Abstract
Background
We recently showed that mechanisms of protein turnover in skeletal muscle are unresponsive to amino acid (AA) infusion in severely burned pediatric patients at 6 months postinjury. In the current study, we evaluated if oxandrolone treatment affects mechanisms of protein turnover in skeletal muscle and whole-body protein breakdown in pediatric burn patients 6 months postinjury.
Methods
At the time of admission, patients were randomized to control or oxandrolone treatments. The treatment regimens were continued until 6 months postinjury, at which time patients (n = 26) underwent study with a stable isotope tracer infusion to measure muscle and whole-body protein turnover.
Results
Protein kinetics in leg muscle were expressed in nmol/min/100 ml leg volume (mean±SE). During AA infusion, rates of protein synthesis in leg muscle were increased (p < .05) in both groups (Basal vs. AA: control, 51±8 vs. 86±21; oxandrolone, 56±7 vs. 96±12). In the control group, there was also a simultaneous increase in breakdown (Basal vs. AA: 65±10 vs. 89±25), which resulted in no change in the net balance of leg muscle protein (Basal vs. AA: − 15±4 vs. − 2±10). In the oxandrolone group, protein breakdown did not change (Basal vs. AA: 80±12 vs. 77±9), leading to increased net balance (Basal vs. AA: − 24±7 vs. 19±7, p < .05). Protein breakdown at the whole-body level was not different between the groups.
Conclusion
Long-term oxandrolone treatment increased net deposition of leg muscle protein during AA infusion by attenuating protein breakdown, but did not affect whole-body protein breakdown.
Keywords: Burn, children, protein metabolism, oxandrolone treatment, stable isotopes
INTRODUCTION
With advances in acute burn care and the associated increases in survival, improving long-term outcomes and quality of life have become a clinically relevant challenge. The hypermetabolic and catabolic condition leading to muscle wasting persists years after burn injury1,2. Recently, we showed that amino acid (AA) infusion does not increase the net balance of muscle protein in pediatric patients at 6 months after burn injury as it does in healthy young adults3. In that study, AA infusion increased synthesis rates of leg muscle protein in both healthy and burn groups. In the healthy group, the increase in synthesis translated into a gain in the net balance. However, in the burn group, a simultaneous (albeit non-significant) increase in protein breakdown occurred, resulting in no change in the net protein balance. In the same study, we measured whole-body protein breakdown and consequently calculated the rates of whole-body muscle and non-muscle protein breakdown. In healthy control subjects, AA infusion significantly decreased the whole-body protein breakdown rate by decreasing the rates of both whole-body muscle and non-muscle protein breakdown. In contrast, in burn patients, we observed no changes in the parameters of whole-body protein breakdown following AA infusion. Thus, the difference in protein metabolism during AA infusion between normal and burn subjects lies in the response of protein breakdown. This catabolic state of the patients may be explained by long-lasting increases in catabolic hormones and the persistence of insulin resistance1,2,4. In this clinical situation, nutrition alone may not be sufficient for burn children to resume normal growth, and additional interventions such as anabolic agents could be indicated. For this reason, we have investigated whether oxandrolone improves muscle protein metabolism.
Oxandrolone, a synthetic analog of testosterone, has minimal virilizing activity and hepatotoxicity compared to testosterone5,6. In skeletal muscle, oxandrolone binds to the androgen receptor and migrates to the cell nucleus, where it stimulates protein synthesis and anabolism. Oxandrolone also exerts its anabolic effects by counteracting the catabolic effects of cortisol through competitive inhibition of the glucocorticoid receptors7.
Clinically, oxandrolone has been used successfully to treat muscle wasting and conditions of growth disorders8-10. Recently, studies have been conducted to evaluate the effects of oxandrolone on the catabolic state resulting from burn injury and have show that this drug has beneficial effects11-20. Hart et al.13 demonstrated that, during the acute postburn phase in pediatric patients, oxandrolone treatment increases protein synthesis in skeletal muscle. Consistent with this finding, Murphy et al.17 showed that long-term oxandrolone treatment increases lean body mass compared to the placebo treatment at 6 – 24 months post-injury17. Similar results have been obtained in studies conducted on adult burn patients11,12.
The current study was conducted on pediatric burn patients at 6 month postinjury to investigate the effect of long-term oxandrolone treatment on protein turnover in leg muscle in response to AA infusion (as assessed using stable-isotope techniques) and on body composition (as assessed using dual X-ray absorptiometry (DEXA). We also quantified the effect of oxandrolone treatment on whole-body total protein and whole-body non-muscle protein breakdown. We hypothesized that long-term (6 months) oxandrolone treatment has an anabolic effect on muscle and whole-body protein in pediatric burn patients when an adequate AA supply is provided.
METHODS
Patients
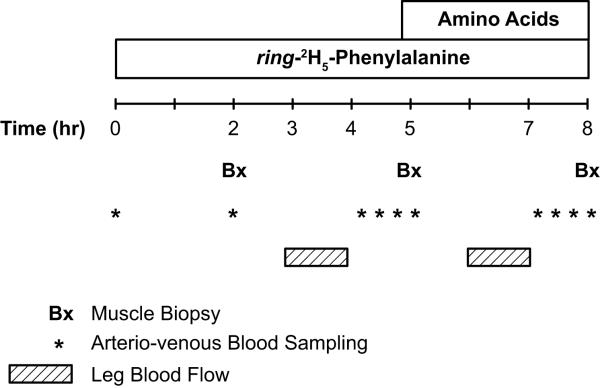
Patients admitted to Shriners Hospitals for Children, Galveston, TX during the acute phase of burn injury were eligible for this study. Inclusion criteria were as follows: age ≤ 18 years at admission, ≥ 40 % of total body surface area burned, participation in the control (standard of care) or oxandrolone treatment regimens from acute admission until 6 months postinjury, and participation in a metabolic study to evaluate protein kinetics at postabsorptive and AA infusion periods, as depicted in Figure 1. This project was a part of a larger clinical trial (Clinical Trial Registration Number: NCT00675714) evaluating the effect of anabolic agents on muscle mass preservation after burn injury.
Figure 1.
Stable isotope infusion protocol.
The study protocol was approved by the Institutional Review Board of the University of Texas Medical Branch, Galveston, TX, and the study was conducted at Shriners Hospitals for Children. Child assent and parental permission were obtained from each patient and patient’s guardian before enrollment into the study. At acute admission, patients were randomized into control or oxandrolone treatment groups. These treatment regimens were continued after discharge as long-term treatment regiments, meaning oxandrolone group patients continued oxandrolone treatment (0.1 mg/kg twice daily) and control patients continued placebo treatment until 6 months after burn injury.
Study design
Patients who returned for their 6-month follow-up/reconstructive surgery visit participated in an 8-hour stable isotope tracer study (Fig. 1). All stable isotope studies were performed in the postabsorptive state (fasted overnight, 6 – 12 hour). Baseline blood samples were drawn for chemistry analyses and background measurement of AA enrichment. Thereafter, a primed continuous infusion of L-[ring-2H5]-phenylalanine (Cambridge Isotope laboratories, Andover, MA) was initiated with a priming dose of 3.2 μmol/kg and a continuous infusion rate of 0.08 μmol/kg per min (Fig. 1). Blood samples to measure plasma phenylalanine enrichment were collected simultaneously from the femoral artery and vein during the last hour of each period (i.e., postabsorptive or AA infusion period) (Fig. 1). Biopsies of the vastus lateralis muscle for measurement of muscle protein fractional synthesis rate (FSR) were obtained after 2, 5 and 8 hour of tracer infusion using a 5-mm Bergström needle (Stille, Stockholm, Sweden) (Fig. 1). The muscle samples were frozen immediately in liquid nitrogen and stored at −80°C until further analysis. A continuous infusion of indocyanine green (Akron, Buffalo Grove, IL) at 0.05 mg/min was used for measurement of leg blood flow (Fig. 1) as described previously21.
After the basal period (0 – 5 hour), a primed (0.45 ml/kg) continuous infusion of unlabeled AA (10 % Travasol, Clintec Nutrition, Deerfield, IL: total AA = 100 mg/ml) was initiated and maintained at the rate of 1.35 ml/kg per min for 8 hour. One hundred milliliters of Travasol solution contained 730 mg leucine, 600 mg isoleucine, 580 mg lysine, 580 mg valine, 560 mg phenylalanine, 480 mg histidine, 420 mg threonine, 400 mg methionine, 180 mg tryptophan, 2.07 g alanine, 1.15 g arginine, 1.03 g glycine, 680 mg proline, 500 mg serine, and 40 mg tyrosine. At the end of the study, peripheral intravenous lines and femoral catheters were kept in place for reconstructive surgery.
Analytical methods
Blood AA enrichment
Blood samples for the measurement of phenylalanine enrichment and concentration were collected as described previously21. Briefly, arterial and venous blood samples were collected in preweighed tubes containing 15 % sulfosalicylic acid. L-[U-13C9,15N]phenylalanine was added as the internal standard for measurement of blood phenylalanine concentrations. After deproteinization, blood AAs were separated using cation exchange chromatography21. The enrichments and the concentrations of phenylalanine in arterial and venous blood samples were determined on the tert-butyldimethylsilyl (t-BDMS) derivatives using gas chromatography-mass spectrometry (GC-MS HP 5989; Hewlett-Packard, Palo Alto, CA) with electron impact ionization21. The serum concentration of indocyanine green was determined by means of spectrophotometry at λ = 805 nm.
Muscle
Muscle samples were weighed and protein precipitated with 800 μl of 10 % perchloric acid. The supernatant was collected after homogenization of the tissue and centrifugation. The AAs in the supernatant were then separated using cation exchange chromatography21. The isotopic enrichment of intracellular phenylalanine was determined on the t-BDMS derivatives by GC-MS in electron impact mode. The remaining pellet was washed as described previously21, dried at 50°C overnight, and hydrolyzed in 6 N HCl at 110°C for 24 h. The hydrolysate was then passed over a cation exchange column, and phenylalanine enrichment was measured by GC-MS in the same manner as the intracellular portion21.
Calculations
Kinetics of leg muscle protein
Because phenylalanine is neither synthesized nor oxidized in the peripheral tissues, its rates of appearance (Ra) and disappearance (Rd) across the femoral arterial and venous plasma pools reflect breakdown and synthesis of leg skeletal muscle protein, respectively. The assumptions and limitations of the kinetic model have been described elsewhere21. Because calculations of the model kinetics require steady-state, physiologic conditions, we infused the AA mixture (i.e., Travasol) at a constant rate after a priming dose. Finally, arterial and venous blood sampling took place in the last hour of the 3-h AA infusion when blood AA concentrations and enrichments achieved steady state. The phenylalanine kinetic rates in the leg were calculated and standardized for leg volume.
Kinetics of whole-body phenylalanine
The Ra of whole-body AAs, represented by phenylalanine plasma kinetics, was calculated according to the standard equation, i.e., tracer infusion rate divided by arterial enrichment. This quantity is a direct reflection of whole-body protein breakdown, because phenylalanine is not synthesized in the body and therefore its appearance in plasma can only originate from protein breakdown in the fasted state. For the AA infusion period, whole-body Ra was corrected for the rate of the infused phenylalanine. The Ra for whole-body muscle AAs was calculated from the Ra for leg muscle AAs, corrected for the whole-body muscle mass by assuming that the total muscle mass is 4.5 times the amount in one leg21. Whole-body non-muscle phenylalanine Ra was calculated by subtracting whole-body muscle Ra from total whole-body Ra.
Muscle protein FSR
Calculation of muscle protein FSR was based on the precursor-product method, using intracellular free and bound protein tracer enrichments in the muscle biopsy as precursor and product, respectively. The calculation of synthesis, breakdown, and net balance of muscle protein was done according to a previously described two-pool model21.
The specific equations for the calculations described above are as follows:
Leg muscle protein net balance: NB = (CA – CV) • BF
Leg muscle protein rate of disappearance: lRd = (CA • EA – CV • EV) / EA • BF
Leg muscle protein rate of appearance: lRa = Rd – NB
Whole-body protein rate of appearance: wbRa = IR / EA
Whole-body muscle protein Ra: wbmRa = Leg Ra • Leg Volume • 4.5 / Weight
Whole-body endogenous Ra: wbeRa = wbRa – wbmRa
Fractional synthesis rate: FSR = [(Emt2 – Emt1) / (Ef • t)] • 60 • 100 • 24
In these equations, NB is the net balance of protein in leg muscle; lRd and lRa are Rd and Ra of phenylalanine in the leg, respectively; CA and CV are free phenylalanine concentrations in blood from the femoral artery and vein; EA and EV are free phenylalanine enrichments (tracer/tracee ratio) in blood from the femoral artery and vein; BF is leg blood flow in ml/min per 100 ml of leg volume; wbRa is whole-body Ra of phenylalanine; IR is the infusion rate of phenylalanine tracer; wbmRa is whole-body muscle Ra of phenylalanine; wbeRa is whole-body endogenous Ra of phenylalanine; Ef and Em are the enrichments of free and bound AA, respectively; and t is the time interval in minutes between two sequential biopsies. Leg and whole-body protein Rd, Ra, and NB were expressed in nmol Phe/min per 100 ml leg volume and μmol Phe/min per kg, respectively. FSR was expressed in percent per hour (%/h).
DEXA
At discharge and at the 6-month return visit, lean body mass and bone mineral content were measured by DEXA (Hologic Model QDR-4500A DEXA; Hologic Inc, Waltham, Mass). The percentage change occurring from discharge to 6 months was calculated and normalized for time.
Data presentation and statistical analysis
Data are presented as mean ± SE. A two-way repeated ANOVA with factors of time and treatment was performed with Tukey’s post-hoc correction to assess differences in measurements. Differences in percent change in DEXA parameters and blood clinical chemistry parameters were analyzed by t test. A p value less than .05 was considered statistically different. We have previously found that oxandrolone improves muscle protein balance by 64 ± 44 nmol/min/100 ml leg volume (mean ± SD) relative to control patients in the acute stage postinjury13, so we assumed a similar change would occur in the current study. Assuming an alpha of .05 and a desired power of 80%, 10 patients per group would be sufficient to detect a similar difference in the present study.
RESULTS
Demographics of patients are presented in Table 1. There was no statistically significant difference in any of these parameters between the groups (p > .05). Thirteen patients in each group agreed to participate in the 6-month study. Due to technical problems during the infusion protocol and sample analyses, three studies in the control and one in the oxandrolone group were excluded from analysis. Therefore, we present data from 10 controls and 12 oxandrolone-treated patients.
Table 1.
Patient demographics
| Control | Oxandrolone | p value | |
|---|---|---|---|
| Number | 10 | 12 | |
| Age, years | 7.4±1.2 | 10.4±1.4 | NS |
| Months after injury | 6.1±0.2 | 6.1±0.2 | NS |
| Months after discharge* | 4.9±0.3 | 4.6±0.4 | NS |
| Weight, kg | 27±6 | 36±5 | NS |
| Height, cm | 120±8 | 134±9 | NS |
| BMI, kg/m2 | 18±1 | 19±1 | NS |
| Sex, M/F | 4/6 | 10/2 | NS |
| TBSA (%) | 58±5 | 53±2 | NS |
| Third degree (%) | 49±8 | 30±6 | NS |
| Type of burn | Flame 8/Scald 2 | Flame 10/Elect 2 | NS |
| LOS/TBSA | 0.5±0.1 | 0.4±0.1 | NS |
Data are presented as mean±SE.
, months after acute admission discharge. LOS/TBSA, data from acute admission.
Blood clinical chemistry results are presented in Table 2. In both groups, all parameters, except alkaline phosphatase activity and phosphorus concentrations, were within the reference ranges. Although the alanine aminotransferase activity was within the reference range, the values were 50% greater in the oxandrolone group than in the control group. This result was strongly affected by the value from one patient (alanine aminotransferase = 241 U/L). Exclusion of this result from the analysis brought the average alanine aminotransferase value in the oxandrolone group to 24.9 U/L, which is the same level seen in the control group. Subsequent measurement of alanine aminotransferase activity in this particular patient showed that the alanine aminotransferase activity was 38.5 U/L. Alkaline phosphatase activity and serum phosphorus concentrations were greater than the reference values in both groups.
Table 2.
Blood clinical chemistry results
| Groups |
||||
|---|---|---|---|---|
| Parameters | Reference values | Control | Oxandrolone | p |
| ALT (U/L) | 9–51 | 25±3 | 45±20 | NS |
| AST (U/L) | 13–40 | 26±2 | 30±2 | NS |
| Total Bilirubin (mg/dl) | 0.1–1.1 | 0.33±0.07 | 0.37±0.05 | NS |
| ALP (U/L) | 34–122 | 189±21# | 200±22# | NS |
| GGT (U/L) | < 40 (58)* | 27±10 | 27±6 | NS |
| BUN (mg/dL) | 7–23 | 11±1.3 | 9±0.6 | NS |
| Albumin (g/dL) | 3.5–5 | 3.6±0.2 | 3.8±0.1 | NS |
| Glucose (mg/dL) | 70–110 | 96±8 | 88±3 | NS |
| Potassium (mmol/L) | 3.5–5 | 4±0.1 | 4±0.1 | NS |
| Magnesium (mg/dL) | 1.7–2.4 | 1.9±0.1 | 1.7±0.1 | 0.02 |
| Phosphorus (mg/dL) | 2.8–4.9 | 5.2±0.2# | 5.3±0.2# | NS |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyltransferase. Data are presented as mean±SE.
Statistical significance between the groups was evaluated by t test, NS, non-significant.
, the value in the parenthesis is for males;
, the averages are higher than the reference values.
Leg blood flow was not different between the groups during the postabsorptive period (control vs. oxandrolone: 8.6±1.7 vs. 9.8±1.2 ml min−1 100 ml leg volume−1, p > .05) or during AA infusion (control vs. oxandrolone: 12.3±4.1 vs. 9.6±1.1 ml min−1 100 ml leg volume−1, p > .05).
Phenylalanine enrichments (Table 3) and concentrations (Table 4) in the femoral artery and vein were in steady-state conditions over the last hour of each study period in both groups. When the phenylalanine concentration increased (p < .001) during AA infusion (Table 4), phenylalanine enrichments in the femoral artery and vein decreased (p < .001) (Table 3).
Table 3.
Plasma phenylalanine enrichment
| Femoral Artery | Femoral Vein | |
|---|---|---|
| Control (Basal) | 0.078±0.009 | 0.074±0.009 |
| Control (AA) | 0.044±0.004* | 0.042±0.003* |
| Oxandrolone (Basal) | 0.079±0.006 | 0.067±0.004 |
| Oxandrolone (AA) | 0.047±0.002* | 0.043±0.002* |
Enrichment data are presented as mean±SE and expressed as a tracer-to-tracee ratio.
, significantly different from enrichment during basal period (p < .001).
Table 4.
Plasma phenylalanine concentrations
| Femoral Artery | Femoral Vein | |
|---|---|---|
| Control (Basal) | 66±4 | 67±4 |
| Control (AA) | 133±10* | 133±9* |
| Oxandrolone (Basal) | 53±5 | 55±5 |
| Oxandrolone (AA) | 130±13* | 130±13* |
Concentration data are presented as mean±SE and expressed as μmol/L.
, significantly different from concentrations during basal period (p < .001).
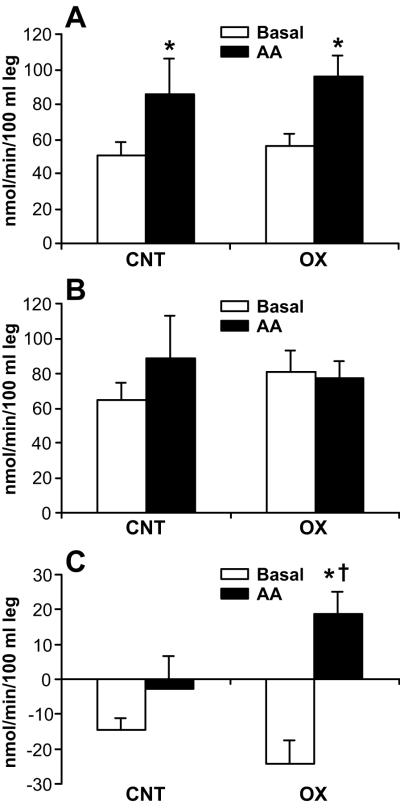
During the postabsorptive period, no significant differences in the parameters of leg protein metabolism were noted between the groups (Fig. 2). In both groups, muscle protein synthesis, as represented by Rd, was higher after AA infusion than during the basal period (p < .05) (Fig. 2A). AA infusion did not significantly affect leg muscle protein breakdown, as represented by Ra, in either group (Fig. 2B). In the control group it increased by 36 % (p = .19, basal vs. AA infusion period), whereas in the oxandrolone group it decreased by 4 % (p = .85, basal vs. AA infusion period). As a result, during AA infusion, net deposition of muscle protein did not change in the control group but increased in the oxandrolone group (p < .05) (Fig. 2C). There was a significant interaction between group and period (p < .05).
Figure 2.
Leg muscle protein kinetics. Data are presented as mean±SE. In both groups, muscle protein synthesis, as represented by rate of disappearance (Rd), was greater following amino acid (AA) infusion than during basal period (A). AA infusion did not affect leg muscle protein breakdown, as represented by rate of appearance (Ra) in either group (B). In the control group, it increased by 36% (p = .19, basal period vs. AA infusion), and in the oxandrolone group, it decreased by 4% (p = .85, basal period vs. AA period infusion). As a result, during AA infusion, the net deposition of muscle protein did not change in the control group but significantly increased in the oxandrolone group (C). *, p < .05, significantly different from basal period.
Consistent with the leg balance data, muscle protein FSR increased during AA infusion by 40 % in the control group and 19 % in the oxandrolone group (control: 0.12 ± 0.04 vs. 0.16 ± 0.04 and oxandrolone: 0.13 ± 0.02 vs. 0.15 ± 0.04 %/h, Basal vs. AA, respectively); however, these increases did not reach statistical significance (p > .05).
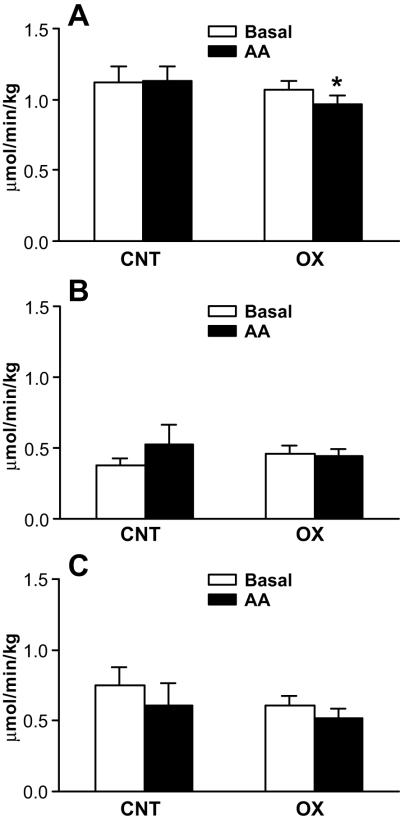
Whole-body protein breakdown data are presented in Figure 3. There were no differences in parameters between the groups during the postabsorptive state (Fig. 3). In the oxandrolone group, whole-body protein Ra was lower following AA infusion (p < .05) than during the basal period (Fig. 3A). AA infusion did not affect either whole-body muscle Ra (Fig. 3B) or whole-body non-muscle protein Ra in either group (Fig. 3C).
Figure 3.
Whole-body endogenous phenylalanine kinetics. Data are presented as mean±SE. A, Rate of appearance (Ra) of whole-body protein (which is a reflection of protein breakdown). In the oxandrolone group, whole-body protein Ra was significantly (p < .05) lower following AA infusion than during basal period. B, Whole-body muscle protein Ra. C, whole-body non-muscle protein Ra. AA infusion did not affect whole-body muscle Ra (B) or whole-body non-muscle protein Ra in either group (C). *, p < .05, significantly different from basal period.
Seven patients in the control group and ten patients in the oxandrolone group underwent DEXA measurements at discharge and at 6 months after injury. The mean percent change in lean body mass normalized for time was not different between the groups (control vs. oxandrolone, 0.4 ± 0.6 vs. 0.7 ± 0.5 %/month, p = .68). Change in bone mineral content was increased in oxandrolone but decreased in the control group, and the difference between the groups was significant (control vs. oxandrolone, −1.3 ± 1.0 vs. 1.0 ± 0.5 %/month, p < .05).
DISCUSSION
Long-term recovery from massive burn injury is often affected by the catabolic and hypermetabolic state, which lead to muscle breakdown and a negative net protein balance in skeletal muscle2. This problem occurs at a time when pediatric patients would normally be in an anabolic state because of developmental growth.
Recently, we have shown that, in pediatric patients at 6 months after burn injury, leg muscle protein turnover is unresponsive to the anabolic signaling and whole-body protein turnover is unresponsive to AA infusion, that occur in contrast to the responses seen in healthy young adults3. We proposed that burn patients need anabolic agents to restore their muscle protein response to AA stimulation, which would increase net protein deposition in muscle during the rehabilitation period.
Oxandrolone has been reported to be a safe and beneficial anabolic agent in patients with muscle wasting and growth delay5-7. Several groups have reported that oxandrolone has beneficial effects in preserving and/or increasing lean body mass in burn patients during acute and rehabilitation periods11-20. Demling et al.12 reported that, during the acute admission, adult burn patients randomized to receive oxandrolone have less weight and nitrogen loss. Similar results have been obtained in pediatric burn patients13,14. Hart et al.13 and Wolf et al.14 evaluated the kinetics of muscle protein in oxandrolone-treated pediatric patients during the acute phase after burn injury. They demonstrated that oxandrolone treatment increases the rate of muscle protein synthesis without changing the breakdown rate, resulting in improved net deposition of muscle protein13,14. Positive results from these acute-phase studies have led to long-term studies investigating the ability of oxandrolone to ameliorate muscle loss during the rehabilitation period. Another work of Demling et al.11 demonstrated that, in adult burn patients, oxandrolone treatment, in combination with proper nutrition and exercise, increases lean body mass, promotes body weight gain, increases muscle strength, restores functional capacity of the patients, and decreases the duration of rehabilitation. In pediatric patients, oxandrolone treatment increases lean body mass at 6 – 24 months post injury17,22.
In the current study, we hypothesized that long-term oxandrolone treatment restores AA utilization in skeletal muscle and whole-body tissue and consequently improves net balance of muscle protein in the pediatric burn patient population. To determine the interaction of oxandrolone treatment with feeding (AA utilization), we studied patients in the fasted state and during infusion of a commercial AA solution (Travasol). After 6 months of oxandrolone treatment, children demonstrated a significant negative net balance in muscle protein in the fasted state, similar to the control group. In contrast, children who received oxandrolone demonstrated the expected muscle anabolism following AA infusion with a positive net balance in muscle protein. On the other hand, the children in the control group failed to respond to AA administration, and their net balance in muscle protein remained negative. These results are in agreement with the finding that administration of oxandrolone during the acute phase after burn injury enables restoration of the anabolic effects of feeding on skeletal muscle13,14. They also agree with results obtained in healthy older women24. The current results, however, bring some new insights into the mechanism underlying the effect of oxandrolone treatment on muscle AA kinetics in pediatric burn patients.
Our previous work in adult burn patients during the acute phase after injury showed that muscle breakdown decreased by almost 50 % when patients were treated with testosterone, and this decrease was accompanied by a corresponding improvement in net protein balance23. In pediatric patients, however, 5 days of oxandrolone treatment during the acute phase increased muscle protein synthesis without affecting protein breakdown13,14. This observation suggests that, while the outcome of oxandrolone therapy may be similar in adults and pediatric burn patients, the mechanism of action is different. A possible explanation for the difference between children and adults could be that children are still growing and therefore have a greater capacity for muscle protein synthesis. From the current results, it is evident that 6 months of oxandrolone treatment restores the suppression mechanisms of protein breakdown normally seen with feeding. The reason for the discrepancy between the results from the current and previous studies in pediatric burn patients13,14 could be that it takes a greater duration of oxandrolone treatment to reverse the catabolic state in children than in adults25. Nevertheless, our current results confirm our hypothesis that long-term oxandrolone treatment improves utilization of AAs in muscle protein in the pediatric burn patient population.
The biochemical mechanism by which oxandrolone exerts its anabolic action cannot be elucidated in the current study; however, we speculate that the decrease in protein breakdown may be related to the ability of oxandrolone (testosterone) to antagonize glucocorticoid-induced activation of muscle protein breakdown. Burn injury affects the hormonal profile by increasing levels of catabolic hormones. For example, in pediatric burn patients, the serum cortisol concentration increases shortly after the injury26 and remains increased for up to 2 years after injury4. Hypercortisolemia has been shown to affect endogenous production of testosterone27,28. Testosterone has a high binding affinity for the glucocorticoid receptor29 and exerts its anabolic activity by interacting with the glucocorticoid receptor to antagonize the catabolic activity of endogenous glucocorticoids30. Therefore, oxandrolone treatment may diminish the catabolic effects of glucocorticoids (cortisol) on skeletal muscle through a mechanism involving the glucocorticoid receptor.
The AA infusion did not affect whole-body protein kinetics in either group, with one exception. In the long-term oxandrolone group, whole-body protein breakdown was significantly lower following AA infusion than during basal period. We assume that statistical significance was attained because of the paired analysis we conducted. The difference between the periods within the long-term oxandrolone group was 10%, whereas the difference between groups within the AA period was 15 %. These data suggest that a 6-month oxandrolone treatment does not affect the protein breakdown rate at the level of whole-body total protein and whole-body non-muscle protein. This result is consistent with our previous study23 showing that fibrinogen protein synthesis is unchanged following 2 weeks of testosterone treatment in adult burn patients. Additionally, we speculate that the altered response of whole-body total protein and whole-body non-muscle protein to AA infusion may relate to the underlying burn-related hypermetabolic condition, which is not affected by oxandrolone treatment18; however, further experiments would be required to address this issue.
Previously, we have reported that long-term treatment with oxandrolone increases lean body mass at 6 months post injury17. When we looked at a subgroup of patients who underwent DEXA measurement in the current study, the mean percent change in lean body mass in the oxandrolone group was 89 % greater than in the control group; however, the difference did not reach statistical significance. Sample size analysis demonstrates that 418 patients per group would be required to reach a statistical significance with α = .05 and β = 80 %. Oxandrolone treatment increased bone mineral content (p < .05), which is consistent with the previous report17.
The results of liver function tests suggest that there was no detectable hepatotoxic side effects in oxandrolone-treated patients. However, previous studies have shown that liver enzyme activities are increased in oxandrolone-treated groups16,20. Therefore, careful consideration in future studies is advisable. The average serum alkaline phosphatase activity value was greater than the reference range in both groups. In general, alkaline phosphatase activity tends to be greater in children and adolescents than in adults, because their bones are growing and alkaline phosphatase activity is often very high during a growth spurt, which occurs at varying ages. The serum phosphorus concentration was slightly greater than the reference range in both groups. Although we do not have an explanation for this phenomenon, we speculate that the increased concentration of serum phosphorus is related to the high level of alkaline phosphatase and the high rate of bone growth in these children. The serum magnesium concentration was significantly less in the oxandrolone group, but the averages in both groups were within the reference ranges. Therefore, we do not believe that this result relates to the treatment regimen or has any pathophysiologic importance.
In conclusion, 6 months of oxandrolone treatment improved net deposition of skeletal muscle protein in pediatric burn patients by restoring leg muscle AA utilization. However, whole-body protein breakdown was not affected by oxandrolone treatment.
Acknowledgements
The authors wish to thank the study volunteers and their families for their patience and dedication as well as the clinical and research staff of Shriners Hospitals for Children, Galveston, TX for their help in conducting the clinical portion of the study; special thanks to Cindy Locklin and Deb Benjamin for performing metabolic infusion studies; Tara Cocke, Christopher Danesi, and Ann Hightower for excellent technical performance with sample processing; Guy Jones and Jariwala Guarang for performing GC-MS analyses; and Steve Schuenke for an excellent assistance with manuscript preparation.
This study was supported by NIH grant P50 GM 60338, Shriners Mass Spectrometry Core Grant 84090, and Shriners grant 87600.
Abbreviations used
- AA
amino acid(s)
- FSR
fractional synthesis rate
- t-BDMS
tert-butyldimethylsilyl
- GC-MS
gas chromatograph-mass spectrometry
- Ra
rate of appearance
- Rd
rate of disappearance
- DEXA
Dual X-ray absorptiometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Reiss E, Pearson E, Artz CP. The metabolic response to burns. J Clin Invest. 1956;35:62–77. doi: 10.1172/JCI103253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 3.Tuvdendorj D, Chinkes DL, Zhang XJ, Sheffield-Moore M, Herndon DN. Skeletal muscle is anabolically unresponsive to an amino acid infusion in pediatric burn patients 6 months postinjury. Ann Surgery. 2011 Jan 21; doi: 10.1097/SLA.0b013e31820d9a63. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–64. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox M, Minot AS, Liddle GW. Oxandrolone: a potent anabolic steroid of novel chemical configuration. J Clin Endocrinol metab. 1962;22:921–4. doi: 10.1210/jcem-22-9-921. [DOI] [PubMed] [Google Scholar]
- 6.Karim A, Ranney Re, Zagarella J, Maibach HI. Oxandrolone disposition and metabolism in man. Clin Pharmacol Ther. 1973;14:862–9. doi: 10.1002/cpt1973145862. [DOI] [PubMed] [Google Scholar]
- 7.Orr R, Singh M Fiatarone. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64:725–50. doi: 10.2165/00003495-200464070-00004. [DOI] [PubMed] [Google Scholar]
- 8.Berger J, Pall L, Hall CD, Simpson DM, Perry PS, Dudley R. Oxandrolone in AIDS-wasting myopathy. AIDS. 1996;10(14):1657–62. doi: 10.1097/00002030-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Stahnke N, Keller E, Landy H. Favorable final height outcome in girls with Ullrich-Turner syndrome treated with low-dose growth hormone together with oxandrolone despite starting treatment after 10 years of age. J Pediatr Endocrinol Metab. 2002;15:129–38. doi: 10.1515/jpem.2002.15.2.129. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DM, McCauley E, Brown DR, Dudley R. Oxandrolone therapy in constitutionally delayed growth and puberty. Pediatrics. 1995;96:1095–100. [PubMed] [Google Scholar]
- 11.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997;43:47–51. doi: 10.1097/00005373-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000;15:12–7. doi: 10.1053/jcrc.2000.0150012. [DOI] [PubMed] [Google Scholar]
- 13.Hart DW, Wolf SE, Ramzy PI, Chinkes DL, Beauford RB, Ferrando AA, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233(4):556–64. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf SE, Thomas SJ, Dasu MR, Ferrando AA, Chinkes DL, Wolfe RR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237(6):801–11. doi: 10.1097/01.SLA.0000071562.12637.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrow RE, Dasu MRK, Ferrando AA, Spies M, Thomas SJ, Perez-Polo JR, et al. Gene expression patterns in skeletal muscle of thermally injured children treated with oxandrolone. Ann Surg. 2003;237(3):422–8. doi: 10.1097/01.SLA.0000055276.10357.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg. 2007;246:351–62. doi: 10.1097/SLA.0b013e318146980e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy KD, Thomas S, Mlcak RP, Chinkes DL, Klein GL, Herndon DN. Effects of long-term oxandrolone administration in severely burned children. Surgery. 2004;136:219–24. doi: 10.1016/j.surg.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Przkora R, Jeschke MG, Barrow RE, Suman OE, Meyer WJ, Finnerty CC, et al. Metabolic and hormonal changes of severely burned children receiving long-term oxandrolone treatment. Ann Surg. 2005;242:384–91. doi: 10.1097/01.sla.0000180398.70103.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas S, Wolf SE, Murphy KD, Chinkes DL, Herndon DN. The long-term effect of oxandrolone on hepatic acute phase proteins in severely burned children. J Trauma. 2004;56:37–44. doi: 10.1097/01.TA.0000108636.63225.63. [DOI] [PubMed] [Google Scholar]
- 20.Wolf SE, Edelman LS, Kemalyan N, Donison L, Cross J, Underwood M, et al. Burn applied research team. Effects of oxandrolone on outcome measurements in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res. 2006;27(2):131–9. doi: 10.1097/01.BCR.0000202620.55751.4F. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research. John Wiley & Sons, Inc., publication; New Jersey: 2005. [Google Scholar]
- 22.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007;119(1):106–19. doi: 10.1542/peds.2006-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care med. 2001;29:1936–42. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Sheffield-Moore M, Paddon-Jones D, Casperson SL, Gilkison C, Volpi E, Wolf SE, et al. Androgen therapy induces muscle protein anabolism in older women. J Clin Endocrin Metab. 2006;91(10):3844–9. doi: 10.1210/jc.2006-0588. [DOI] [PubMed] [Google Scholar]
- 25.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14(9-10):553–60. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeschke MG, Chinkes DL, Finnerty CC, Kulp GA, Suman OE, Norbury WB, et al. Pathophysiological response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doerr P, Pirke KM. Cortisol-induced suppression of plasma testosterone in normal adult males. J Clin Endocrinol Metab. 1976;43:622–9. doi: 10.1210/jcem-43-3-622. [DOI] [PubMed] [Google Scholar]
- 28.Welsh TH, Bambino TH, Hsueh AJ. Mechanism of glucocorticoid-induced suppression of testicular androgen biosynthesis in vitro. Biol Reprod. 1982;27:1138–46. doi: 10.1095/biolreprod27.5.1138. [DOI] [PubMed] [Google Scholar]
- 29.Dahnaive PA, Rousseau GG. Binding of glucocorticoid antagonist to androgen and glucocorticoid hormone receptros in rat skeletal muscle. J Steroid Biochem. 1986;24:481–7. doi: 10.1016/0022-4731(86)90109-3. [DOI] [PubMed] [Google Scholar]
- 30.Dahnaive PA, Rousseau GG. Evidence for sex-dependent anabolic response to androgenic steroids mediated by muscle glucocorticoid receptors in the rat. J Steroid Biochem. 1988;29:575–81. doi: 10.1016/0022-4731(88)90154-9. [DOI] [PubMed] [Google Scholar]