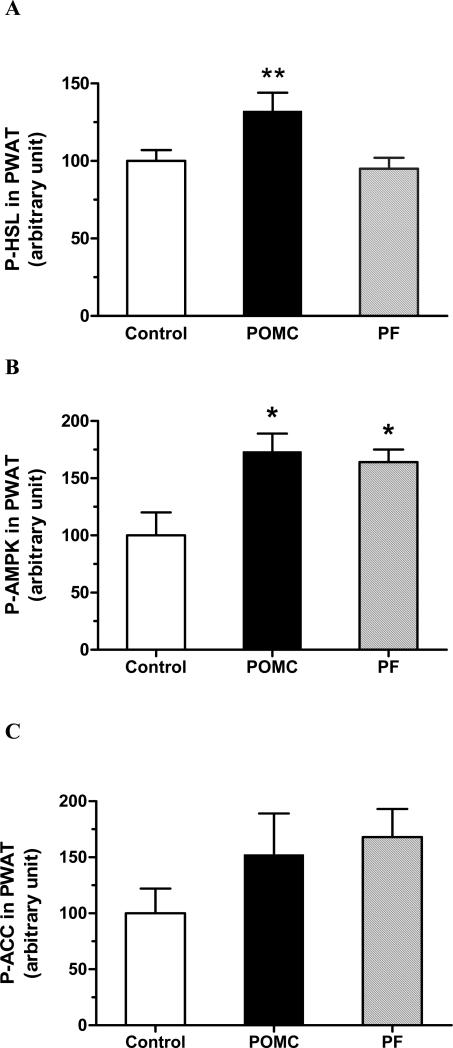
We examined phosphorylation of the hormone-sensitive lipase (HSL) at Ser-563 in PWAT at sacrifice as one measurement of fat mobilization. A 32% increase in P-HSL (Fig. 2A) was observed with POMC treatment compared to either the control or PF animals (F2,18 = 5.58, P = 0.013 by one-way ANOVA; P < 0.05 for POMC vs. Control or vs. PF by Tukey). Since activation of AMPK and/or inactivation of ACC upon phosphorylation are often considered indicators for augmented fatty acid oxidation and/or diminished fatty acid synthesis (Kim, 1997), we also determined phosphorylation of AMPK and ACC in soleus muscle and PWAT. Neither POMC nor pair feeding changed P-AMPK and P-ACC versus the control levels in soleus muscle (data not shown). In PWAT, however, P-AMPK was increased with both POMC and pair feeding (Fig. 2B; F2,18 = 6.12, P = 0.0094 by one-way ANOVA; P < 0.05 for Control vs. POMC or vs. PF by Tukey). There was an apparent increasing trend but no statistical significance with respect to P-ACC with either treatment (Fig. 2C).
Fig 2.
P-HSL (A), P-AMPK (B) and P-ACC (C) in perirenal white adipose tissue at day 86. Data represent the means ± SE of 7 rats per group. Significance was found for P-HSL (F2,18 = 5.58, P = 0.013) and P-AMPK assessment (F2,18 = 6.12, P = 0.0094) by one-way ANOVA, but not for P-ACC analysis. ** P < 0.05 for difference between the POMC and Control or PF rats with P-HSL assessment, and * P < 0.05 for difference between the POMC or PF versus Control for P-AMPK by Tukey analysis.
Wheel running
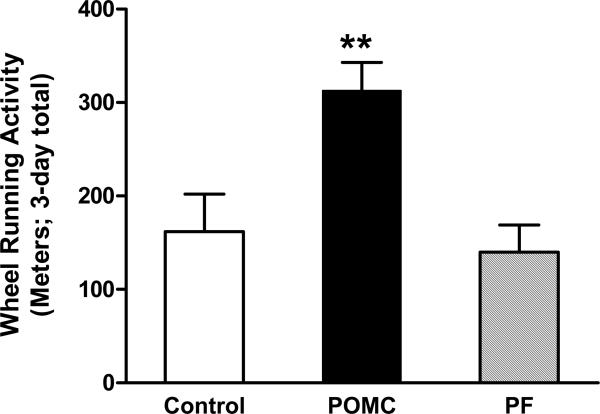
Physical activity constitutes one aspect of energy expenditure. One measure of volitional physical activity is voluntary wheel running. On day 75 after the POMC gene delivery, running wheels were placed in each of the home cages and voluntary WR assessed for three consecutive days. During this assessment, the rats had access to food at all times. The PF rats were fed the same amount of food consumed by the POMC rats (21.1 ± 0.8 g/day). This quantity of food was not statistically different than the amount of food consumed by the control rats immediately prior to wheel running (22.1 ± 0.9 g/day). Food intake and body weights remained similar during the 3-day running period in all three groups (data not shown). The animals were not trained or coerced in any way to run. The control and PF rats ran to the same extent, whereas the POMC-treated animals ran twice the distance of the other two groups (Fig. 3; F2,15 = 7.96, P = 0.0044 by one-way ANOVA; P < 0.05 for POMC vs. Control and P < 0.01 for POMC vs. PF by Tukey). A separate study demonstrated that 6-month-old young rats treated with the control vector for the same length of time ran 5 times the amount of the aged control rats (1084 ± 131 m/3-days). In all groups, the majority of the voluntary WR occurred at night (data not shown).
Fig 3.
Cumulative wheel running activity over a three-day period from days 75 to 78 post vector delivery. Data represent the means ± SE of 6 rats per group. The mean distance ran was significantly different (F2,15 = 7.96 and P = 0.0044) by one-way ANOVA. ** indicated difference between the POMC and Control, P < 0.05, and between the POMC and PF group, P < 0.01 by Tukey post-hoc analysis.
Because muscle quality could be a factor that affects wheel running, we measured forelimb grip strength at day 65 post viral injection (Carter et al., 2004). There were no significant differences between forelimb grip strength among the three experimental groups (3.11 ± 0.16 kg force, control; 3.34 ± 0.13, PF; and 3.22 ± 0.08, POMC).
GFP, POMC and AgRP expression in the hypothalamus and brainstem
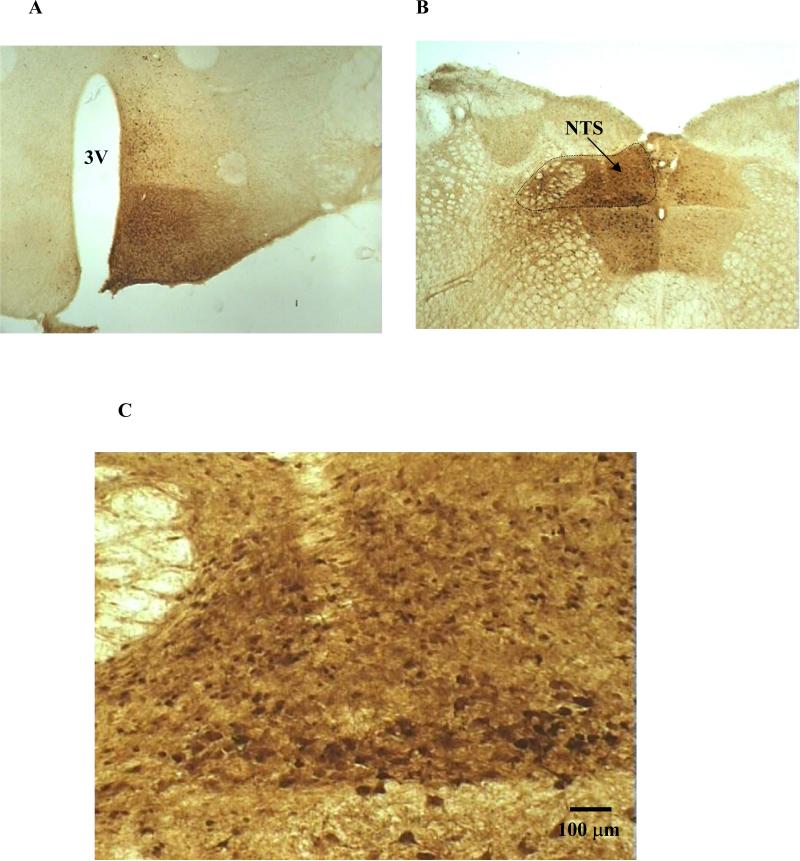
To evaluate plausible rAAV-mediated transgene delivery at the brain targeting sites, we injected rAAV control vector containing the GFP reporter gene and analyzed GFP expression pattern by immunohistochemistry analysis. Hypothalamic GFP labeling was centered on the ipsilateral arcuate nucleus, posterior to the optic chiasm and rostral to the mammillary complex (Fig. 4A). Transduction was highest near the ventral aspect of the 3rd ventricle and diminished dorsally and laterally, and contained within the ventromedial hypothalamus. Some neurons in contralateral arcuate were transduced. Scattered neurons were located in dorsal medial hypothalamus adjacent to the injection track (Fig. 4A). GFP transduction in dorsal medial brainstem extended from the caudal medulla to the middle cerebellar peduncle (Fig. 4B). Neurons were heavily transduced in the NTS and some in hypoglossal, vestibular, and tegmental nuclei. Transduction was predominantly ipsilateral but considerable expression was also observed contralaterally (Fig. 4B). We have demonstrated previously that rAAV5 virtually transduces neurons exclusively (Klein et al., 2002). The size and morphology of the GFP-transduced cells in this study indicate neuronal cells, consistent with the previous finding (Fig. 4C). Based on these observations, we expected the POMC delivery intended for the ARC and NTS areas to infect virtually all POMC neurons in the ipsilateral side of the ARC and NTS and some in the contralateral side. The virus infection was also expected to involve neurons in the ventromedial hypothalamus, other areas besides the NTS in the caudal medulla and parts of cerebellar peduncle. However, the nature of the infected neurons is unknown (no POMC neurons in these sites). We speculate that the nonselective infection outside the ARC and NTS regions might not generate proper POMC production and/or processing to cause meaningful physiological consequences.
Fig 4.
GFP immunohistochemistry post rAAV-GFP delivery to the ARC region of the hypothalamus (A) and the NTS of the brainstem (B). Representation of GFP expression highlighting neuronal cells is shown in C. GFP-positive cells were prominent along the 3rd ventricle (3V) and centered on the ipsilateral arcuate nucleus, posterior to the optic chiasm and rostral to the mammillary complex. Transduction was highest near the ventral aspect of the 3rd ventricle and diminished dorsally and laterally, and contained within the ventromedial hypothalamus (A). GFP was heavily expressed in the NTS and some in hypoglossal, vestibular, and tegmental nuclei, and the transduction extended from the caudal medulla to the middle cerebellar peduncle. GFP expression was mainly ipsilateral but also clearly visible contralaterally (B). The size and morphology of the GFP-transduced cells, viewed at 10x magnification, indicate neuronal cells (C).
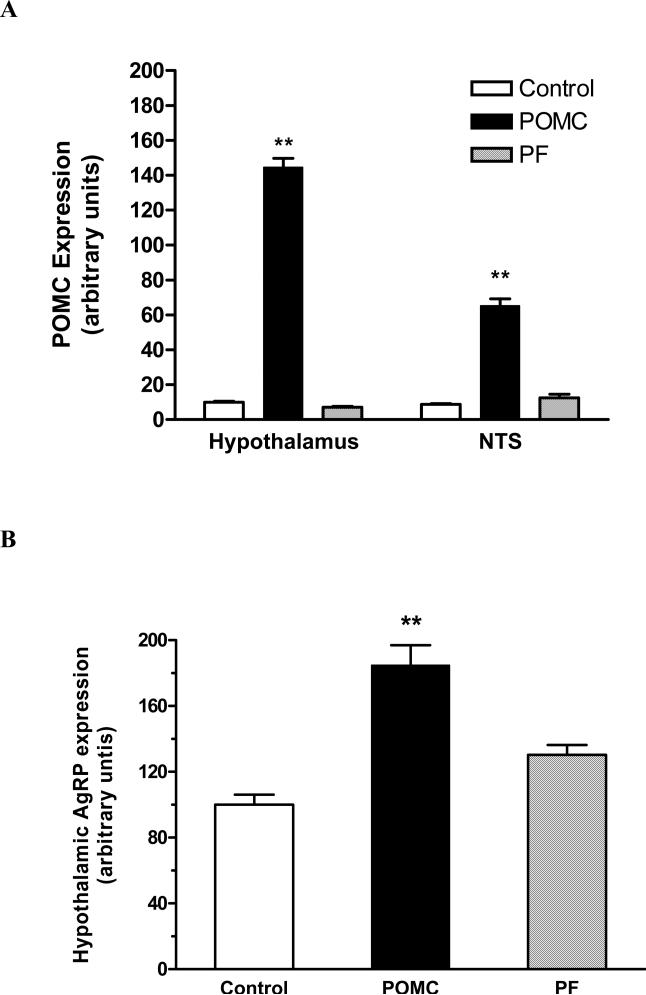
At the termination of the experiment, POMC mRNA levels were elevated nearly 14-fold in the hypothalamus and 7.5 fold in the NTS in rAAV-POMC rats compared with rAAV-GFP animals (Fig. 5A; in hypothalamus: F2,17 = 4696, P < 0.0001 by one-way ANOVA; P < 0.001 for POMC vs. Control or vs. PF by Tukey analysis; in NTS: F2,17 = 949.1, P < 0.0001 by one-way ANOVA; P < 0.001 for POMC vs. Control or vs. PF by Tukey). There was a trend towards a decrease in POMC expression in the hypothalamus of the PF rats but no change in the NTS relative to the control rats (Fig. 5A). Hypothalamic AgRP expression was increased only with the POMC treatment (Fig. 5B; F2,17 = 23.47, P < 0.0001 by one-way ANOVA; P < 0.001 for POMC vs. Control or vs. PF by Tukey).
Fig 5.
POMC expression 86 days after rAAV-POMC or control vector delivery in both the NTS and hypothalamus (A) and AgRP expression in the hypothalamus only (B). POMC and AgRP mRNA levels were measured by relative quantitative RT-PCR with 18S rRNA as an internal standard. Data are means ± SE from 6-7 rats per group. The mean values in control rats were set to 10 (A) and 100 (B) with SE adjusted proportionally. The mean POMC expression was significantly different among the groups whether measured in the hypothalamic (F2,17 = 4696, P < 0.0001) or NTS samples (F2,17 = 949.1, P < 0.0001) by one-way ANOVA. Tukey post-hoc analysis revealed ** P < 0.001 for difference in POMC expression in either the hypothalamus or NTS between the POMC group compared to either the control or PF animals. The hypothalamic AgRP expression also differed among groups (F2,17 = 23.47, P < 0.0001), and Tukey analysis showed ** P < 0.001 between the POMC and either the Control or PF group.
Discussion
Activation of the MC system is an effective weight reducing strategy in rodents, yet limited by its transient nature (Pierroz et al., 2002; Li et al., 2004; Zhang et al., 2004). Chronic studies with transgenic mice overexpressing the N-terminal POMC transgene indicated significant metabolic improvements in transgenic animals fed a regular or high-fat diet (Savontaus et al., 2004; Lee M, 2007). However, multi-organ transgene expression confounds mechanistic interpretations of these functional studies. Alternatively, we employed neuro-site directed gene transfer approach and achieved POMC overexpression and increased α-MSH production by delivering POMC directly to either the hypothalamus (Li et al., 2005) or the brainstem NTS region (Li et al., 2007). In the current study, we targeted the two POMC neuron-bearing sites concurrently and assessed the combined contribution of melanocortin activation from both regions to the long-term energy balance regulation.
It is known that the hypothalamic POMC expression or the levels of POMC-derived neuropeptides in hypothalami decrease with age (Nelson et al., 1988; Lloyd et al., 1991; Mobbs et al., 2001). Our earlier study also demonstrates impairment in the induction of POMC by exogenous leptin in the aged-obese F344xBN rats compared to their young-lean counterparts (Scarpace et al., 2002). Conceivably, insufficient MC activation, whether due to lower MC levels and/or improper MC receptor responses, is associated with aging and may contribute to age-related obesity. In light of this recognition, our investigation of chronic stimulation of both the hypothalamic and brainstem MC systems in aged-obese rats becomes physiologically relevant and important.
The dual-site POMC gene delivery caused a prolonged but transient decrease in food consumption and yet, a sustained reduction in body weight lasting till termination. In comparison to the two previous studies with the single-site POMC gene transfer, the concurrent POMC overexpression in the hypothalamus and brainstem produced a larger and near 60% reduction of food intake at the nadir versus near 30% in the POMC_NTS study (Li et al., 2007) or near 20% in the POMC_hypothalamus study (Li et al., 2005). The duration of hypophagia was about 60 days in this study versus that of 20 days or so in the POMC_hypothalamus study (Li et al., 2005) or that of 42 days in the POMC_NTS study (Li et al., 2007), although the POMC anorectic effect could last longer in the NTS study if the experiment were to be carried out longer than 42 days. In general, the effect on energy intake with the dual-site POMC treatment seemed to be additive relative to with the single-site POMC gene transfer. Moreover, the net extent of the body mass decrease, if using day 40 post-gene transfer as the reference point (the time at which the two earlier studies were about to terminate), also appeared additive (~ 70g in this study) relative to the individual-site POMC treatment (~ 30g from the hypothalamus study and ~ 40g from the NTS study). Additionally, the dual-site POMC treatment significantly elevated physical activity reflected as an increase in the propensity for voluntary wheel running. This aspect was not examined in the two previous studies. These results imply that the hypothalamic and brainstem MC pathways make quantitative and dissociable contributions to the overall regulation of energy homeostasis.
We used the short-term voluntary WR as a measure for physical activity in the current study rather than an intervention for reducing obesity, in which case, long-term WR is commonly employed. When provided the opportunity, the POMC-treated rats ran more than twice the distance as either control or PF rats. This enhanced running activity appeared to be appetite independent because all three groups had similar food consumption at the time of WR. Among other factors that may affect volitional WR activity, we ruled out the impact of muscle strength since all three groups of animals scored equally in the grip strength test. Reduced body size or adiposity could be potentially stimulatory towards WR, but exhibited no effect in this case since the leaner and lighter PF animals ran the same distance as the fatter and heavier control rats. Neuropeptide AgRP has been indicated as one factor that can suppress physical activity (Tang-Christensen et al., 2004). We detected augmented hypothalamic AgRP expression in the POMC-treated rats, a likely compensatory response to the amplified POMC expression. Nevertheless, in the presence of this elevated opposing factor, the POMC rats still ran to a greater extent. The AgRP-related antagonism to melanocortin activation is absent in the NTS due to the lack of AgRP neurons. POMC overexpression in and around the NTS could contribute importantly to melanocortinergic stimulation of WR activity, which may be dependent or independent from the POMC effect in the hypothalamus. Separate studies are needed to examine these various possibilities.
It remains unclear which nuclei are involved to enhance physical activity in response to the dual-site POMC gene transfer. The neurobiological regulation of physical activity, especially with respect to voluntary wheel running, is poorly understood at present time. POMC overexpression in the hypothalamus increases the principle melanocortin α-MSH level (Li et al., 2007), which can modulate lateral hypothalamic orexin expression (López et al., 2007) and potentially affect WR activity (Kotz et al., 2002). Since POMC neurons in the ARC project extensively in the CNS (Leibel, 2006), POMC gene transfer targeted to the ARC could impact the motivational aspect of physical activity via modulating reward/addiction circuitry involving dopaminergic tegmentum, nucleus accumbens and cortex (Lenard et al., 2008; Chen et al., 2010). By speculation, POMC overexpression in the NTS area could activate the rostral ventrolateral medulla as the α-MSH-containing neural fibers originating from the NTS innervate this site and consequently increase physical activity (Grill, 2006; Mueller, 2007; Skibicka et al., 2009). Much more work is needed to address these speculations. Compared to young F344xBN rats, the aged control rats run only one fifth of the distance by our observation, demonstrating a severe decline in propensity for voluntary WR that is possibly related to both age and obesity. The POMC gene therapy did not fully restore WR activity, but did increase it by two-fold. This moderate increase, if sustained, may generate a cumulative effect in the long run to reduce weight and adiposity. Future investigations are warranted to examine if the modest increase in WR with POMC treatment persists over time, and thus, impacts chronic weight maintenance.
Pair feeding helped derive some evidence for POMC stimulation of energy expenditure, but it only became evident after 45 days post-viral delivery. During the period of 10 to 34 days, however, the body mass decrease was larger in the PF than the POMC animals. There may be several reasons for this unintuitive result. It has been our experience that when MC activation-evoked anorexia is significant as it was for the first 40 days or so following the POMC treatment in this experiment, the anorexia dominates the weight loss, and any influence on body mass from changes in energy expenditure is usually masked (Zhang et al., 2004). Pair feeding can be problematic because the daily amount of food given to pair-fed animals is based on the average food intake from the previous day of the POMC-treated group. A couple of POMC rats consumed less food than usual on various days during this period. Although the reduced intake values were not low enough to be excluded from calculating the mean value, they did lessen the mean that guided the pair feeding next day. Thus, the PF animals as a group were somewhat underfed on some days, leading to more body mass loss than the POMC group. Despite this interesting twist, the body weights of the POMC and PF groups began to diverge beginning at day 45 albeit the two groups ate the same amounts of food, and were further separated from each other by terminus of the experiment. Adiposity was also reduced further in the POMC rats. These facts imply a specific POMC-related impact on body fat and body mass independent of food intake. Indeed, the protein level of BAT UCP1 was elevated only in the POMC rats compared to either the control or PF animals, suggesting a BAT thermogenesis-mediated increase in overall energy expenditure separate from the food effect.
The POMC-related decrease in adiposity may involve enhanced lipolysis in white adipose tissue. Phosphorylation of hormone sensitive lipase at Ser-563 by protein kinase A is a major regulator of HSL activity and the rate-limiting step for lipolysis in white fat (Anthonsen et al., 1998; Carmen & Victor, 2006). P-HSL was significantly elevated in PWAT in the POMC versus either control or PF animals at sacrifice, indicative of enhanced HSL activity and consequentially augmented lipolysis. Moreover, this result confirmed the continued cellular responses to the POMC treatment until the termination of the study. Because POMC-evoked food suppression was already attenuated during the 25 or so days prior to the end of the experiment, the elevated WAT lipolysis may explain in part why the POMC rats maintained their reduced body weights whereas the PF animals gradually gained weight during this last period. There have been reports on MC4R-related central modulation of lipid mobilization, probably via augmentation of CNS sympathetic outflow to white fat (Matsumura et al., 2002; Rahmouni et al., 2003; Williams et al., 2003; Song et al., 2005), and our observation is consistent with these reports. Both the PF and POMC rats lost weight and fat mass relative to control animals, the elevated P-AMPK and P-ACC (tendency to increase) in PWAT suggest increased fat metabolism and decreased fatty acid synthesis in these animals, providing one underlying mechanism.
When tested at day 71 post rAAV-POMC delivery, POMC markedly reduced fasting glucose and insulin levels and significantly improved the QUICKI (Katz et al., 2000). Either value remained unchanged in the PF rats possessing comparable degree of weight reduction relative to the POMC rats at this point of intervention. The dramatic POMC-related improvements in these parameters could involve direct suppression of insulin release from the pancreas and subsequent enhancement of glucose metabolism resultant from direct central MC receptor activation (Fan et al., 2000; Obici et al., 2001), but more experimentation is required to investigate this issue.
In summary, concurrent central POMC gene transfer to both the hypothalamus and brainstem yielded prolonged suppression in food intake, persistent body weight reduction, enhanced lipolysis, improved insulin sensitivity, and increased voluntary wheel running activity in aged-obese F344xBN rats. Besides the POMC-mediated anorectic response that eventually attenuated, the POMC effects on the propensity for volitional WR, BAT thermogenesis and WAT lipolysis were apparent towards or at the end of the experiment. Our findings demonstrate the importance of POMC influence from the two brain regions on long-term body weight maintenance and generate new interest for exploring long-term efficacy of melanocortin activation combined with voluntary wheel running in alleviating adult-onset obesity.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs, NIH grants AG20985 and AG026159, and Claude D. Pepper Older Americans Independence Center NIH Grant # 1 P30 AG028740.
Abbreviations
- POMC
pro-opiomelanocortin
- rAAV-POMC
recombinant adeno-associated virus-mediated POMC gene transfer
- rAAV-Control
rAAV encoding GFP
- MC
melanocortin
- MC3R
melanocortin 3 receptor
- MC4R
melanocortin 4 receptor
- α-MSH
α-melanocyte stimulating hormone
- MTII
melanotan II
- AgRP
agouti-related protein
- NPY
neuropeptide Y
- HF
high fat
- DIO
diet-induced obese or diet-induced obesity
- WR
wheel running
- ARC
arcuate nucleus
- NTS
nucleus of the solitary track
- UCP1
uncoupling protein 1
- BAT
brown adipose tissue
- WAT
white adipose tissue
- P-HSL
phospho-hormone sensitive lipase
- P-AMPK
phospho-AMP activated kinase
- P-ACC
phospho-acetyl CoA carboxylase
- PWAT, RTWAT and EWAT
perirenal, retroperitoneal and epididymal white adipose tissue
- QUICKI
quantitative insulin sensitivity check index
References
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- Cettour-Rose P, Rohner-Jeanrenaud F. The leptin-like effects of 3-d peripheral administration of a melanocortin agonist are more marked in genetically obese Zucker (fa/fa) than in lean rats. Endocrinology. 2002;143:2277–2283. doi: 10.1210/endo.143.6.8871. [DOI] [PubMed] [Google Scholar]
- Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010;1187:129–39. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Ball MJ, Morris MJ. Enhanced inhibitory feeding response to alpha-melanocyte stimulating hormone in the diet-induced obese rat. Brain Res. 2001;892:130–137. doi: 10.1016/s0006-8993(00)03246-7. [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am.J.Physiol Regul.Integr.Comp Physiol. 2001;281:R444–R451. doi: 10.1152/ajpregu.2001.281.2.R444. [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- Kim KH. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr. 1997;17:77–99. doi: 10.1146/annurev.nutr.17.1.77. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Klein RL, Hamby ME, Sonntag CF, Millard WJ, King MA, Meyer EM. Measurements of vector-derived neurotrophic factor and green fluorescent protein levels in the brain. Methods. 2002;28:286–292. doi: 10.1016/s1046-2023(02)00234-7. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim A, Chua SC, Obici S, Wardlaw SL. Transgenic MSH Overexpression Attenuates the Metabolic Effects of a High Fat Diet. Am J Physiol Endocrinol Metab. 2007;293:E121–131. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- Leibel RL. The molecular genetics of the melanocortin pathway and energy homeostasis. Cell Metab. 2006;3:79–81. doi: 10.1016/j.cmet.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;(Suppl 3):S11–22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Klein RL, Matheny M, King MA, Meyer EM, Scarpace PJ. Induction of uncoupling protein 1 by central interleukin-6 gene delivery is dependent on sympathetic innervation of brown adipose tissue and underlies one mechanism of body weight reduction in rats. Neuroscience. 2002;115:879–889. doi: 10.1016/s0306-4522(02)00447-5. [DOI] [PubMed] [Google Scholar]
- Li G, Mobbs CV, Scarpace PJ. Central pro-opiomelanocortin gene delivery results in hypophagia, reduced visceral adiposity, and improved insulin sensitivity in genetically obese Zucker rats. Diabetes. 2003;52:1951–1957. doi: 10.2337/diabetes.52.8.1951. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang Y, Wilsey JT, Scarpace PJ. Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol. 2004;182:123–132. doi: 10.1677/joe.0.1820123. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang Y, Wilsey JT, Scarpace PJ. Hypothalamic pro-opiomelanocortin gene delivery ameliorates obesity and glucose intolerance in aged rats. Diabetologia. 2005;48:2376–2385. doi: 10.1007/s00125-005-1943-8. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY, Scarpace PJ. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab. 2007;293:E252–258. doi: 10.1152/ajpendo.00451.2006. [DOI] [PubMed] [Google Scholar]
- Li H, Matheny M, Nicolson M, Tumer N, Scarpace PJ. Leptin gene expression increases with age independent of increasing adiposity in rats. Diabetes. 1997;46:2035–2039. doi: 10.2337/diab.46.12.2035. [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Scarbrough K, Weiland NG, Wise PM. Age-related changes in proopiomelanocortin (POMC) gene expression in the periarcuate region of ovariectomized rats. Endocrinology. 1991;129:1896–902. doi: 10.1210/endo-129-4-1896. [DOI] [PubMed] [Google Scholar]
- López M, Lage R, Tung YC, Challis BG, Varela L, Virtue S, O'Rahilly S, Vidal-Puig A, Diéguez C, Coll AP. Orexin expression is regulated by alpha-melanocyte-stimulating hormone. J Neuroendocrinol. 2007;19:703–707. doi: 10.1111/j.1365-2826.2007.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I, Iida M. Central alpha-melanocyte-stimulating hormone acts at melanocortin-4 receptor to activate sympathetic nervous system in conscious rabbits. Brain Res. 2002;948:145–148. doi: 10.1016/s0006-8993(02)03045-7. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am.J.Physiol Regul.Integr.Comp Physiol. 2000;279:R695–R703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Bray GA, Atkinson RL, Bartke A, Finch CE, Maratos-Flier E, Crawley JN, Nelson JF. Neuroendocrine and pharmacological manipulations to assess how caloric restriction increases life span. J Gerontol A Biol Sci Med Sci. 2001;56:34–44. doi: 10.1093/gerona/56.suppl_1.34. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Bender M, Schachter BS. Age-related changes in proopiomelanocortin messenger ribonucleic acid levels in hypothalamus and pituitary of female C57BL/6J mice. Endocrinology. 1988;123:340–344. doi: 10.1210/endo-123-1-340. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J.Clin.Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51:1337–1345. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savontaus E, Breen TL, Kim A, Yang LM, Chua SC, Jr., Wardlaw SL. Metabolic effects of transgenic melanocyte-stimulating hormone overexpression in lean and obese mice. Endocrinology. 2004;145:3881–3891. doi: 10.1210/en.2004-0263. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y, Tumer N, Frase CD, Shek EW, Hong B, Prima V, Zolotukhin S. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology. 2002;42:548–561. doi: 10.1016/s0028-3908(02)00003-5. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–61. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschop M. Central administration of ghrelin and agouti-related protein (83-132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145:4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
- Uhler M, Herbert E. Complete amino acid sequence of mouse pro-opiomelanocortin derived from the nucleotide sequence of pro-opiomelanocortin cDNA. J.Biol.Chem. 1983;258:257–261. [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144:4692–4697. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Matheny M, Tumer N, Scarpace PJ. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol Aging. 2004;25:1349–1360. doi: 10.1016/j.neurobiolaging.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]