Abstract
Background
Selective mutism (SM), considered an early-onset variant of social anxiety disorder (SAD), shares features of impaired social interaction and communication with autism spectrum disorders (ASDs) that suggest a possible shared pathophysiology. We examined the association of a susceptibility gene, contactin-associated protein-like 2 (CNTNAP2), for ASDs and specific language impairment (SLI) with SM and social anxiety-related traits.
Methods
Sample 1 subjects were 99 nuclear families including 106 children with SM. Sample 2 subjects were young adults who completed measures of social interactional anxiety (SIAS; N = 1028) and childhood behavioral inhibition (RSRI; N = 920). Five SNPs in CNTNAP2 (including rs7794745 and rs2710102, previously associated with ASDs) were genotyped.
Results
FBAT analyses revealed nominal significance (p = 0.018) for association of SM with rs2710102 which, with rs6944808, was part of a common haplotype associated with SM (permutation p = 0.022). Adjusting for sex and ancestral proportion, each copy of the rs2710102*a risk allele in the young adults was associated with increased odds of being >1SD above the mean on the SIAS (OR = 1.33, p = 0.015) and RSRI (OR = 1.40, p = 0.010).
Discussion
Although association was found with rs2710102, the risk allele (“a”) for the traits studied here is the non-risk allele for ASD and SLI (“g”). These findings suggest a partially shared etiology between ASDs and SM, but raise additional questions about specific aspects of these syndromes (i.e., language impairment and/or social anxiety) potentially influenced by CNTNAP2 and mechanism(s) by which these influences may be conveyed.
Keywords: genetics, anxiety disorders, speech, childhood, autism, autism spectrum, social anxiety, behavioral inhibition
INTRODUCTION
Selective mutism (SM) is a relatively rare childhood disorder (prevalence < 1%) (1) in which the child consistently fails to speak in one or more social settings (e.g., school) despite speaking normally in other settings (e.g., home). By definition, language development is within normal limits, though some studies find subtle expressive and/or receptive language problems in up to 25% of cases (2–7). The vast majority of children with SM have markedly elevated levels of social anxiety and/or meet diagnostic criteria for Social Anxiety Disorder (SAD) (3;8–11), and these two disorders co-occur in families at a much higher than chance rate (8;12) suggesting a shared familial (possibly genetic) basis (13). The extensive phenomenological and familial overlap between SM and SAD has led to a proposal for DSM-5 that SM be reclassified as a childhood form of SAD (14), though other experts believe that SM has a complex, multi-determined etiology and caution against making this assumption (15). One area, in particular, that bears further scrutiny is the relationship between SAD, SM, and a group of disorders that includes language and social communication deficits among its symptoms: autism spectrum disorders (ASD (MIM 209850), also known as Pervasive Developmental Disorders (PDDs).
Rates of SAD and trait levels of social anxiety are markedly elevated in children with autism spectrum disorders (ASDs) (16;17) and in their families (18;19). In fact, SAD symptoms are so common in children with ASDs that they may present a dilemma in differential diagnosis, especially in high-functioning children (20–22). One study found equally high levels of social anxiety symptom severity in children with ASD and comorbid SAD compared with children with ASD alone, leading the investigators to comment on the possibility that this finding may reflect overlapping etiologies between ASD and SAD (21). Several other authors have similarly commented on the high rates of impairing anxiety (84% in one study) (23) among children with ASDs, pointing to the possibility that phobic and social anxiety symptoms may be core symptoms of ASDs (22).
Interestingly, although associations between SAD and SM, and between SAD and ASDs have been widely recognized in the literature, little if any work has focused on a possible relationship between SM and ASDs. Although a diagnosis of ASD would supersede a diagnosis of SM according to DSM-IV, the common features of impaired social interaction and communication problems raise questions about possible shared pathophysiology and a partially shared genetic basis for these symptoms. A longitudinal community-based twin study concluded that autism-like communication deficits made a significant contribution to anxious “internalizing” traits (24), though genetic influences on these traits were largely independent (25). To our knowledge, no other studies have addressed this question experimentally, and none have specifically examined genetic covariation of social anxiety and autism-like traits.
A rare mutation at the contactin-associated protein-like 2 (CNTNAP2) locus was implicated in a syndrome including focal epilepsy, cortical dysplasia, and autism-spectrum-like symptoms in the Old Order Amish (26). Rare variants at this locus, the protein product of which is expressed in the developing human cortex, also occur at increased frequency in subjects with autism (27). Common polymorphisms at the same locus, which encodes a member of the neurexin superfamily, have recently been shown to be associated with increased susceptibility to ASDs (28;29) and, perhaps more precisely, to language impairment within ASDs (30). A pathway involving the FOXP2 transcription factor and its regulatory effect on CNTNAP2 has been suggested to provide a functional link between a variety of clinically distinct syndromes involving disrupted language (31;32). Interestingly, investigators have recently demonstrated a relationship between common genetic variants in CNTNAP2 and frontal lobar connectivity in autism, suggesting a mechanism by which the gene may act to influence risk (33).
In aggregate, these observations demonstrate a growing awareness among the research community that anxiety – perhaps especially social anxiety, although some studies note a broader relationship with internalizing symptoms (34) – is a common feature of ASDs. The extant literature also suggests the possibility that language and/or other communication deficits are a shared feature of ASDs and anxious/internalizing disorders, and may have at least a partially shared genetic basis. These observations led us to hypothesize and test for a possible genetic association of variation in CNTNAP2 with SM in a family-based association study of children with SM. We also examined the association of social anxiety-related traits with the same CNTNAP2 single nucleotide polymorphisms (SNPs) in a sample of young adults. The rationale for this second comparison was that if an association was found in the SM sample, then the finding of an association with these same SNPs and social anxiety-related traits could provide some clues about the nature of a partially shared etiology of SM, SAD, and ASDs.
METHODS
1. Selective Mutism Family-Based Study
The family-based samples described here overlap with those reported earlier (12), consisting of those subjects for whom DNA was available and to which several new trios were added. A nationwide sample was recruited through two sources: 1) a website sponsored by a non-profit organization for children with selective mutism (the Selective Mutism Group~Child Anxiety Network [SMG~CAN]), and 2) parent oriented conferences organized by this same non-profit group. The SMG~CAN website (http://www.selectivemutism.org/) receives approximately 500,000 hits per month from parents, professional and educators. Interested parents completed a consent-to-contact form and were thereafter sent study consents/child assents and contacted by telephone or consented in person if recruited from the conferences.
Measure
Those families that returned their consent forms were screened over the telephone by masters or doctoral level interviewers with the Selective Mutism (SM) module of the Anxiety Disorders Interview Schedule for Children Parent Report (ADIS-P/C) (35) and the Selective Mutism Questionnaire (SMQ), a measure of selective mutism severity (36;37). For a proband to be assigned a diagnosis of SM, parental report needed to indicate symptoms consistent with a lifetime diagnosis of SM for the child, and at least a moderate amount of impairment in one of the domains (i.e., speaking behaviors at home, school, and in other social situations) assessed by the SMQ. Screening questions to exclude children with psychotic, developmental or communication disorder diagnoses were also taken from the ADIS-P/C and augmented by supplemental questions to adequately rule out these conditions. Two of the authors (DAC and MBS) discussed all cases that were equivocal or excluded from the study. In those instances, where diagnoses remained questionable, one of the authors (ESB) – a child psychiatrist with extensive clinical experience with children with SM – was asked to review a videotape of the child speaking at home and/or to confer with another clinician who had clinically assessed the child to rule out the presence of a pervasive developmental disorder (PDD) or significant communication disorder; this was necessary for five cases and resulted in the exclusion of two cases.
Participating families received a $50 gift card from a national bookseller. All study procedures were approved by the Human Research Protection Program at the University of California San Diego (UCSD).
Participants
The participation of both biological parents and a child with SM between the ages of 3–11 were initial requirements for this study. Additional affected siblings (up to age 14) were included if available. The current sample was comprised of 99 nuclear families (parent-child trios) where SM in the proband had been diagnostically confirmed; among these, 5 families included an additional affected sibling and 1 family included 2 additional affected siblings. A total of 106 affected children were included of whom 67 (63.2%) were female and 39 (36.8%) were male. Mean age of the affected children was 6.8 (SD 2.5; range 3–14) years.
Sample Collection, Preparation and Genotyping
Salivary DNA was collected using Oragene kits (http://www.dnagenotek.com) that were mailed back to UCSD and subsequently transferred to Yale University School of Medicine where DNA was extracted and stored frozen and genotyping of five SNPs in CNTNAP2 was conducted. Custom genotyping assays (Applied Biosystems, Inc, [ABI] Foster City, CA) were used for TaqMan genotyping using the ABI PRISM 7900 Sequence Detection System. CNTNAP2 is a very large gene (2.3 megabases); we examined only a few markers in CNTNAP2 in order to limit the number of multiple comparisons made given our very limited sample size and power. Two of these SNPs were chosen because they were found to be associated with ASDs in previous studies: rs7794745 – either alone (28) or as part of a haplotype (29) (also recently shown to be associated with normal variation in brain circuitry and structure [(38)]) – and rs2710102 (as a tagging SNP [(30)]); the other 3 SNPs were chosen because of their proximity to these two SNPs.
Statistical Analysis
Allelic association of individual SNPs was examined using the Family-Based Association Test (FBAT) method (39;40) assuming an additive genetic model under the null hypothesis of no linkage and no association, diallelic mode, minimum number of informative families of 10 for each analysis and offset of zero. Haplotypic association was also calculated by FBAT with the ‘hbat’ command, which assumed a diallelic mode for haplotype-specific association and multi-allelic mode for global association to test all haplotypes as a whole. Under the null hypothesis of no linkage and no association, p-values for ‘hbat’ global association test in this study were computed using a permutation test (100,000 iterations). The significance level of haplotype-specific tests was adjusted using a Bonferroni correction, i.e., division by the number of major haplotypes with frequency greater than 5.0%.
It was decided a priori that only SNPs found to be associated with SM in the family-based analysis would be carried forward to the analysis of association with social anxiety-related traits in the young adult sample (below).
2. Young Adult (Undergraduate College Student) Study
Participants (N = 1028) were 732 female and 296 male (mean age 18.87 [95% CI 18.75 – 18.99] years) undergraduate psychology students at San Diego State University (SDSU). Subjects came for a scheduled appointment at which a blood sample (60 ml) was drawn for genetic studies and questionnaires were completed. Subjects gave informed, written consent to participate in the study, which was approved by the Human Research Protection Programs at both SDSU and UCSD. Subjects received $25 for providing the blood sample.
Measures
All subjects completed a panel of self-report measures relevant to the study of personality and anxiety-related traits. Measures that were examined for this report are:
Retrospective Self-Report of Inhibition (RSRI), a retrospective measure of behavioral inhibition in childhood, a heritable, early risk factor for (or indicator of) anxiety proneness (41;42). Total scores range from 30–150.
Social Interactional Anxiety Scale (SIAS), a unifactorial, widely used measure of social interactional anxiety (43). Scores range from 0–80.
Genotype Analysis
Genomic DNA was extracted from whole blood and genotyping of the CNTNAP2 SNP rs2710102 was conducted as described above.
Ancestral Proportion Scores
Subjects ancestries were estimated using a set of unlinked genetic markers by Bayesian cluster analysis, using the procedures and software developed by Pritchard and colleagues (http://pritch.bsd.uchicago.edu/software.html) (44–46). Pritchard’s software program STRUCTURE implements Bayesian cluster modeling that can recognize cryptic population genetic patterns without prior information of population origins. Data were submitted to the program STRUCTURE using 35 ancestry informative markers (AIMs) of short tandem repeat (STR) with models specified as “admixture” and “allele frequencies correlated” and 500,000 burn-in and 500,000 MCMC (Markov Chain Monte Carlo) iterations. The markers were the set of STRs described previously (47).
A three-cluster solution classified the 86.0% of self-identified European American (EA) subjects (N = 555) in this sample in complete concordance with their self-reported ethnicity. Within the EA sample, distribution of rs2710102 alleles was G 53.3% and A 46.7%, and genotype observations were consistent with no significant deviation from HWE expectations (chi-square = 1.69, df = 1, p = 0.19). Nearly identical allele frequencies for this marker are reported in Hapmap for CEU (http://www.hapmap.org): G, 52.7% and A, 47.3%.
Statistical Analyses
Analyses were conducted using the biostatistical analysis program STATA (Version 11) (48). Linear regression techniques in STATA (“rreg”) that are considered robust to outliers were used to model the relationship between CNTNAP2 rs2710102 genotype (coded as 0 for GG, 1 for AG, and 2 for AA) and SIAS and RSRI scores, adjusting for ancestry using two covariates (as described above) and sex. Logistic regression (adjusted for ancestry and sex) was also used to estimate odds of being > 1SD above the mean on each of these measures.
RESULTS
1. Selective Mutism Family-Based Study
Single SNP testing with FBAT (49) revealed one SNP (rs2710102) that was nominally (p = 0.018) associated with SM (Table 1). The risk allele for rs2710102 was the minor “a” allele, which had a frequency of 0.483 in this sample.
Table 1.
Markers studied in CNTNAP2 on Chromosome 7
| Marker | dbSNP | Chromosome Position | Distance* (bp) | MAF** | p-value |
|---|---|---|---|---|---|
| C1 | rs986062 | 146085354 | --- | 0.301 | 0.311 |
| C2 | rs10952659 | 146088800 | 3,446 | 0.283 | 0.553 |
| C3 | rs7794745 | 146120539 | 31,793 | 0.386 | 0.335 |
| C4 | rs6944808 | 146883481 | 762,942 | 0.328 | 0.588 |
| C5 | rs2710102 | 147205323 | 321,842 | 0.483 | 0.018 |
Distance from antecedent SNP in base-pairs.
MAF = Minor Allele Frequency
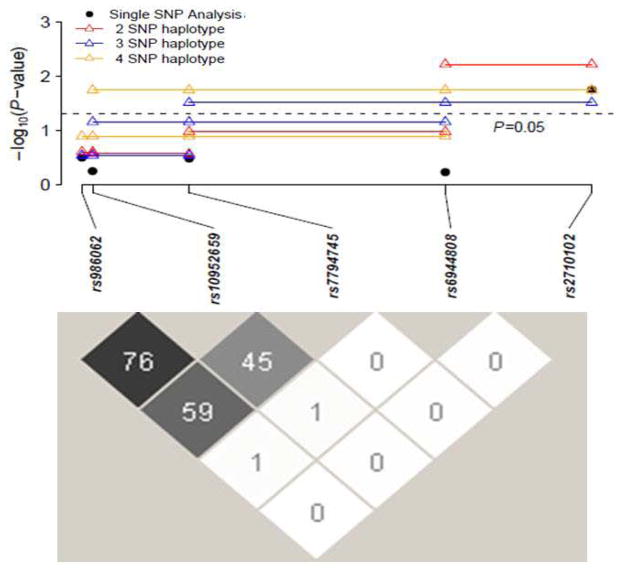
Haplotype analysis was conducted using a sliding window for various combinations of all 5 CNTNAP2 markers as shown in Table 2, in which the smallest p value for a haplotype-specific test within each haplotype analysis was listed in the table. The global association test was statistically significant (empirical p < 0.05) for the haplotype analyses of rs6944808 and rs2710102 (p = 0.022). Haplotypes containing the “a” allele for rs6944808 and the “a” allele for rs2710102 were all nominally (p < 0.05) significantly associated with SM, but after Bonferroni correction within each haplotype, only the haplotype consisting of exclusively these two SNPs was significantly (p = 0.006) associated with SM. These data are shown pictorially on a logarithmic scale (Figure 1), along with the extent of linkage disequilibrium (LD) between the chosen markers for the probands, expressed as D′.
Table 2.
Haplotype analysis by sliding window
| Markers in Haplotype | Marker Alleles | No. of Informative Families | freq | Z | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| C1 C2 | A | g | 10 | 0.042 | −1.16 | 0.248 | |||
| C2 C3 | g | t | 27 | 0.123 | −1.11 | 0.269 | |||
| C3 C4 | t | g | 35 | 0.123 | −1.62 | 0.105 | |||
| C4 C5 | a | a | 54 | 0.337 | 2.75 | 0.006* | |||
| C1 C2 C3 | G | g | a | 53 | 0.599 | 1.06 | 0.288 | ||
| C2 C3 C4 | g | t | g | 19 | 0.067 | −1.82 | 0.069 | ||
| C3 C4 C5 | a | a | a | 49 | 0.228 | 2.05 | 0.040 | ||
| C1 C2 C3 C4 | G | g | t | g | 15 | 0.05 | −1.53 | 0.127 | |
| C2 C3 C4 C5 | g | a | a | a | 46 | 0.219 | 2.37 | 0.018 | |
| C1 C2 C3 C4 C5 | G | g | a | a | a | 42 | 0.213 | 2.48 | 0.013 |
Significant after Bonferroni correction within each haplotype
Each haplotype-specific allele listed was the one with the smallest p-value among all the haplotypic alleles for each haplotype.
FIGURE 1.
Family Based Association of the Five CNTNAP2 SNPs in Selective Mutism and Linkage Disequilibrium Plot among the SNPs
There were no statistically significant parent-of-origin or sex-specific effects (See Supplementary Material).
2. Young Adult (Undergraduate College Student) Study
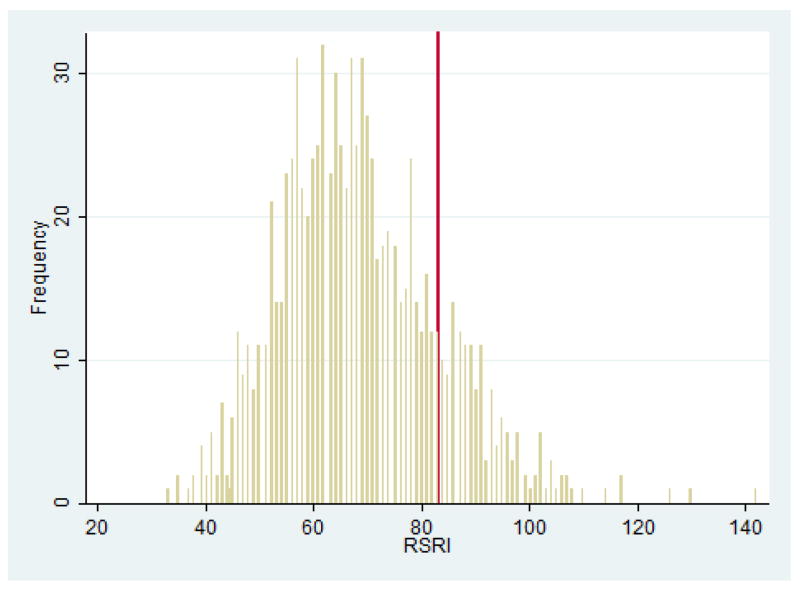
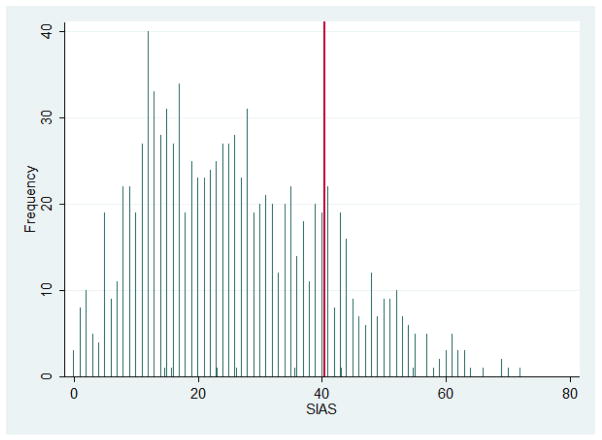
It had been decided a priori to only conduct analyses in the young adult sample with SNP(s) – if any – found to be significantly associated with SM in the family-based sample. As noted above, rs2710102 was the only SNP associated – as a single marker and as part of a haplotype – with SM, hence this was the only SNP tested for association with social anxiety-related traits in the young adult sample. Distribution of RSRI and SIAS scores in the sample are shown in Figures 2 and 3.
FIGURE 2.
Distribution of Retrospective Self-Report of Inhibition (RSRI) Scores in Student Sample. Vertical (red) line indicates 1 SD above the sample mean.
FIGURE 3.
Distribution of Social Interactional Anxiety Scale Scores (SIAS) in Student Sample. Vertical (red) line indicates 1 SD above the sample mean.
In linear regression analyses, using models adjusting for sex and for ancestral proportion scores (to reduce possibility of confounding by population stratification) each copy (0–2) of the risk allele (“a”) of rs2710102 was significantly associated with higher retrospective behavioral inhibition (RSRI) and current social interactional anxiety (SIAS) scores (Table 3).
Table 3.
Linear regression of CNTNAP2 (rs2710102) and social anxiety-related Traits
| rs2710102g | | Coef. | Std. Err. | t | P>|t| | [95% Conf Interval] |
|---|---|---|---|---|---|
| RSRI Total Score| | 1.424 | .686 | 2.08 | 0.038 | [.078 2.77] |
| SIAS Total Score | | 1.904 | .649 | 2.94 | 0.003 | [.632 3.18] |
Coefficients (beta) are adjusted for sex and two ancestry informative marker (AIM) cluster scores. Each copy (0–2) of the “a” allele is associated with increased RSRI and SIAS scores.
Expressed as the odds of being 1 standard deviation (SD) above the sample mean, in logistic regression analyses adjusting for ancestral proportion scores and sex, each copy of the risk allele (“a”) for rs2710102 was associated with an increase in the adjusted odds of being >1SD above the mean on RSRI (OR = 1.40, 95% CI 1.08–1.80, p = 0.010) and on SIAS (OR = 1.33, 95% CI 1.06–1.66, p = 0.015).
DISCUSSION
This is the first study, to the best of our knowledge, to look at genetic susceptibility factors for selective mutism (SM). We considered investigating genes that might be implicated in SM through its presumed etiological links with anxiety disorders such as Social Anxiety Disorder (SAD) (1;12) but no replicated susceptibility variants for SAD have yet been reported (50). Instead, we elected to capitalize on recent advances in autism genetics (51–53) and the possible relationship of autism spectrum disorders (ASDs) to SM in order to initiate our search for SM susceptibility genes.
We found that common variation in CNTNAP2 – a gene that has been implicated in autism (27–29) and, perhaps more specifically, in the developmental language delayed component of autism (30;31) as well as in various forms of specific language impairment (31;54) – is associated with risk for SM in a family-based sample and with social anxiety-related traits (behavioral inhibition and social anxiety) in a separate sample of young adults. Although our coverage of CNTNAP2 (one of the largest genes in the human genome, encompassing almost 1.5% of chromosome 7) was not intended to be comprehensive, one of the five SNPs genotyped – rs2710102, which had been selected on the basis of its recently reported association (as a tagging SNP) with language impairment in ASDs (30) – provided consistent evidence of association across these two samples. These dual findings of CNTNAP2 association with SM in a family-based sample and with social anxiety-related traits in an unrelated young adult sample can be considered reasonably strong evidence against the spuriousness of either finding alone. Nonetheless, strict replication of these findings in larger samples is very much needed, in concert with broad-based phenotypic assessment of disorder characteristics that can help resolve the specificity of these associations for particular developmental language and/or anxiety-related traits.
Our reliance on telephone screening interviews to confirm SM and to rule out PDD diagnosis is a limitation of this study. In-person clinician administered structured interviews, behavioral observations, and speech/language assessments are the gold standard for diagnostic assessment, but we were limited in this regard by the wide geographic distribution of our sample. All questionable screening interviews were reviewed for consensus by one of the authors (DAC), a licensed clinical psychologist, and a board-certified Psychiatrist (MBS). When diagnoses were still in doubt, videotapes provided by the family were reviewed by a child psychiatrist with special expertise in SM (ES-B). Children whose diagnostic status was unclear were not included in the study. Thus, while we believe that this very conservative approach to diagnosis offered some protection against misdiagnosis of PDD as SM, future attempts to replicate this work will optimally make use of standardized screening instruments (e.g., Autism Screening Questionnaire [ASQ] [(55)]) followed by in-person semi-structured interviews (e.g., Autism Diagnostic Observation Schedule [ADOS] [(56)]). Further, our samples were predominantly female (which is usually the case for SM), whereas an excess of males is virtually always observed in ASDs; future attempts to replicate this work should, ideally, include more males.
Several prior studies have examined childhood anxiety-related characteristics (e.g., shyness or behavioral inhibition) that could be considered central to SM (57). These studies found associations of shyness with 5HTTLPR, though contradictory findings about whether the short (“s”) allele increases or decreases risk have rendered this literature difficult to interpret (58;59). In contrast, evidence of association of the corticotropin releasing hormone gene (CRH) with behavioral inhibition (60) and with one of the anxiety disorders, panic disorder (61), is more compelling and suggests that genetic variation in CRH should be further evaluated. It is likely that SM is genetically complex, and that CNTNAP2 may interact with other genes (e.g., CRH) to influence expression of the phenotype. Another limitation of our work pertains to sparse coverage of CNTNAP2; future studies will want to genotype this gene, particularly the region around rs2710102, much more densely.
Current evidence strongly supports the idea that autism is not one disorder but, rather, a syndrome whose manifestations can vary. In this regard, one might hypothesize that different genes influence particular syndromal characteristics of ASDs (e.g., language deficits; social communication difficulties) (62). In this regard, CNTNAP2 and its pathway connections with FOXP2 have been considered most likely to underlie the developmental language problems in ASDs (31;32). Given that the central feature of SM is failure to speak (albeit in particular situations), and the numerous observations of subtle developmental language problems in at least a subset of children with SM (5;15), it would be reasonable to infer a partially shared genetic etiology for ASDs and SM through CNTNAP2 and its influence on language development that may underlie both conditions.
On the other hand, anxiety – especially social anxiety – is extremely common among children with ASDs. In one recent population-based study, SAD was found to be the most common comorbid psychiatric disorder at a prevalence of 29.2% in children with ASDs (17). Accordingly, it is possible that CNTNAP2 more directly influences the social anxiety component (rather than the language component) that ties together SM and many cases of ASDs. Given that the risk allele in CNTNAP2 SNP rs2710102 for language impairment appears to the “g” allele (31), our finding that the risk allele associated with higher social anxiety and higher ratings of childhood behavioral inhibition in young adults is the “a” allele, is more consistent with this latter hypothesis. This interpretation fits well with the recent observation that CNTNAP2 variants show a varying pattern of association with different autism traits, with the “a” allele of rs2710102 showing a positive association with “social inhibition” (63).
We can only speculate as to why the same SNP, but opposite alleles, might be associated with ASDs and SM, respectively. This was not a finding we had anticipated in designing the study. We can note, though, that we are aware of at least one other precedent for this opposite allele phenomenon: Numerous GWAS studies have shown variants that map to a chromosome 15 nicotinic receptor cluster influence risk for nicotine dependence. One rs16969968 allele was originally reported to be associated with nicotine dependence (64), but subsequently the opposite allele was shown (65) and since replicated (66) to be associated with cocaine dependence. Thus, we have another example where the same SNP, but opposite alleles, are associated with traits that are themselves highly comorbid within individuals. The biological explanation and phenotypic ramifications of this type of phenomenon remain to be determined.
At this juncture, it is premature to make strong inferences about the nature of the relationship between SM and ASDs on the basis of a common association with CNTNAP2 variation. Whereas a case can be made for tying together these childhood disorders by virtue of their common dysfunction in communication/language and/or social anxiety, CNTNAP2 has also been associated with other neuropsychiatric disorders such as schizophrenia (67), to which phenomenological ties are less obvious (though it should be noted that social anxiety symptoms are extremely common in schizophrenia [(68–70)]). However, the finding of CNTNAP2 emerging as a genetic risk factor for ASDs and schizophrenia, disorders which had previously been considered neurodevelopmentally distinct, provides new insights into shared pathogenic mechanisms that might not otherwise have been recognized. In fact, the finding of common genetic determinants of other “distinct” neuropsychiatric disorders such as schizophrenia and bipolar disorder (71) is forcing a rethinking of diagnostic conceptualizations that will be based on a better understanding of neurobiological pathways than our current nosological systems (72). In this regard, it is conceivable that genes that code for neurexin proteins such as CNTNAP2, which play a critical role in cell adhesion and neural differentiation (23), could have pleiotropic effects that extend across a broad array of traits and neurodevelopmental disorders including, perhaps, social anxiety, other internalizing traits and disorders, and SM.
Finally, these preliminary findings raise a cautionary flag about the merits of reclassifying SM under SAD in DSM-5. Although SM is frequently accompanied by social anxiety symptoms, it may be that there is considerable heterogeneity in the SM syndrome such that some forms – perhaps those with subtle, but measurable developmental language problems or other even less well-studied social communication deficits – are more closely allied with the ASD spectrum and its association with CNTNAP2. Although this initial study cannot address these possibilities, it does raise questions that should slow the call to judgment on the reclassification of SM under SAD in DSM-5 (14), pending further research. In the interim, clinicians are advised to be aware of potential language impairments and delays and to assess these appropriately when working with children with SM. Further, interventions that focus on improving language skills may be needed to augment the standard behavioral and anxiety management approaches to treatment (4).
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS AND FINANCIAL DISCLOSURE
The authors are grateful to Shadha Hami-Cissell MSW, Adrienne Means-Christensen PhD, Jack Maser PhD, and Sonya Norman PhD for conducting diagnostic interviews, to Sarah Sullivan at UCSD for having assisted with database management and sample storage and retrieval, and to Ann Marie Lacobelle and Greg Kay at Yale for excellent technical assistance. We appreciate the efforts of Selective Mutism Group~Child Anxiety Network (SMG~CAN) to publicize the study among their membership and help with recruitment. Many thanks to Matthew W. State MD, PhD, Yale Child Study Center, New Haven, CT for this thoughtful review of and comments on an earlier draft of this manuscript.
This study was supported in part by NIMH grant MH64122 to MBS, NIDA career development award K01 DA024758 to BZY, NIMH K01 MH072952 to DAC, a VA MERIT grant to JG, the VA Connecticut REAP and MIRECC, and by an unrestricted research grant from GlaxoSmithKline. None of the study funders had any role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Stein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergman RL, Piacentini J, McCracken JT. Prevalence and description of selective mutism in a school-based sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:938–946. doi: 10.1097/00004583-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Arie M, Henkin Y, Lamy D, Tetin-Schneider S, Apter A, Sadeh A, Bar-Haim Y. Reduced auditory processing capacity during vocalization in children with Selective Mutism. Biol Psychiatry. 2007;61:419–421. doi: 10.1016/j.biopsych.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Cohan SL, Chavira DA, Shipon-Blum E, Hitchcock C, Roesch SC, Stein MB. Refining the classification of children with selective mutism: a latent profile analysis. J Clin Child Adolesc Psychol. 2008;37:770–784. doi: 10.1080/15374410802359759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohan SL, Price JM, Stein MB. Suffering in silence: why a developmental psychopathology perspective on selective mutism is needed. J Dev Behav Pediatr. 2006;27:341–355. doi: 10.1097/00004703-200608000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Manassis K, Tannock R, Garland EJ, Minde K, McInnes A, Clark S. The Sounds of Silence: Language, Cognition, and Anxiety in Selective Mutism. J Am Acad Child Adolesc Psychiatry. 2007;46:1187–1195. doi: 10.1097/CHI.0b013e318076b7ab. [DOI] [PubMed] [Google Scholar]
- 6.McInnes A, Fung D, Manassis K, Fiksenbaum L, Tannock R. Narrative skills in children with selective mutism: an exploratory study. Am J Speech Lang Pathol. 2004;13:304–315. doi: 10.1044/1058-0360(2004/031). [DOI] [PubMed] [Google Scholar]
- 7.Steinhausen H-C, Juzi C. Elective mutism: An analysis of 100 cases. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:606–614. doi: 10.1097/00004583-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Black B, Uhde TW. Elective mutism as a variant of social phobia. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;31:1090–1094. doi: 10.1097/00004583-199211000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Dummit ESI, Klein RG, Tancer NK, Asche B, Martin J, Fairbank JA. Systematic assessment of 50 children with selective mutism. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:653–660. doi: 10.1097/00004583-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 11.Carbone D, Schmidt LA, Cunningham CC, McHolm AE, Edison S, St Pierre J, Boyle MH. Behavioral and socio-emotional functioning in children with selective mutism: A comparison with anxious and typically developing dhildren across multiple informants. J Abnorm Child Psychol. 2010;38:1057–1067. doi: 10.1007/s10802-010-9425-y. [DOI] [PubMed] [Google Scholar]
- 12.Chavira DA, Shipon-Blum E, Hitchcock C, Cohan S, Stein MB. Selective mutism and social anxiety disorder: all in the family? J Am Acad Child Adolesc Psychiatry. 2007;46:1464–1472. doi: 10.1097/chi.0b013e318149366a. [DOI] [PubMed] [Google Scholar]
- 13.Gelernter J, Stein MB. Heritability and genetics of anxiety disorders. In: Antony MM, Stein MB, editors. Handbook of Anxiety Disorders. New York: Oxford University Press; 2009. pp. 87–96. [Google Scholar]
- 14.Bogels SM, Alden L, Beidel DC, et al. Social anxiety disorder: questions and answers for the DSM-V. Depress Anxiety. 2010;27:168–189. doi: 10.1002/da.20670. [DOI] [PubMed] [Google Scholar]
- 15.Viana AG, Beidel DC, Rabian B. Selective mutism: a review and integration of the last 15 years. Clin Psychol Rev. 2009;29:57–67. doi: 10.1016/j.cpr.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila ML, Ebeling H, et al. Social anxiety in high-functioning children and adolescents with Autism and Asperger syndrome. J Autism Dev Disord. 2008;38:1697–1709. doi: 10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- 17.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 18.Smalley SL, McCracken J, Tanguay P. Autism, affective disorders, and social phobia. Am J Med Gen. 1995;60:19–26. doi: 10.1002/ajmg.1320600105. [DOI] [PubMed] [Google Scholar]
- 19.Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. Am J Psychiatry. 1999;156:557–563. doi: 10.1176/ajp.156.4.557. [DOI] [PubMed] [Google Scholar]
- 20.Hartley SL, Sikora DM. Which DSM-IV-TR criteria best differentiate high-functioning autism spectrum disorder from ADHD and anxiety disorders in older children? Autism. 2009;13:485–509. doi: 10.1177/1362361309335717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cath DC, Ran N, Smit JH, van Balkom AJ, Comijs HC. Symptom overlap between autism spectrum disorder, generalized social anxiety disorder and obsessive-compulsive disorder in adults: a preliminary case-controlled study. Psychopathology. 2008;41:101–110. doi: 10.1159/000111555. [DOI] [PubMed] [Google Scholar]
- 22.Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, et al. Parent-rated anxiety symptoms in children with pervasive developmental disorders: frequency and association with core autism symptoms and cognitive functioning. J Abnorm Child Psychol. 2008;36:117–128. doi: 10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- 23.O'Dushlaine C, Kenny E, Heron E, Donohoe G, Gill M, Morris D, et al. Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Hallett V, Ronald A, Rijsdijk F, Happe F. Association of autistic-like and internalizing traits during childhood: a longitudinal twin study. Am J Psychiatry. 2010;167:809–817. doi: 10.1176/appi.ajp.2009.09070990. [DOI] [PubMed] [Google Scholar]
- 25.Hallett V, Ronald A, Happe F. Investigating the association between autistic-like and internalizing traits in a community-based twin sample. J Am Acad Child Adolesc Psychiatry. 2009;48:618–627. doi: 10.1097/CHI.0b013e31819f7116. [DOI] [PubMed] [Google Scholar]
- 26.Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 27.Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arking Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Hu Z, He Y, Xiong Z, Long Z, Peng Y, et al. Association analysis of CNTNAP2 polymorphisms with autism in the Chinese Han population. Psychiatr Genet. 2010;20:113–117. doi: 10.1097/YPG.0b013e32833a216f. [DOI] [PubMed] [Google Scholar]
- 30.Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher SE, Scharff C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009;25:166–177. doi: 10.1016/j.tig.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, et al. Altered functional connectivity in frontal lobe circuits Is associated with variation in the autism risk gene CNTNAP2. Sci Transl Med. 2010;2:56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pine DS, Guyer AE, Goldwin M, Towbin KA, Leibenluft E. Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:652–661. doi: 10.1097/CHI.0b013e31816bffa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman WK, Saavedra LM, Pina AA. Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent versions. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:937–944. doi: 10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Bergman RL, Keller ML, Piacentini J, Bergman AJ. The development and psychometric properties of the selective mutism questionnaire. J Clin Child Adolesc Psychol. 2008;37:456–464. doi: 10.1080/15374410801955805. [DOI] [PubMed] [Google Scholar]
- 37.Letamendi AM, Chavira DA, Hitchcock CA, Roesch SC, Shipon-Blum E, Stein MB. Selective Mutism Questionnaire: Measurement Structure and Validity. J Am Acad Child Adolesc Psychiatry. 2008;47:1197–1204. doi: 10.1097/CHI.0b013e3181825a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan GC, Doke TF, Ashburner J, Wood NW, Frackowiak RS. Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. Neuroimage. 2010;53:1030–1042. doi: 10.1016/j.neuroimage.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype-phenotype associations. European Journal of Human Genetics. 2001;9:301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- 40.Horvath S, Xu X, Lake SL, Silverman EK, Weiss ST, Laird NM. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet Epidemiol. 2004;26:61–69. doi: 10.1002/gepi.10295. [DOI] [PubMed] [Google Scholar]
- 41.Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral inhibition and their relation to adult mental health. Developmental Psychopathology. 1992;4:301–321. [Google Scholar]
- 42.Robinson JL, Kagan J, Reznick JS, Corley RP. The heritability of inhibited and uninhibited behavior: A twin study. Developmental Psychology. 1992;28:1030–1037. [Google Scholar]
- 43.Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav Res Ther. 1998;36:455–470. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- 44.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pritchard JK, Stephens M, Rosenberg NA, Donnely P. Association mapping in structured populations. Am J Hum Genet. 2000;67:170–181. doi: 10.1086/302959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: Characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol. 2005;28:302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
- 48.StataCorp. Stata Statistical Software Version 11. College Station, TX: Stata Corporation; 2009. [Google Scholar]
- 49.Lange C, Laird NM. Power calculations for a general class of family-based association tests: dichotomous traits. Am J Hum Genet. 2002;71:575–584. doi: 10.1086/342406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smoller JW, Gardner-Schuster E, Covino J. The genetic basis of panic and phobic anxiety disorders. Am J Med Genet C Semin Med Genet. 2008;148:118–126. doi: 10.1002/ajmg.c.30174. [DOI] [PubMed] [Google Scholar]
- 51.Geschwind DH. Autism: many genes, common pathways? Cell. 2008;135:391–395. doi: 10.1016/j.cell.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar RA, Christian SL. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:188–197. doi: 10.1007/s11910-009-0029-2. [DOI] [PubMed] [Google Scholar]
- 53.Weiss LA, Arking DE, Daly MJ, Chakravarti A Gene Discovery Project of Johns Hopkins & the Autism Consortium. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newbury DF, Fisher SE, Monaco AP. Recent advances in the genetics of language impairment. Genome Med. 2010;2:6. doi: 10.1186/gm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 56.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 57.Stein MB, Gelernter J, Smoller JW. Genetic Aspects of Social Anxiety and Related Traits. In: Bandelow B, Stein DJ, editors. Social Anxiety Disorder: More than Shyness. New York, NY: Marcel Dekker, Inc; 2004. pp. 197–214. [Google Scholar]
- 58.Arbelle S, Benjamin J, Golin M, Kremer I, Belmaker RH, Ebstein RP. Relation of shyness in grade school children to the genotype for the long form of the serotonin transporter promoter region polymorphism. Am J Psychiatry. 2003;160:671–676. doi: 10.1176/appi.ajp.160.4.671. [DOI] [PubMed] [Google Scholar]
- 59.Battaglia M, Ogliari A, Zanoni A, Fagnani C, Partiarca V, Cirrincione R, et al. Influence of the serotonin transporter promoter gene and shyness on children’s cerebral responses to facial expressions. Arch Gen Psychiatry. 2005;62:85–94. doi: 10.1001/archpsyc.62.1.85. [DOI] [PubMed] [Google Scholar]
- 60.Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, et al. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- 61.Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, et al. The Corticotropin-Releasing Hormone Gene and Behavioral Inhibition in Children at Risk for Panic Disorder. Biol Psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steer CD, Golding J, Bolton PF. Traits contributing to the autistic spectrum. PLoS One. 2010;5(9):e12633. doi: 10.1371/journal.pone.0012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bierut LJ, Stitzel JA, Wang JC Hinrichs AL, Frucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, et al. Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology. 2010;35:1921–1931. doi: 10.1038/npp.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedman JI, Vrijenhoek T, Markx S, Janseen IM, van der Vliet WA, Faas BH, et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- 68.Pallanti S, Quercioli L, Hollander E. Social anxiety in outpatients with schizophrenia: a relevant cause of disability. Am J Psychiatry. 2004;161(1):53–58. doi: 10.1176/appi.ajp.161.1.53. [DOI] [PubMed] [Google Scholar]
- 69.Lysaker PH, Hammersley J. Association of delusions and lack of cognitive flexibility with social anxiety in schizophrenia spectrum disorders. Schizophr Res. 2006;86:147–153. doi: 10.1016/j.schres.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 70.Voges M, Addington J. The association between social anxiety and social functioning in first episode psychosis. Schizophr Res. 2005;76:287–292. doi: 10.1016/j.schres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cross-Disorder Phenotype Group of the Psychiatric GWAS Consortium. Craddock N, Kendler K, Neale M, Nurnberger J, Purcell S, et al. Dissecting the phenotype in genome-wide association studies of psychiatric illness. Br J Psychiatry. 2009;195:97–99. doi: 10.1192/bjp.bp.108.063156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.