Abstract
An effective HIV vaccine requires strong systemic and mucosal, cellular and humoral immunity. Numerous non-human primate studies have investigated memory T cells, but not memory B cells. Humoral immunologic memory is mediated by long-lived antibody-secreting plasma cells and differentiation of memory B cells into short-lived plasma blasts following re-exposure to immunizing antigen. Here we studied memory B cells in vaccinated rhesus macaques. PBMC were stimulated polyclonally using CD40 Ligand, IL-21 and CpG to induce B cell proliferation and differentiation into antibody secreting cells (ASC). Flow cytometry was used for phenotyping and evaluating proliferation by CFSE dilution. B cell responses were quantified by ELISPOT. Methodology was established using PBMC of vaccinated elite-controller macaques that exhibited strong, multi-functional antibody activities. Subsequently, memory B cells elicited by two replicating Ad-recombinant prime/envelope boost regimens were retrospectively evaluated pre- and post- SIV and SHIV challenges. The vaccine regimens induced SIV and HIV Env-specific IgG and IgA memory B cells. Prior to challenge, IgA memory B cells were more numerous than IgG memory B cells, reflecting the mucosal priming immunizations. Pre- and post-challenge memory B cells were correlated with functional antibody responses including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cell-mediated viral inhibition (ADCVI) and transcytosis inhibition. Post-challenge, Env-specific IgG and IgA memory B cells were correlated with reduced chronic viremia. We conclude that functional antibody responses elicited by our prime/boost regimen were effectively incorporated into the memory B cell pool where they contributed to control of viremia following re-exposure to the immunizing antigen.
Keywords: memory B cells, rhesus macaque, simian immunodeficiency virus, vaccine, replication-competent adenovirus recombinant
Introduction
Vaccination, by providing primary antigen exposure and inducing cellular and humoral immunological memory, is highly effective in preventing infectious diseases. Immunologic memory leads to a more rapid secondary response following a subsequent exposure. The cellular arm of the adaptive immune system involves T-cells that produce cytokines and kill infected cells, whereas adaptive humoral immunity encompasses antibodies and the B cells involved in their production. These two components have different functions within the immune system, but there is extensive crosstalk between them. CD4 T cell help, for example, is essential for induction of antibody responses and subsequent development of memory B cells1,2.
Humoral immunologic memory encompasses circulating antibodies, present in blood and continuously secreted by long-lived plasma cells residing in bone marrow, and antibody made by memory B cells upon re-exposure to antigen3–6. When naïve B cells are stimulated by antigen to produce antibodies, the majority differentiate into short-lived plasmablasts. However, a small percentage traffic to germinal centers of B cell follicles where immunoglobulin genes undergo somatic hypermutation and class switch recombination7 leading to differentiation into memory B cells and plasma cells. Memory B cells mainly reside in bone marrow and spleen but also circulate in peripheral blood6.
Long-term, stable antibody responses with half-lives of over 50 years are elicited by live attenuated vaccines such as vaccinia, polio, and measles, whereas non-replicating protein antigens, such as tetanus and diphtheria toxins, elicit antibody responses with much shorter half-lives 3, 8, 11. While several factors important for B cell expansion and survival in germinal centers and bone marrow have been identified4, 9, it is generally unclear how vaccination elicits such long-lived memory B cells. Also unknown is the size of antigen-specific pools of memory B cells, and their longevity in the absence of antigen re-exposure10.
While cytotoxic T cells can reduce the severity of viral disease by killing infected cells or reducing viral spread through release of immune mediators, it is generally accepted that vaccine-induced antibodies provide the best protection against initial infection. In characterizing vaccine-elicited antibody responses, antibody titer often correlates with vaccine protective efficacy, however, memory B cell and serum antibody levels do not always correlate3. In the case of HIV infection, envelope-specific plasma antibody and memory B cell levels may or may not correlate11, 12. Further, protection may be mediated by circulating antibodies and memory B cells in combination with other adaptive immune responses including T cells. Hence, to fully assess vaccine-elicited protective immunity and the contribution of the humoral immune component, it is important to quantify memory B cell responses. Since memory B cell responses at systemic and mucosal sites are regulated by distinct processes13–15, evaluation of both IgG and IgA memory B cells is warranted.
Our vaccine approach involves priming with replication-competent Ad-recombinants and boosting with envelope protein in order to elicit humoral, cellular, and mucosal immunity. Our studies in chimpanzee and rhesus macaque models16–20 have shown induction of potent, durable protection and strong antibody responses that mediate neutralizing antibodies and/or nonneutralizing, functional activities associated with protective efficacy, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cell mediated viral inhibition (ADCVI), and transcytosis inhibition21–25. To further evaluate humoral immunity elicited by this approach assessment of memory B cells was needed. Human memory B cells have been extensively studied10, 11, 26, however, only recently have rhesus macaque memory B cell studies been undertaken27, 28.
Here we report development of methodology for assessing macaque memory B cells and results of retrospective evaluations of vaccinated macaque PBMC for both HIV and SIV Env-specific memory B cells. We demonstrate that our vaccine regimen incorporates functional antibodies into memory B cell pools, and that these Env-specific IgG and IgA memory B cells are significantly correlated with reduced chronic phase viremia post-challenge. This humoral immunologic memory is a critical component needed for long-term vaccine efficacy.
MATERIALS and METHODS
Study animals
PBMC were obtained from vaccinated, long-term elite controller rhesus macaques, #5, #7, and #9, that resisted sequential SIVmac251 challenges, and exhibited strong, persistent antibody responses19, 20. Recently, they were re-challenged with SIVE660. Macaque #858, a naïve control for the SIVE660 re-challenge, served as an unvaccinated, infected control here. PBMC were collected during the chronic phase of SIVE660 infection: between 12 and 91 weeks (macaques #5, #7, and #9) and 12 and 20 weeks post-challenge (macaque #858). PBMC from naive macaques #419 and 421 served as uninfected controls. Animals were maintained according to NIH guidelines.
Retrospective evaluations of memory B cells used viably frozen PBMC from two previous preclinical vaccine studies in rhesus macaques. The first was a comparative study of mucosal priming regimens, involving two sequential oral priming immunizations (Oral/Oral) or an intranasal followed by an oral immunization (Intranasal/Oral) with Ad5hr-SIV recombinants encoding SIVsmH4env/rev, SIV239gag and SIV239nefΔ1–1329. Macaques were subsequently boosted twice intramuscularly with SIV gp120 and together with vector/adjuvant controls, challenged intrarectally with SIVmac251. Here, PBMC were evaluated 2 weeks following the second protein boost and 4 weeks after the challenge.
A second retrospective analysis made use of PBMC from a study that compared priming with Ad5hr-recombinants encoding HIVenv89.6P, SIV239gag and SIV239nefΔ1–13 alone to regimens in which priming was followed by boosting with HIV89.6P gp140 or an HIV “peptomer”, a polypeptide representing the CD4 binding site of gp12017. PBMC obtained 2 weeks after the second booster immunization and 4 weeks following intravenous SHIV89.6P challenge were evaluated.
Polyclonal stimulation of macaque B cells
PBMC (105 cells/200µl/well) were cultured in R-10 medium (RPMI 1640 containing 10% FBS (Hyclone), 200mM L-glutamine (Invitrogen), 100U/ml penicillin and 100µg/ml streptomycin (Invitrogen)) or R-10 medium supplemented with 1µg/ml CpG (ODN-2006) (Operon), 0.5µg/ml recombinant-Human sCD40L (Peprotech), and 50ng/ml recombinant-Human IL-21 (Peprotech), and incubated at 37°C in 5% CO2. At 3 and 5 days, cells were collected, spun at 1200 rpm for 7 minutes and washed with R-10. Viability was assessed using trypan blue.
Quantification of ASC
Total IgG and IgA ASC were quantified by ELISPOT. Multiscreen 96-well plates (MSIPS4510; Millipore) were activated with 70% ethanol, washed three times with PBS, and coated overnight at 4°C with 100µl of 10µg/ml goat anti-monkey IgG or IgA (KPL). The plates were blocked 2–4 hr at 37°C with milk (KPL; 200µl/well diluted 1:20 in R-10). PBMC were added (2×104/well in triplicate) and incubated at 37°C overnight. Following 4 washes in PBS containing 0.05% Tween 20 (Sigma) (PBST) the plates were incubated for 2 h at 37°C with 1µg/ml of bioatinylated goat anti-monkey IgG or IgA (Rockland) in PBST + 1% FBS, washed four times with PBST, and incubated for 1 h at room temperature (RT) with 5 µg/ml HRP-avidin-D conjugate (Vector Laboratories, Burlingame, CA) in PBST + 1% FBS. Subsequently, the plates were washed three times with PBST, three times with PBS, and developed using 3 amino-9 ethyl-carbazole (AEC, Sigma). After washing twice with water, spots were counted using an automated ELISPOT reader (Axioplan 2 imaging; Zeiss, Munchen, Germany).
To quantify Env-specific IgG and IgA ASC, plates were coated with 300 ng/well SIVmac251 gp120 (Advanced BioScience Laboratories, Inc.) or HIV89.6P gp140 and washed in R-10. PBMC (2×105/well in triplicate) were added and incubated at 37°C overnight. Following washing, the plates were blocked with 200µl/well R-10 plus 3% BSA (fraction V, Sigma) for 2 hr at 37°C. After washing with PBST, the procedure continued as above beginning with goat anti-monkey IgG or IgA incubation. For both total and specific IgG and IgA, mean ASC from triplicate wells were recorded.
Flow cytometric proliferation assay
PBMC (2×106/ml) were incubated with 0.125 mM CFSE (Invitrogen) at 37°C for 15 min, washed with PBS containing 0.1% BSA, then R10, and cultured at 105 cells/200 µl/well in 96-well U botton plates (Nunc) with or without polyclonal stimulation. At days 0, 3, and 5 PBMC were additionally labeled with polyclonal rabbit anti-human IgD PE (Dako), mouse anti-human CD27 PE-Cy7 (ebioscience, clone O323), and mouse anti-human CD20-APC-Cy7 (Biolegend, clone 2H7) for 15 min at RT, washed with PBS, and fixed with 1% ultrapure formaldehyde (Tousimis). Samples were acquired on a LSRII flow cytometer using FACS DIVA 6.0 software (BDBiosciences). Data analysis was performed using FlowJo 8.8.6 software (TreeStar).
Statistical analyses
Repeated measures analysis of variance was applied to comparisons between log-transformed pre- and post-challenge percent Env-specific IgG and IgA memory B cells from macaques in the comparative mucosal priming study. P values were corrected for multiple comparisons by the method of Hochberg. Changes from pre- to post-challenge within immunization groups in the second study were analyzed by analysis of variance of paired differences in square-root transformed percentages, with left-censoring of differences between 0’s and with Hochberg p value correction. Comparisons of responses between immunization groups used analysis of variance of square-root transformed percentages with left-censoring at 1 (or 0) of IgG (or IgA values assayed as 0’s, with Hochberg correction. Relationships between ASC and ADCC titer or percent killing, percent ADCVI, percent transcytosis inhibition, and viral loads were evaluated using the Spearman rank correlation test with exact p value calculation.
Results
Polyclonal stimulation of memory B cells
B lymphocytes can proliferate and differentiate in the absence of antigens by polyclonal stimulation of Toll-like receptor 9, expressed on activated B cells30, by unmethylated single-stranded DNA motifs such as CpG oligonucleotides, and in a non-cognate fashion via CD40 ligand26. Following CD40 engagement, IL-21 induces extensive proliferation and differentiation of ASC in humans31. A mixed regimen of CpG ODN-2006, CD40Ligand and IL-21 was found optimal for triggering macaque B lymphocyte proliferation and differentiation, as illustrated using PBMC from three vaccinated elite controller macaques known to possess anti-SIV envelope antibodies19, 20, and from an unvaccinated SIV infected macaque (Fig. 1). Within the CD20+ population memory B cells were distinguished from naïve B cells by expression of CD27, and subsets of memory B cells by expression of IgD. Memory B cells are predominantly IgM-secreting (unswitched; CD20+CD27+IgD+) or IgG, IgA, and IgM-secreting (switched; CD20+CD27+IgD−)31–33. Both subsets and naïve B cells (CD20+IgD+CD27−) were readily detected. Regardless of vaccination status, the proportion of switched and unswitched B cell subsets among total memory cells (combined upper two CD27+ quadrants) in the 4 macaques was remarkably similar.
Figure 1.
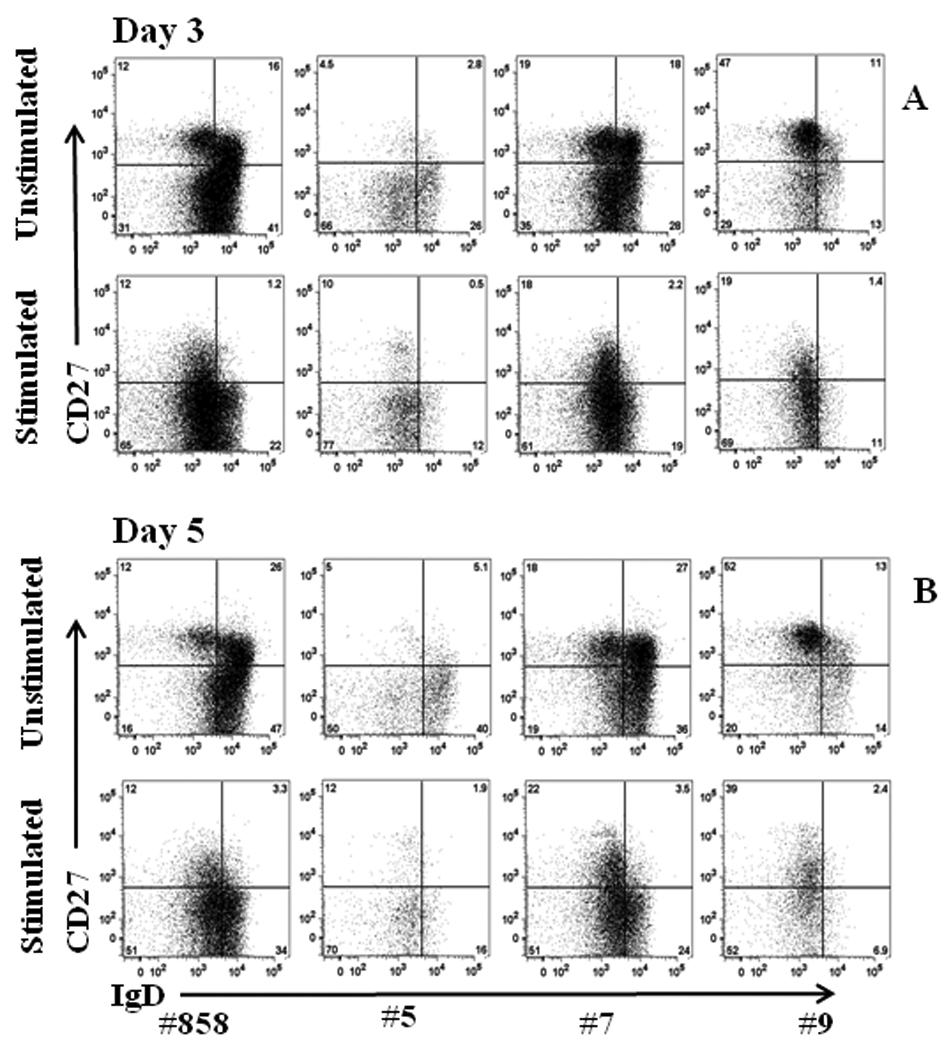
Representative staining of memory B cell subpopulations of PBMC from vaccinated (#5, #7, #9) and control (#858) macaques, with and without polyclonal stimulation for 3 and 5 days. CD20+ cells were gated for analysis.
In fresh PBMC, memory B cell subsets were 47±3% switched, 53±4% unswitched, and remained unchanged following culture for 3 and 5 days without stimulation (51±5% and 46±4% switched; 49±5% and 54±4% unswitched; respectively). With polyclonal stimulation, memory B cells differentiated, displaying a greater percentage of switched memory B cells after 3 (87±1%) compared to 5 days (77±3%) of culture. The mean ratio of switched/unswitched memory B cells in fresh, unstimulated cultures was 1.2±1, whereas 3 and 5 day stimulated cultures exhibited elevated mean ratios of 11.3±2.9 and 5.8±2.2, respectively.
Flow cytometric analysis confirmed proliferation of macaque memory B cells following polyclonal stimulation (Fig. 2). Mean proliferation of B cells in all 4 macaques after 3 and 5 days of stimulation were 24% and 35%, respectively. In the absence of polyclonal stimulation, B cells did not proliferate.
Figure 2.
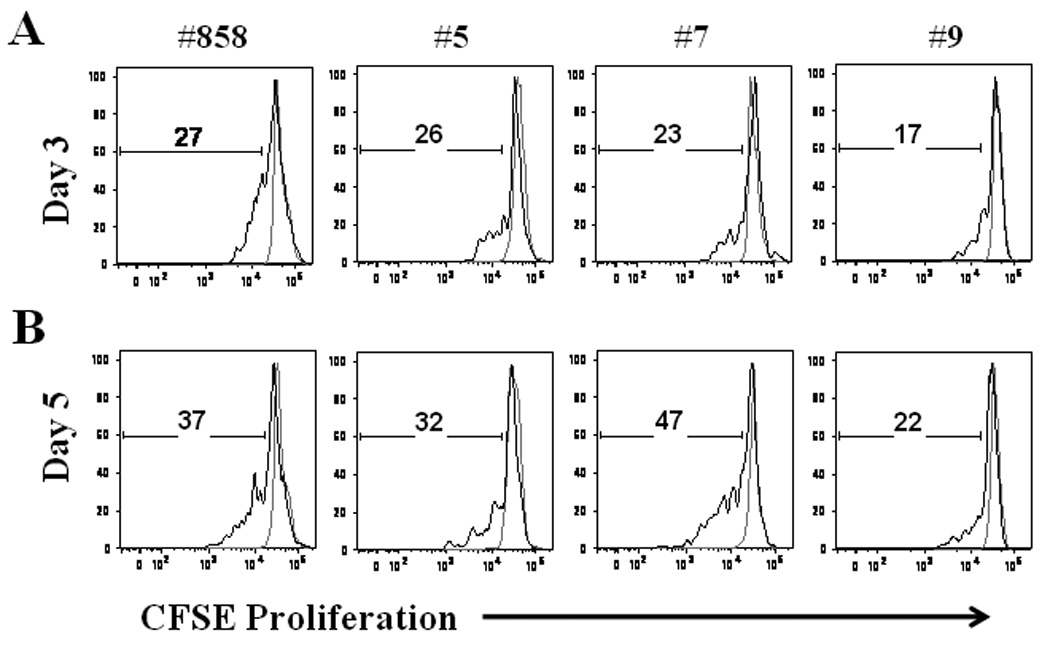
Proliferation of memory B cells. Total PBMC were stained with CFSE and cultured with media alone (gray line) or with polyclonal stimulation (black line). After 3 (A) and 5 (B) days of stimulation PBMC were collected, washed and stained with anti-CD20 and anti-CD27. The percentage of memory B cells (CD20+CD27+) that had entered division was calculated from CFSE dilution. Representative results from 1 of 2 experiments are shown.
Quantification of memory B cells
Total and SIV Env-specific IgG and IgA memory B cells in PBMC from the vaccinated elite controllers, the unvaccinated infected, and 2 naïve macaques were quantified with and without polyclonal stimulation. In fresh, unstimulated PBMC, total IgG and IgA ASC were low, but increased dramatically with polyclonal stimulation (Table 1). Mean total IgG ASC of all 6 macaques increased 35- and 43-fold after 3 and 5 days of stimulation respectively, while mean total IgA ASC increased 12- and 14-fold respectively.
Table 1.
Quantitation of total and SIV Env-specific IgG and IgA memory B cells in PBMC of rhesus macaquesa.
| Total IgG ASC | Total IgA ASC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unstimulated PBMC | Stimulated PBMC | Unstimulated PBMC | Stimulated PBMC | |||||||
| Days of culture | 0 | 3 | 5 | 3 | 5 | 0 | 3 | 5 | 3 | 5 |
| All Macaques | 788 ± 219 | 476 ± 182 | 541 ± 173 | 16852 ± 2799 | 23354 ± 3646 | 90 ± 38 | 85 ± 33 | 100 ± 31 | 991 ± 128 | 1446 ± 507 |
| Env-Specific IgG ASC | Env-Specific IgA ASC | |||||||||
| Naïve Macaques | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unvaccinated infected | 1 ± 1 | 13 ± 7 | 40 ± 31 | 296 ± 46 | 305 ± 49 | 0 | 0 | 2 ± 2 | 15 ± 8 | 38 ± 28 |
| Vaccinated controllers | 2 ± 2 | 10 ± 8 | 49 ± 41 | 637 ± 293 | 311 ± 107 | 0 | 1 ± 1 | 6 ± 3 | 281 ± 106 | 82 ± 19 |
| IgG Specific Activity | IgA Specific Activity | |||||||||
| Naïve Macaques | 0 | 0 | 0 | 0 | ||||||
| Unvaccinated infected | 1.3 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.5 | 3.4 ± 2.4 | ||||||
| Vaccinated controllers | 5.4 ± 0.8 | 2.4 ± 0.2 | 43.1 ± 11.6 | 30.7 ± 10.8 | ||||||
Total and Env-specific IgG and IgA ASC were enumerated by ELISPOT as described in materials and Methods. IgG and IgA specific activity was calculated as (Env-specific ASC /total memory ASC) × 100. Experiments were repeated 2 to 4 times. Mean values ±standard error of the mean are reported for 2 naive macaques (#419 and 421), an unvaccinated, infected macaque (#858) and for 3 vaccinated elite controllers (#5, #7, and #9).
SIV Env-specific IgG and IgA ASC were assessed using aliquots of the same PBMC used to evaluate total memory. No Env-specific IgG or IgA ASC were detected in PBMC of naïve macaques, either stimulated or unstimulated (Table 1). Minimal IgG and IgA ASC were seen in fresh, unstimulated PBMC of the unvaccinated infected macaque, #858, and the vaccinated elite controllers, #5, #7, and #9. In contrast Env-specific IgG ASC increased 23- and 8-fold upon 3 and 5 days of stimulation of PBMC from macaque #858, and 64- and 6-fold for macaques #5, #7, and #9, respectively. Similarly, after 3 and 5 days of stimulation, IgA ASC for macaque #858 increased more than 15- and 19-fold respectively, while for macaques # 5, #7, and #9, mean levels increased 281- and 14-fold respectively.
Percent SIV Env-specific IgG and IgA activity levels (Table 1) were consistent with the immunization and challenge history of the macaques. The naïve macaques were negative, while IgG and IgA specific activity levels were elevated in the elite controller macaques, reflecting their vaccination and repeated SIV exposures which allowed for greater induction of memory B cells. The elevated levels of Env-specific IgA memory B cells most likely results from several factors including the initial mucosal priming immunizations, the repeated intrarectal SIV challenges, and the known propensity of SIV to replicate at intestinal sites.
Retrospective evaluation of vaccine-elicited memory B cells
Using the methodology established, induction of memory B cells by replicating Ad-recombinant prime/envelope boost regimens was retrospectively evaluated using viably frozen PBMC from two pre-clinical vaccine studies. We first examined responses in PBMC of all immunized macaques from a comparative study that evaluated Oral/Oral versus Intranasal/Oral Ad-SIV recombinant priming. As previously reported29, all immunized macaques developed systemic and mucosal antibody responses to SIV envelope over the course of immunization and following intrarectal SIVmac251 challenge that mediated ADCC, ADCVI, and transcytosis inhibition23. Using a 3 day culture period which, as described above, showed enhanced switched memory B cells compared to 5 days of stimulation, pre- and post-challenge PBMC were evaluated. Memory B cells were elicited by both the Oral/Oral and Intranasal/Oral immunization regimens. SIV Env-specific IgG ASC (expressed as the percent of total IgG ASC) remained relatively constant in both immunization groups at both time points (Fig. 3A). However, Env-specific IgA memory B cells in Intranasal/Oral-primed animals decreased 5.4 fold after the intrarectal challenge (p = 0.014; Fig. 3B). A smaller non-significant decrease was seen in Env-specific IgA ASC in Oral/Oral-primed macaques. These decreases may reflect homing of the IgA ASC to the mucosal site of challenge. Overall, SIV envelope-specific IgA ASC pre-challenge were higher than IgG ASC (Fig. 3A, B), significantly so for Intranasal/Oral primed macaques (p = 0.0051). The elevation in IgA ASC is consistent with the mucosal priming regimen.
Figure 3.
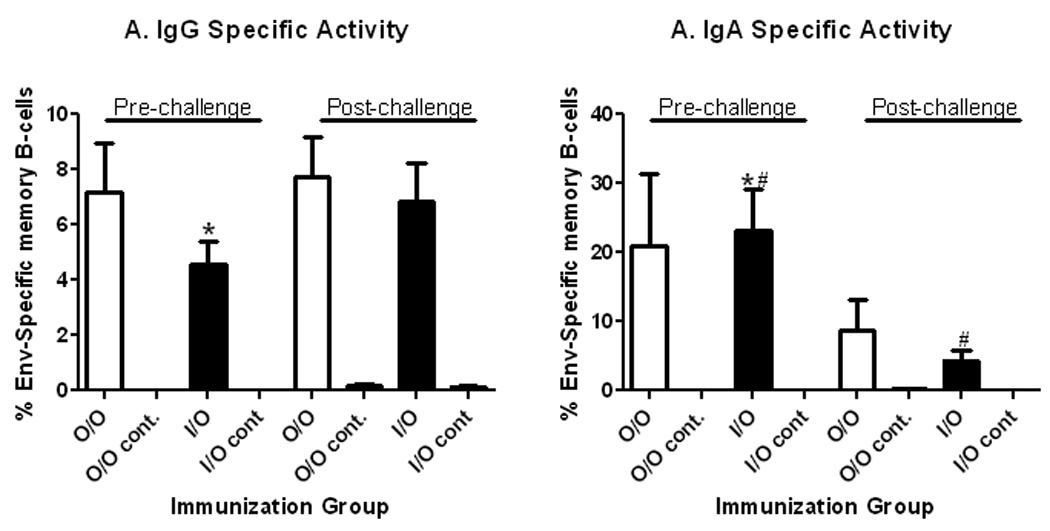
Quantification of SIV envelope-specific IgG and IgA memory B cells in rhesus macaques mucosally primed by Oral/Oral or Intranasal/Oral routes29. PBMC obtained pre-challenge, 2 weeks after the second boost (week 38) and 4 weeks post-challenge (week 46) were polyclonally stimulated for 3 days. Total and SIV gp120-specific IgG and IgA ASCs were enumerated by ELISPOT assay. Duplicate assays were run for each macaque. The results for either IgG or IgA were calculated as percent SIV envelope-specific memory B cells by calculating gp120-specific ASC/total ASC × 100. Mean values for each immunization group (7 macaques/group) or control group (2 macaques/group) ± SEM are shown.
*: pre-challenge IgG versus IgA specific activity in the Intranasal/Oral group (p = 0.0051).
#: pre- versus post-challenge IgA specific activity in the Intranasal/Oral group (p = 0.014).
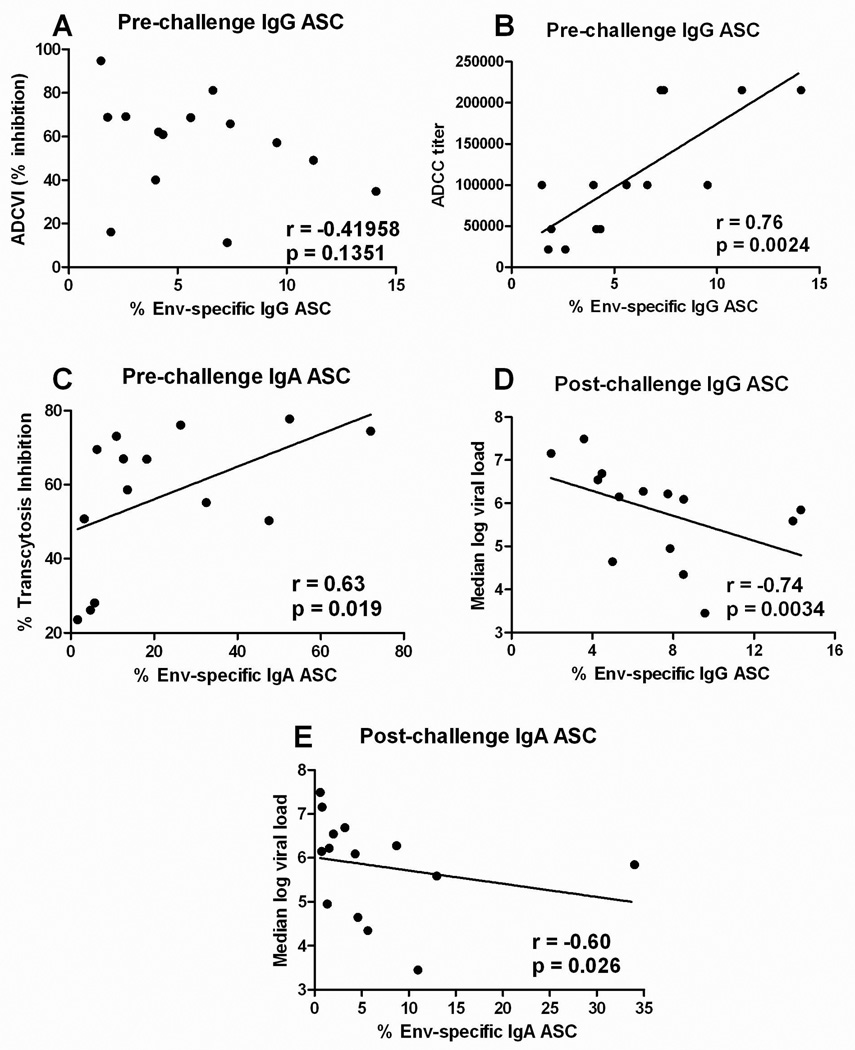
Previously, the Intranasal/Oral-primed macaques exhibited lower acute viremia post-challenge than the Oral/Oral and control macaques29, inversely correlated with pre-challenge anti-Env antibodies that mediated ADCC (r = −0.71, p = 0.0059) and with anti-Env antibodies pre- and post-challenge that mediated ADCVI (r = −0.69, p = 0.0083 and r = −0.59, p = 0.0030, respectively23). In contrast, chronic phase viremia was controlled to similar extents by both the Intranasal/Oral and Oral/Oral groups29. Serum antibody levels reflect antibody secretion by plasma cells in the bone marrow, but don’t necessarily reflect short-term antibody production by memory B cells following antigen exposure. We therefore assessed SIV Env-specific IgG and IgA ASC in PBMC for correlations with antibody activities and post-challenge viral loads.
Memory B cells were not correlated with serum binding antibody titers to gp120 (data not shown) nor with ADCVI activity (Fig. 4A). However, SIV Env-specific IgG ASC post-immunization were correlated with the serum ADCC titer at the same timepoint (Fig. 4B). Further, post-challenge Env-specific IgG ASC were significantly correlated with reduced chronic viremia over weeks 8 to 36 (Fig. 4D). A lesser but significant negative correlation of post-challenge Env-specific IgA ASC with median viremia over weeks 8 to 36 post-challenge was also seen (Fig. 4E).
Figure 4.
Correlation of memory B cells in PBMC of immunized macaques from the comparative priming study with functional antibody activities and chronic phase protection. (A) Lack of correlation of SIV Env-specific IgG specific activity 4 weeks pre-challenge with ADCVI activity. (B) A significant correlation of SIV Env-specific IgG specific activity 4 weeks pre-challenge with serum ADCC titer. (C) A significant correlation of SIV Env-specific IgA specific activity 4 weeks pre-challenge with transcytosis inhibition by rectal secretions at the same time point. (D) SIV Env-specific IgG and (E) IgA specific activities 4 weeks post-challenge are inversely correlated with chronic phase viremia over weeks 8 to 36 post-challenge.
The Intranasal/Oral and Oral/Oral regimens also were previously shown to elicit antibodies in rectal secretions pre- and post-challenge that mediated transcytosis inhibition of SIVmac251 across an epithelial cell barrier29. Here pre-challenge peripheral blood Env-specific IgA ASC correlated with percent transcytosis inhibition at the same timepoint (Fig. 4C), indicating maturation of antibodies mediating this activity. Overall the retrospective analyses of PBMC from macaques primed by the Oral/Oral or Intranasal/Oral routes documented that the vaccination regimens induced SIV Env-specific memory B cells which incorporated important functional antibody activities into immunologic memory.
We next examined whether a similar immunization regimen induced HIV Env-specific memory B cells. A second pre-clinical study previously evaluated the effect of envelope boosting on immunogenicity and protective efficacy. Macaques primed mucosally with Ad- HIVenv, Ad-SIVgag and Ad-SIVnef recombinants and boosted with HIV gp140 exhibited significantly reduced acute viremia compared to controls and to macaques that were primed but either not-boosted or boosted with an HIV peptomer. The gp140-boosted group also displayed a 3-log decrease in chronic viremia following SHIV89.6P challenge17. The better protection in the gp140 group was correlated with antibodies displaying increased avidity and mediating ADCC, ADCVI, and transcytosis inhibition25. Here, our retrospective analysis showed that only gp140-boosted macaques developed Env-specific memory B cells pre-challenge, both IgG and IgA (group II, Fig. 5A,B). However, by four weeks post-challenge, all macaques exhibited memory B cells. Both IgG and IgA Env-specific memory B cells were elevated above pre-challenge levels in the gp140-boosted macaques, significantly so for IgA memory B cells (Fig. 5B). Moreover, both IgG and IgA memory B cells were significantly elevated in vaccinated macaques above levels in control animals (group IV), indicating the strong effect of vaccination on memory B cell development. Notably, macaques that were primed but not boosted (group I), or primed and boosted with peptomer (group III) displayed elevated post-challenge memory B cell levels (Fig. 5A, B), indicating that the Ad-recombinant priming positively influenced development of both IgG and IgA HIV Env-specific memory B cells.
Figure 5.
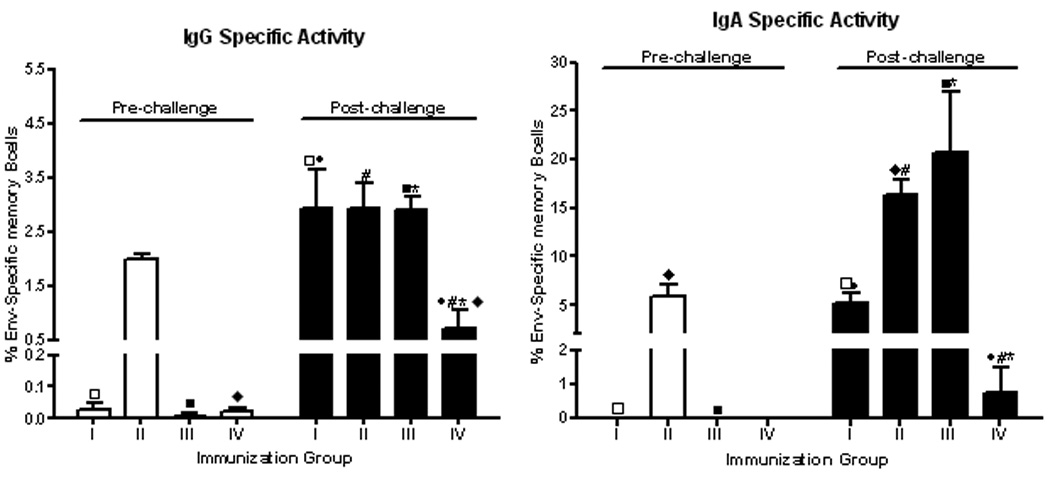
Quantification of HIV envelope-specific IgG and IgA memory B cells in rhesus macaques mucosally primed by replicating Ad-HIV and Ad-SIV recombinants and subsequently not boosted (group I), or boosted with HIV gp140 (group II) or an HIV peptomer (group III).Group IV represents controls (17). Each immunization and control group contained 6 macaques. PBMC obtained pre-challenge, 2 weeks after the second boost (week 38) and 4 weeks post-challenge (week 48) were polyclonally stimulated for 3 days. Total and HIV Env-specific IgG and IgA ASCs were enumerated by ELISPOT assay. Duplicate assays were run for each macaque. The results for either IgG or IgA were calculated as described in the legend to Figure 3. Fresh, unstimulated PBMC were negative for IgG and IgA ASC. Significant differences pre-versus post-challenge by immunization groups for IgG memory B cells: □ and ■, p < 0.0001, ◇ p = 0.034 and for IgA memory B cells: □ and ■, p < 0.0001, ◆ p = 0.0042. Significant differences between immunization groups for IgG memory B cells post-challenge: ●, p = 0.0003, # and *, p = 0.0002 and for IgA memory B cells post-challenge: ●, p = 0.0003, # and *, p < 0.0001.
Similarly to results obtained in comparison of priming regimens, in this study investigating envelope boosts, a correlation of memory B cells with serum antibody binding titers to gp140 was not observed (data not shown). However, as functional antibody activities were shown to play a role in protective efficacy in this second study25, we investigated correlations of memory B cells with several antibody activities. Previously, post-challenge ADCC activity was correlated with reduced median chronic viremia over weeks 8 to 40 (r = −0.56, p = 0.0051). Here, we observed that post-challenge IgG and IgA Env-specific memory B cells were correlated with percent ADCC killing at the same timepoint (Fig. 6A). Correlations were also seen with IgG and IgA memory B cells separately (r = 0.45, p = 0.027; r = 0.64, p = 0.0007; respectively; data not shown). Similarly, ADCVI activity post-challenge was previously correlated with reduced median chronic viremia (r = −0.64, p = 0.001125). Here again, post-challenge IgG and IgA memory B cells were correlated with ADCVI activity at the same timepoint (Fig. 6B). The correlation with ADCVI was also seen for IgG and IgA ASC separately (r = 0.47, p = 0.021; r = 0.75, p <0.0001; respectively; data not shown). Thus these two systemic antibody activities were effectively incorporated into the memory B cell pool.
Figure 6.
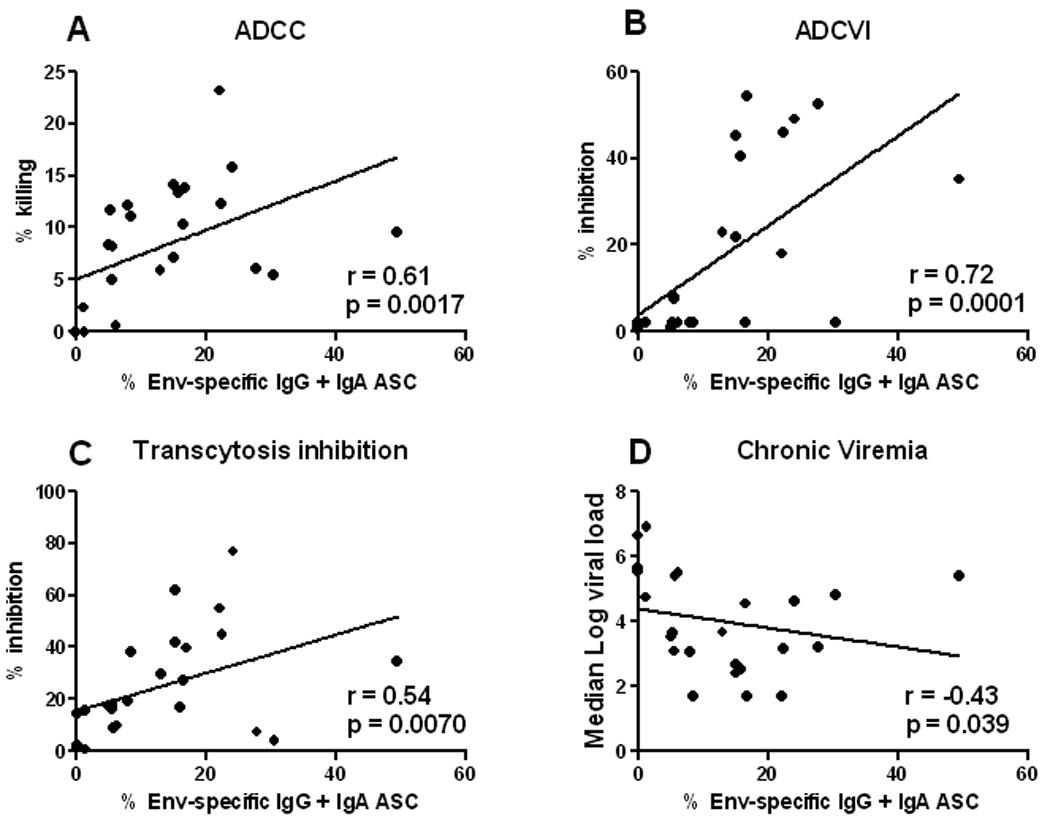
Correlation of memory B cells in the immunized macaques from the study evaluating envelope boosting with functional antibody activities and chronic phase protection. HIV Env-specific IgG and IgA specific activities 4 weeks post-challenge are directly correlated with ADCC titer (A), ADCVI (B), and transcytosis inhibition (C) at the same time point. Post-challenge HIV Env-specific IgG and IgA specific activities are inversely correlated with chronic phase viremia over weeks 8 to 40 post-challenge (D).
HIV is transmitted mucosally, and replicates extensively in the intestinal tract. Therefore, mucosal immunity is believed to be critical for an effective HIV vaccine. Rectal secretions from the immunized macaques in this second study were previously shown to inhibit transcytosis of SHIV89.6P across an epithelial cell barrier25. This activity post-challenge was correlated with reduced median chronic viremia (r = −0.58, p = 0.01325). Here, we observed that post-challenge IgG and IgA memory B cells in peripheral blood were correlated with the level of transcytosis inhibition at the same timepoint (Fig. 6C). Correlations with IgG and IgA separately were also observed (r = 0.51, p = 0.012; r = 0.53, p = 0.0073; respectively; data not shown). Therefore, the immunization regimen elicited a humoral memory response important for mucosal protection.
In view of these strong correlations with functional antibody activities, it was not unexpected that levels of IgG and IgA Env-specific ASC post-challenge were also correlated with reduced chronic viremia over weeks 8 to 40 (Fig. 6D). Similar correlations were seen for IgG and IgA ASC separately (r = −0.48, p = 0.018; r = −0.46, p = 0.025 respectively; data not shown).
Taken together, these observations demonstrate that our prime/boost regimen effectively elicits memory B cells specific for both SIV and HIV envelope, that the cells preserve functional antibody activities, and that they positively influence the outcome of challenge exposures.
Discussion
Humoral immunologic memory is provided by long-lived plasma cells and memory B cells5, 15, 35, 36. Plasma cells are completely differentiated, non-dividing cells, generated after encountering antigen or derived from previously generated memory B cells. They reside in bone marrow and continuously secrete low quantities of specific antibodies, not needing further contact with stimulating antigen4, 37. They represent the first line of humoral defense upon re-exposure to a pathogen. In contrast, memory B cells must first proliferate and differentiate into ASC following antigen re-exposure, resulting in a rapid increase in antigen-specific circulating antibody levels4, 38.
Polyclonal stimulation has been shown to induce expansion and differentiation of human memory B cells into ASC26, 31, 39–41, now shown here in the macaque system. Subsets of memory B cells are defined by surface markers42, 43. While evidence suggests some memory B cells may lack CD27 expression42, 44, 45, the molecule is considered a universal marker of human memory B cells, and has provided the basis for an IgD/CD27 memory B cell nomenclature32, 33, 42. Based on this categorization, total memory B cells in rhesus macaque blood were equivalent to approximately 46% of circulating B cells, comparable to humans46, 47 (data not shown).
Retrospective analyses of PBMC from vaccine studies in macaques have demonstrated here that our mucosal prime/boost regimen elicits memory B cells (Figs. 3A,B; 5A,B). Envelope booster immunizations resulted in development of Env-specific ASC prior to challenge, while priming immunizations alone led to rapid appearance of memory B cells following exposure to virus (Fig. 5A,B). Both IgG and IgA SIV Env-specific ASC were observed in PBMC, however, pre-challenge IgA ASC levels were higher than IgG ASC levels in both pre-clinical studies (Figs. 3 A,B; 5A,B). This reflects mucosal priming immunizations with Ad-recombinants which preferentially replicate in epithelial cells lining mucosal inductive sites. It would be informative to assess memory B cells following vaccination with a regimen lacking any mucosal component. This would help elucidate whether mucosal immunizations have a benefit with regard to development of IgG and IgA memory B cells and further inform design of HIV vaccines, where mucosal immunity is believed to be necessary for protective efficacy.
Memory B cell profiles in vaccinated macaques differed, depending on the challenge route. Following SIVmac251 intrarectal challenge, IgA ASC in peripheral blood declined relative to pre-challenge levels (Fig. 3B), while macaques challenged intravenously with SHIV89.6P exhibited elevated IgA ASC levels post-challenge (Fig. 5B, group II). The drop in peripheral blood IgA ASC in the first case may have resulted from homing of cells to the mucosal challenge site. Confirmation of this point will require further studies.
In both pre-clinical vaccine studies the percentages of SIV-specific IgG and IgA memory B cells were correlated with functional antibody activities but not with serum binding antibody titers as measured by ELISA. This is perhaps not surprising, as the ELISA titers reflected antibody reactivity to only a single form of envelope (gp120 or gp140 in the comparative priming and envelope boosting studies, respectively), whereas the functional assays would be influenced by antibody binding to particular envelope epitopes exposed on target cells. Further, antibody characteristics, such as avidity to specific antigen and/or Fc receptors, have little effect on ELISA results, but will clearly impact the functional activity evaluated.
In the comparative priming study, all macaques were boosted with envelope protein and all developed memory B cells pre-challenge. Thus there was sufficient statistical power to demonstrate significant correlations of IgG memory B cells with ADCC antibody titers and IgA memory B cells with transcytosis inhibition (Fig. 4A,B), indicating maturation of antibodies mediating functional activities. Notably, both IgG and IgA memory B cell levels post-challenge were inversely correlated with chronic viremia levels over weeks 8 to 36 (Fig. 4C,D), suggesting that post-challenge, vaccine-induced memory B cells were recalled and influenced the course of disease.
In the study investigating envelope boosting, only macaques primed and boosted with gp140 developed memory B cells pre-challenge (Fig. 5A,B), providing too few macaques for correlation analyses. However post-challenge all macaques developed memory B cells, and both IgG and IgA ASC were significantly correlated with percent ADCC killing, ADCVI activity, and transcytosis inhibition (Fig. 6A–C). These correlations with functional antibody activity were stronger with IgA ASC compared to IgG ASC, again perhaps reflecting the mucosal priming immunizations. However, the correlation strength between Env-specific memory B cells and chronic viremia (Fig. 6D) was similar for IgG and IgA ASC (see results section), suggesting less mucosal homing following intravenous challenge.
The fact that memory B cell levels correlated with ADCVI activity in the envelope boosting study but not in the comparative priming study is worthy of comment. ADCVI activity is complex, and can be mediated not only by direct cell killing via ADCC, but also by effector cell secretion of cytokines and by phagocytosis of immune complexed virus. ADCVI activities in the two studies were evaluated separately using freshly obtained macaque effector cells over a two to three year period. Thus, the difference in correlation outcomes might have resulted if the effector cells used in the envelope boosting study exhibited more antibody dependence, and therefore mediated more ADCC activity than other effector functions. However, it is noteworthy that the post-challenge correlation of ADCVI with memory B cells in the envelope boosting study was much stronger with IgA than with IgG memory B cells. As discussed above, the intravenous challenge in this study may have resulted in less homing of ASC to mucosal sites, in contrast to the comparative priming study which received an intrarectal challenge. Thus it is possible that correlation differences in the two studies resulted from homing effects and different levels of IgA memory B cells in peripheral blood, post-challenge. In this regard, the ability of IgG and IgA antibodies to mediate ADCVI merits further investigation.
Overall, the demonstration here of vaccine-induced memory B cells and their correlation with antibody activities and reduced viremia are in concert with our previous results showing a contribution of mature, functional antibody responses to protective efficacy23, 25. Anamnestic antibody responses upon re-exposure to antigen are mediated by differentiation of memory B cells into short-lived plasma cells20, 21, 24, while long-lived antibodies are produced by plasma cells residing in the bone marrow20, 21. Memory B cell induction by candidate vaccines and quantitation of ASC in peripheral blood and bone marrow compartments should be assessed to adequately evaluate humoral immunologic memory.
Acknowledgements
We thank Dr. Shane Crotty for sharing reagents and helpful discussions. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams LJ, McHeyzer-Williams MG. Memory B cell evolution: B cell biology. Adv Exp Med Biol. 2007;596:31–45. doi: 10.1007/0-387-46530-8_3. [DOI] [PubMed] [Google Scholar]
- 3.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 4.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 5.Dorner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Human B cell memory. Curr Opin Immunol. 2009;21:298–304. doi: 10.1016/j.coi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009;39:2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 8.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 9.Vinuesa CG, Tangye SG, Moser G, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 10.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Meth. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Bonsignori M, Moody MA, Parks RJ, Holl TM, Kelsoe G, Hicks CB, et al. HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J Immunol. 2009;183:2708–2717. doi: 10.4049/jimmunol.0901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Sajadi MM, Kamin-Lewis R, Fouts TR, Dimitrov A, Zhang Z, et al. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A. 2009;106:3952–3957. doi: 10.1073/pnas.0813392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahlers JD, Belyakov IM. Strategies for optimizing targeting and delivery of mucosal HIV vaccines. Eur J Immunol. 2009;39:57–69. doi: 10.1002/eji.200939269. [DOI] [PubMed] [Google Scholar]
- 14.Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. 2009;39:1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi Y. Memory B cells in systemic and mucosal immune response: implications for successful vaccination. Biosci Biotechnol Biochem. 2007;71:2358–2366. doi: 10.1271/bbb.70142. [DOI] [PubMed] [Google Scholar]
- 16.Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, et al. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 17.Patterson LJ, Beal J, Demberg T, Florese RH, Malkevich N, Venzon D, et al. Replicating adenovirus HIV/SIV recombinant priming alone or in combination with a gp140 protein boost results in significant control of viremia following a SHIV89.6P challenge in Mamu-A*01 negative rhesus macaques. Virology. 2008;374:322–337. doi: 10.1016/j.virol.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Bogers WMJM, Davis D, Baak I, Kan E, Hofman S, Sun Y, et al. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology. 2008;382:217–225. doi: 10.1016/j.virol.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gómez-Román VR, Wang L, et al. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol. 2004;78:2212–2221. doi: 10.1128/JVI.78.5.2212-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malkevitch NV, Patterson LJ, Aldrich MK, Wu Y, Venzon D, Florese RH, et al. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology. 2006;353:83–98. doi: 10.1016/j.virol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Román VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Romón VR, Florese RH, Peng B, Montefiori D, Kalyanaraman VS, Venzon D, et al. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr. 2006;43:270–277. doi: 10.1097/01.qai.0000230318.40170.60. [DOI] [PubMed] [Google Scholar]
- 23.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83:791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 27.Douagi I, Forsell MNE, Sundling C. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J Virol. 2010;84:1683–1695. doi: 10.1128/JVI.01896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhrt D, Faith S, Hattemer A, Leone A, Sodora D, Picker L, et al. Naïve and memory B cells in the rhesus macaque can be differentiated by surface expression of CD27 and have differential responses to CD40 ligation. J Immunol Meth. 2010 doi: 10.1016/j.jim.2010.09.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Hidajat R, Peng B, Venzon D, Aldrich MK, Richardson E, et al. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIV(mac251) Vaccine. 2007;25:8021–8035. doi: 10.1016/j.vaccine.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 32.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, Agematsu K, Ochs HD, Sugane K. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin Immunol. 2003;108:28–37. doi: 10.1016/s1521-6616(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 34.Agematsu K, Nagumo H, Yang FC, Nakazawa T, Fukushima K, Ito S, et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997;27:2073–2079. doi: 10.1002/eji.1830270835. [DOI] [PubMed] [Google Scholar]
- 35.Shokrgozar MA, Sam MR, Amirkhani A, Shokri F. Frequency analysis of HBsAg-specific B lymphocytes in high-responder individuals to recombinant hepatitis B vaccine: comparison of LDA and ELISPOT assays. Scand J Immunol. 2006;64:536–543. doi: 10.1111/j.1365-3083.2006.01838.x. [DOI] [PubMed] [Google Scholar]
- 36.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. Appearance of peripheral blood plasma cells and memory B-cells in a primary and secondary immune response in humans. Blood. 2009;114:4998–5002. doi: 10.1182/blood-2009-03-211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malaspina A, Moir S, DiPoto AC, Ho J, Wang W, Roby G, et al. CpG oligonucleotides enhance proliferative and effector responses of B Cells in HIV-infected individuals. J Immunol. 2008;181:1199–1206. doi: 10.4049/jimmunol.181.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 41.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 42.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–206. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 43.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. 2006;177:3728–3736. doi: 10.4049/jimmunol.177.6.3728. [DOI] [PubMed] [Google Scholar]
- 45.Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. 2005;35:3433–3441. doi: 10.1002/eji.200535364. [DOI] [PubMed] [Google Scholar]
- 46.Fecteau JF, Roy A, Neron S. Peripheral blood CD27+ IgG+ B cells rapidly proliferate and differentiate into immunoglobulin-secreting cells after exposure to low CD154 interaction. Immunology. 2009;128:e353–e365. doi: 10.1111/j.1365-2567.2008.02976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohnhorst JO, Thoen JE, Natvig JB, Thompson KM. Significantly depressed percentage of CD27+ (memory) B cells among peripheral blood B cells in patients with primary Sjogren's syndrome. Scand J Immunol. 2001;54:421–427. doi: 10.1046/j.1365-3083.2001.00989.x. [DOI] [PubMed] [Google Scholar]