To the Editor:
We appreciate the opportunity to comment on the criticism levied by Abramowitz and colleagues about our recent paper that describes an animal model of obsessive compulsive disorder (OCD). The purpose of animal research is to recapitulate conditions in mammalian systems that are more amenable to study than the human primate, while always striving to achieve both face and construct validity (1, 2). Such validity is demonstrated by homologous (not necessarily identical) behaviors in other species that are produced by neurobiological manipulations, based on etiological theories, and possess pharmacological isomorphism to the targeted disorder. Successful demonstration of these features provides the strongest platform to investigate causal mechanisms and novel treatments.
While we agree with Abramowitz et al. that it is “difficult to imagine a true animal model of this condition [OCD] (especially one involving rodents),” the reported behaviors bear striking similarities with aspects of the clinical disorder. Although the true psychological state of the animal could not be directly assessed, the observed behaviors were both evolutionarily adaptive and affected by state-evoking stimuli – much like the relationship between stress and OCD behaviors in humans (3). In our original paper, developmental exposure to the tricyclic antidepressant, clomipramine, paradoxically produced adult rats that were more anxious (determined by both elevated plus maze and marble burying), hoarded, showed more perseveration, and demonstrated problems with reversal learning and working memory. The latter two behaviors were mediated by some degree of learning impairment that delays task acquisition, which is also observed in OCD patients (4).
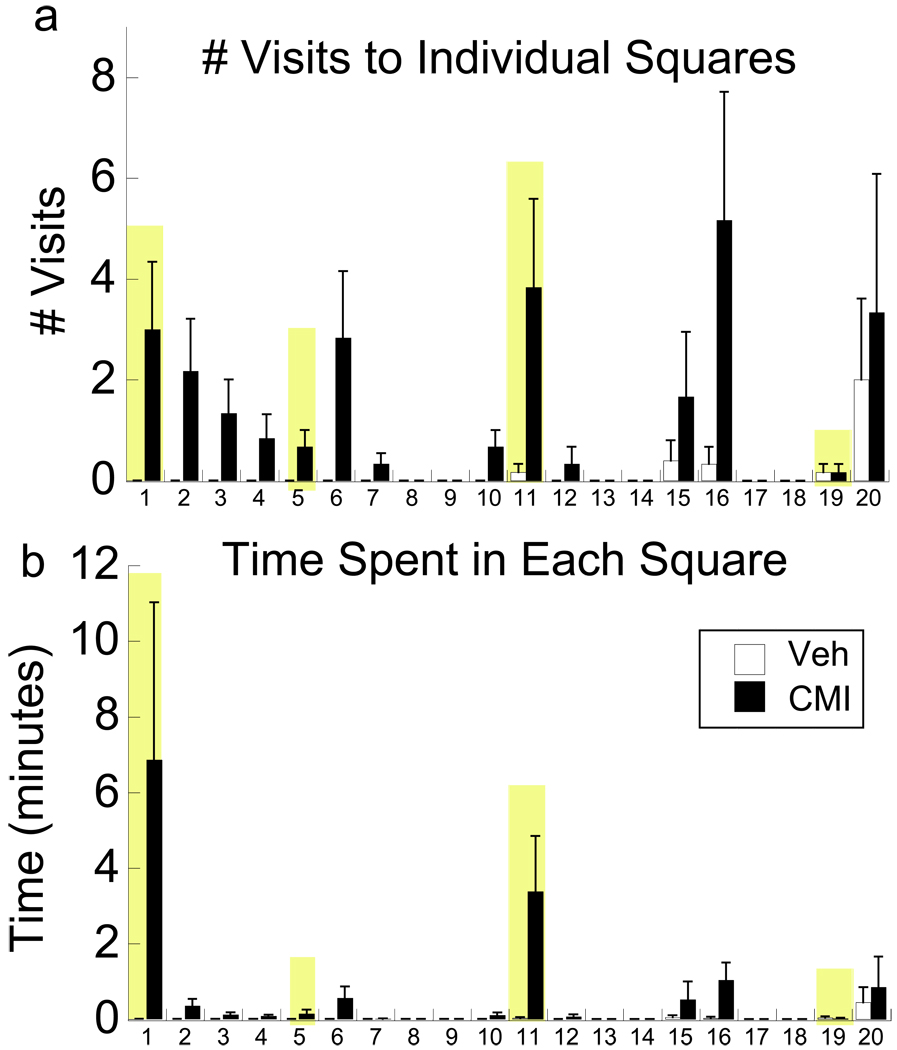
The main argument presented by Abramowitz et al., however, is whether animals can demonstrate true compulsions “…as opposed to some other form of repetitive behavior…”. Certainly, psychiatric disorders are complex and therefore difficult to model in animals. Repetitive behavior demonstrated by OCD patients may relieve uncertainty regarding a feared consequence (5), and thus such behavior is typically related to a specific obsession that is associated with an object or a place (6). On a neurobiological level, repetitive behaviors are believed to reflect supersensitive dopamine D2 receptors in striatal brain regions, amongst other brain changes (7). Early life exposure to clomipramine increases striatal D2 receptors (8), and the present findings clearly demonstrate that these animals repeatedly check novel objects and spend more time in their presence than control subjects (Figure 1). Much like a good clinician, ruling out other causes of behavioral symptoms is important for correct diagnosis, and thus converging evidence from multiple behaviors further strengthens the possible relevance of this model. First, stereotypies, such as repeated grooming and rearing in the rats, and a behavior that is non-specific for OCD, are not present in the clomipramine rats even when provoked with a D2 agonist (unpublished observation). Second, a ‘compulsive’ behavior could result from impaired working memory, where the individual simply does not remember whether a task was completed. The repetitive behaviors observed in OCD do not reflect impaired memory recall (9), and similarly, clomipramine animals are able to choose the correct arm to enter in the delayed working memory task, but have longer response latencies than control animals. Despite these observations that clomipramine-exposed animals show state-dependent checking behaviors, delay in working memory-related tasks, and heightened anxiety in anxiety-eliciting situations, direct assessment of whether checking and delayed responding are due to an obsession in the animals is impossible. However, contrary to the assertion that “It would be nearly impossible to demonstrate whether a rat is experiencing a true obsession versus general anxiety”, these data show that clomipramine-exposed rats demonstrate behaviors that are consistent with the clinical picture.
Figure 1.
Compulsive checking following a single dose of the D2 agonist quinpirole. Based on the modified methods of (13), a single, acute dose of quinpirole (0.5 mg/kg) was administered. Rats were placed in an open field containing four novel objects (shaded bars), allowed to habituate for 25 min, and scored within a 5 x5 square matrix for 30 min. Clomipramine-exposed adult male rats (CMI) made (a) more visits to squares with the novel objects (F1,14=4.71, P<0.05) and (b) spent significantly more time within those squares compared to vehicle-exposed rats (VEH; F1,14=6.34, p<0.05). Note, that time and visits are elevated in squares adjacent to an object (e.g., #6, 16, and 20). Data are based on n=8 rats/group, following the CMI exposure methods as described in (8). Means ± SE are presented for only 20 of the 25 squares for clarity, as subjects spent the remaining time in these squares.
In response to the charge that the clomipramine model is non-specific, anxiety and OCD are often not experienced in isolation, but rather are highly comorbid with depression. Thus, the current data might further underscore their shared common neurobiology and treatment response (10). In addition, the behavioral homologies in clomipramine-exposed rats are further substantiated by selective neurochemical changes in the orbital frontal cortex (5-HT2c receptors) and the striatum (D2 receptors) – two regions and receptors that are consistently implicated in OCD (11).
Rather than working to discredit models based on the impossibility of animal researchers to assess cognition through verbal report, clinicians should be working with preclinical investigators to better translate symptoms into measurable behaviors. The neurobiological underpinnings of complex disorders, including OCD, necessitate that animal models themselves need also be more complex. In our opinion, focusing on a single gene or a single behavior is overly simplistic but is necessary to understand basic mechanisms. In parallel with this endophenotype/genomic approach, animal models also need to be based on externalized, observable behaviors – much like clinicians base diagnoses on multiple symptoms that they observe – and these models ideally should also demonstrate more than one target behavior. By manipulating developmental monoamine systems that are associated with OCD and its treatment, behavioral impairment in numerous domains increases in adulthood. It is by no accident that the very treatment that reduces the aforementioned behaviors can also produce them if provided during sensitive periods of development (12).
Animal research provides an important platform for understanding disorder states, their causes, and a means to test novel treatments. At this stage, current treatments for OCD have significant limitations and none of them are preventive. The greatest promise of the clomipramine model may lie in exploiting the sensitive periods for these (OCD-like) behaviors and their associated biochemistry to develop an intervention that can neurochemically re-transform aberrant circuits to a more normal trajectory. Only future testing will tell.
Susan L. Andersen
Britta Thompson
Acknowledgement
The authors gratefully acknowledge the support of the Judah Family for their support and DA-15403 to SLA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Robbins TW. Homology in behavioural pharmacology: an approach to animal models of human cognition. Behav Pharmacol. 1998;9:509–519. doi: 10.1097/00008877-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Joel D. Current animal models of obsessive compulsive disorder: a critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:374–388. doi: 10.1016/j.pnpbp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Abramowitz JS, Khandker M, Nelson CA, Deacon BJ, Rygwall R. The role of cognitive factors in the pathogenesis of obsessive-compulsive symptoms: a prospective study. Behav Res Ther. 2006;44:1361–1374. doi: 10.1016/j.brat.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Morein-Zamir S, Craig KJ, Ersche KD, Abbott S, Muller U, Fineberg NA, et al. Impaired visuospatial associative memory and attention in obsessive compulsive disorder but no evidence for differential dopaminergic modulation. Psychopharmacology (Berl) 2010;212:357–367. doi: 10.1007/s00213-010-1963-z. [DOI] [PubMed] [Google Scholar]
- 5.Rachman S. A cognitive theory of compulsive checking. Behav Res Ther. 2002;40:625–639. doi: 10.1016/s0005-7967(01)00028-6. [DOI] [PubMed] [Google Scholar]
- 6.Eilam D, Zor R, Szechtman H, Hermesh H. Rituals, stereotypy and compulsive behavior in animals and humans. Neurosci Biobehav Rev. 2006;30:456–471. doi: 10.1016/j.neubiorev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaus S, Antke C, Beu M, Muller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Rev Neurosci. 2010;21:119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- 8.Andersen SL, Greene-Colozzi EA, Sonntag KC. A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry. 68:741–747. doi: 10.1016/j.biopsych.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Savage CR, Deckersbach T, Wilhelm S, Rauch SL, Baer L, Reid T, et al. Strategic processing and episodic memory impairment in obsessive compulsive disorder. Neuropsychology. 2000;14:141–151. doi: 10.1037//0894-4105.14.1.141. [DOI] [PubMed] [Google Scholar]
- 10.Storch EA, Lewin AB, Farrell L, Aldea MA, Reid J, Geffken GR, et al. Does cognitive-behavioral therapy response among adults with obsessive-compulsive disorder differ as a function of certain comorbidities? J Anxiety Disord. 24:547–552. doi: 10.1016/j.janxdis.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Szechtman H, Sulis W, Eilam D. Quinpirole induces compulsive checking behavior in rats: a potential animal model of obsessive-compulsive disorder (OCD) Behav Neurosci. 1998;112:1475–1485. doi: 10.1037//0735-7044.112.6.1475. [DOI] [PubMed] [Google Scholar]