Abstract
Mitral valve closure may be aided by contraction of anterior leaflet (AL) cardiac myocytes located in the annular third of the leaflet. This contraction, observed as a stiffening of the annular region of the AL during isovolumic contraction (IVC), is abolished by beta-blockade (βB). Sub-threshold rapid pacing in the region of aorto-mitral continuity (STIM) also causes AL stiffening, although this increases the stiffness of the entire leaflet during both IVC and isovolumic relaxation (IVR). We investigated whether these contractile events share a common pathway or whether multiple AL contractile mechanisms may be present. Ten sheep had radiopaque-markers implanted: 13 silhouetting the LV, 16 on the mitral annulus, an array of 16 on the AL, and one on each papillary muscle tip. 4-D marker coordinates were obtained from biplane videofluoroscopy during control (C), βB (esmolol) and during βB+STIM. Circumferential and radial stiffness values for three AL regions (Annular, Belly, and free-Edge), were obtained from inverse finite element analysis of AL displacements in response to trans-leaflet pressure changes during IVC and IVR. βB+STIM increased stiffness values in all regions at both IVC and IVR by 35±7% relative to βB (p<0.001). Thus, even when AL myocyte contraction was blocked by βB, STIM stiffened all regions of the AL during both IVC and IVR. This demonstrates the presence of at least two contractile systems in the AL; one being the AL annular cardiac muscle, involving a β-dependent pathway, others via a β-independent pathway, likely involving valvular interstitial cells and/or AL smooth muscle cells.
Keywords: Mitral Valve, Finite Element Analysis, Heterogeneity, Anisotropy, Elastic Modulus
1. INTRODUCTION
In a landmark paper more than 40 years ago, Sonnenblick et al. demonstrated that the anterior mitral valve leaflet (AL) contains active contractile tissue, capable of developing force and thereby stiffening the isolated AL in response to electrical pulse stimulation (Sonnenblick et al., 1967). Subsequent studies in the beating heart have suggested that such AL contraction and stiffening may aid valve closure (Timek et al., 2003) as well as maintain the compound AL shape (Karlsson et al., 1998) in the face of changing cardiac demands which may aid leaflet coaptation as well as provide stable left ventricular (LV) outflow tract geometry.
Recently, we have shown that the AL can exhibit two contractile responses in the beating heart. Itoh et al. demonstrated AL stiffening during isovolumic contraction (IVC), which could be completely abolished by systemic β-adrenergic blockade (Itoh et al., 2009). They also demonstrated that AL stiffness could be increased during both IVC and isovolumic relaxation (IVR) by rapid, sub-threshold electrical stimulation (STIM) in the region of aorto-mitral continuity (also known as the “saddlehorn” region), but IVC stiffness did not change relative to IVR. In that experiment, however, STIM was not added to β-adrenergic blockade and thus they could not determine whether these contractile events shared a common pathway or whether multiple AL contractile mechanisms might be present. Krishnamurthy et al. extended the work of Itoh et al. (Itoh et al., 2009) by measuring stiffness in three separate AL regions –Annular, Belly, and Edge (Krishnamurthy et al., 2009b). They found similar patterns of leaflet stiffening during IVC and relaxation during IVR in all three regions during normal beats. Systemic β-blockade, however, completely abolished Annular IVC stiffening, but did not affect IVR stiffness in any AL region.
We currently know, then, that the AL stiffens during each IVC period of the cardiac cycle and that electrical pulse stimulation can increase overall leaflet stiffness. But we don’t know, from experiments to date, whether these responses arise from a single contractile system, or more than one.
The purpose of the present study, therefore, was to test whether the two contractile (stiffening) responses observed by Itoh et al. (Itoh et al., 2009) share a common underlying pathway, utilizing a common contractile mechanism, or whether more than one contractile system may be functioning. The rationale for the experiment described herein was to block one force-developing pathway with β-blockade, then sub-threshold STIM the saddlehorn region. If leaflet force development could still be altered by electrical stimulation of the β-blocked leaflet, this would provide evidence for more than one contractile system in the AL of the beating ovine heart.
2. METHODS
The methodology used in the present study has been previously described (Krishnamurthy et al., 2008; Krishnamurthy et al., 2009b), although the data presented here are from a new study designed to specifically address this issue.
Data Acquisition
Ten sheep (49±6 kg) had radiopaque markers surgically implanted, with the aid of cardiopulmonary bypass (CPB 135±8.4 mins; cross-clamp 81±10.3 mins), to delineate the left heart and mitral valve. Thirteen markers were implanted to silhouette the LV chamber in each heart, one marker on each papillary muscle tip, 16 around the mitral annulus, 16 on the atrial aspect of the anterior MV leaflet, and one on the central edge of the middle scallop of the posterior mitral leaflet (Figure 1). Echocardiography was performed after separation from CPB and no valve or segmental wall motion abnormalities were identified in any animal. In the catheterization laboratory, approximately 2 hours after recovery from bypass and under open-chest conditions, videofluoroscopic images (60 frames/sec) of all markers were acquired with the heart in normal sinus rhythm and ventilation transiently arrested at end expiration. Left atrial pressure (LAP), left ventricular pressure (LVP), and aortic pressure (AoP) were continuously measured by catheter-tip manometers for all conditions. Data were obtained before (C, Control), after β-blockade with esmolol (βB, 30–60mcg/KG/min infusion to reach 20% reduction in LV ESP) and during rapid, sub-threshold, electrical stimulation of the aorto-mitral continuity (STIM, 320min−1, 1.7±0.9-mA) with ongoing esmolol infusion (βB+STIM). Once the animals were in the catheterization laboratory, no pharmacologic contractility agents were used. Marker coordinates from each view were then merged to yield the 3D coordinates of the centroid of each marker in each frame (Daughters et al., 1989; Niczyporuk and Miller, 1991).
Figure 1.
Radiopaque Marker Array (Not shown: marker on the middle scallop of the posterior mitral leaflet; papillary muscle tip markers.)
Three consecutive beats in sinus rhythm were selected for analysis for all three runs (C, βB, βB +STIM) in each heart. For each of these beats, matched pressure intervals during IVC and IVR were identified using LAP, LVP and leaflet edge marker separation, as previously described (Itoh et al., 2009; Krishnamurthy et al., 2009b).
Inverse Finite Element Analysis
The inverse finite element analysis methodology to determine the material properties of the anterior mitral valve leaflet has been previously described in detail (Krishnamurthy et al., 2008; Itoh et al., 2009; Krishnamurthy et al., 2009b), thus will only briefly be described here.
Finite element models of the AL were individually developed for both IVC and IVR intervals for each of the 30 beats analyzed. The geometry of the AL was initially defined by the 3-D coordinates of the leaflet markers fit with a bi-cubic leaflet surface. The material model of the leaflet was assumed to be orthotropic linear elastic (Krishnamurthy et al., 2009a). The leaflet surface was divided into three regions: Annular, Belly and Edge, as defined by the markers (Figure 2) with each region having two independent material properties (radial elastic modulus (Erad) and circumferential elastic modulus (Ecirc). Two separate shells were used to define the varying thickness of the leaflet regions using data obtained from our histological study of an anterior leaflet from a representative ovine heart. The surface fit of the MV leaflet was then meshed with 2-D plane-stress quadrilateral shell elements. The strut chordae were defined as structures undergoing pure tension. A previously published ex vivo modulus (elastic modulus = 20 N/mm2; cross sectional area = 0.008 cm2) was used for the strut chordae (Kunzelman and Cochran, 1992). Tension-only bar elements were defined as radiating from the papillary muscle tip marker points to leaflet belly insertion positions.
Figure 2.
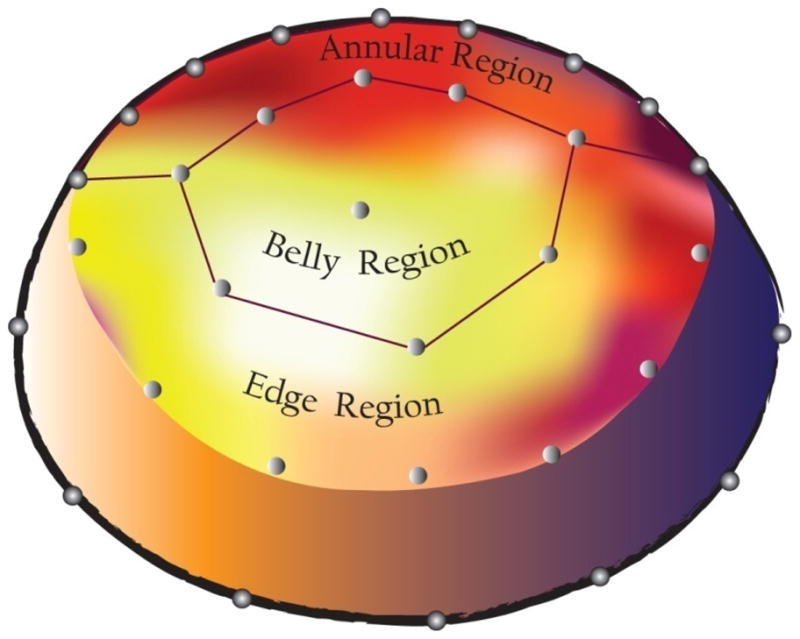
Schematic of the heterogeneous leaflet model showing the anterior mitral leaflet markers delineating the Annular, Belly and Edge leaflet regions.
The boundary conditions (pressures, displacements) were then enforced on the finite element models. The displacements of the annular markers, anterior leaflet free-edge markers and papillary tip markers (Figure 1) were defined using actual marker coordinates and these annular and papillary muscle dynamics are incorporated in the model. Measured leaflet edge displacement boundary conditions account for the effect of marginal chords in the model and changes in coaptation between the anterior and posterior leaflets under the different experimental conditions. The strut chords in the model originate from measured papillary tip positions and are inserted into the anterior leaflet at positions determined from post operative anatomical photographs.
As previously described (Krishnamurthy et al., 2009b), repeated iterative refinement algorithms for the 6 material properties in the 3 AL regions were used to obtain the minimum error between the model-derived and experimentally-derived AL displacements. The values obtained at convergence were taken as the best estimates of the material properties of the three leaflet regions.
Statistical Analysis
All values are given as group mean ± 1 standard deviation (SD). Three-beat averages were used to characterize the data for each animal. Data were compared using Student’s paired t-test (Microsoft Office Excel 2007, Microsoft Corp., Bellingham, WA). Bonferoni correction for multiple comparisons was performed and statistical significance was set at p<0.025.
3. RESULTS
Table 1 shows the expected hemodynamic effects of beta-blockade (βB) on the LV with the decrease in chronotropy and inotropy resulting in increased end-systolic volume and marked decreases in end-systolic pressure and maximum rate of pressure rise (dLVP/dt-max) during IVC. In accordance with experimental design, sub-threshold electrical stimulation in the region of aorto-mitral continuity (STIM) in combination with beta-blockade (βB +STIM) resulted in no significant change in hemodynamic parameters from those measured during βB. Left atrial pressure tracings did not reveal evidence of significant mitral regurgitation in any animal during the study.
Table 1.
Hemodynamics
| Heart Rate | dLVP/dt | ESV | EDV | ESP | EDP | SV | |
|---|---|---|---|---|---|---|---|
| C | 94±10 | 1371±318 | 110±16 | 144±16 | 80±4 | 11±5 | 34±8 |
| βB | 80 ±8 | 753 ±185 | 115 ±17 | 146 ±14 | 63 ±8 | 14 ±6 | 31 ±7 |
| t-test (C vs. βB) | <0.001 | <0.001 | 0.012 | 0.59 | <0.001 | 0.17 | 0.17 |
| βB +STIM | 78 ±9 | 769 ±165 | 115 ±16 | 146 ±14 | 64 ±7 | 13 ±4 | 31 ±7 |
| t-test (C vs. βB +STIM) | <0.001 | <0.001 | 0.05 | 0.59 | <0.001 | 0.07 | 0.27 |
| t-test (βB vs. βB +STIM) | 0.24 | 0.45 | 0.33 | 0.88 | 0.77 | 0.48 | 0.43 |
C—Control; βB —Beta-blocked (intravenous esmolol); βB +STIM— βB with saddle-horn electrical stimulation; HR—Heart Rate (beats/min); LV—Left Ventricular; ESV—LV End Systolic Volume (ml); EDV—LV End Diastolic Volume (ml); ESP—LV End Systolic Pressure (mmHg); EDP—LV End Diastolic Pressure (mmHg); SV—Stroke Volume=EDV-ESV (ml). p<0.025 significant.
Table 2 shows annular dynamics, where βB increased annular septal-lateral and total annular area from C throughout the cardiac cycle, while leaving the commissure-commissure dimension unchanged. Importantly, βB +STIM did not result in a significant change in annular dimensions or area compared with βB alone.
Table 2.
Annular Dynamics
| MAA—ED | MAA—ES | S-L—ED | S-L—ES | C-C—ED | C-C—ES | |
|---|---|---|---|---|---|---|
| C | 13.9±2.2 | 13.4±2.1 | 2.56±0.4 | 2.38±0.3 | 3.41±0.2 | 3.36±0.3 |
| βB | 16.0±2.0 | 15.1±1.5 | 2.80±0.3 | 2.56±0.2 | 3.63±0.5 | 3.58±0.5 |
| t-test (C vs. βB) | 0.01 | 0.01 | <0.001 | 0.002 | 0.15 | 0.16 |
| βB +STIM | 16.0±2.0 | 15.1±0.4 | 2.80±0.3 | 2.57±0.2 | 3.64±0.5 | 3.58±0.5 |
| t-test (C vs. βB +STIM) | 0.01 | 0.01 | <0.001 | 0.002 | 0.15 | 0.17 |
| t-test (βB vs. βB +STIM) | 0.44 | 0.86 | 0.75 | 0.50 | 0.87 | 1 |
C—Control; βB —Beta-blocked (esmolol); βB +STIM— β-blocked (esmolol) with saddle-horn electrical stimulation. MAA—Mitral Annular Area (cm2); S-L—Septal Lateral Dimension (cm); C-C—Commissure-Commissure Dimension (cm); ED—End-diastole; and, ES—End-systole. p<0.025 significant.
Table 3 shows group mean (SD) regional anterior mitral leaflet stiffness values for C, βB, and βB+STIM and Figure 3 depicts these group mean values in graphical form. We have previously shown that leaflet stiffness does not vary with systolic pressure (Krishnamurthy et al., 2009a; Itoh et al., 2009), thus the reduction in pressure from βB should not affect these results.
Table 3.
Valve Stiffness (N/mm2)
| Isovolumic Contraction (IVC) | Isovolumic Relaxation (IVR) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annulus | Belly | Edge | Annulus | Belly | Edge | |||||||
| Ecirc | Erad | Ecirc | Erad | Ecirc | Erad | Ecirc | Erad | Ecirc | Erad | Ecirc | Erad | |
| C | 60±14 | 19±4 | 46±12 | 13±4 | 53±13 | 16±4 | 46±12 | 13±3 | 35±11 | 9±3 | 40±11 | 11±3 |
| % IVR vs. IVC | 131 | 147 | 132 | 154 | 133 | 150 | ||||||
| t-test IVR vs. IVC | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| βB | 45±12 | 13±3 | 41±11 | 11±3 | 53±13 | 16±4 | 45±13 | 13±3 | 35±10 | 9±3 | 40±11 | 11±2 |
| % IVR vs. IVC | 99 | 97 | 116 | 129 | 132 | 149 | ||||||
| t-test IVR vs. IVC | 0.68 | 0.34 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| % C vs. βB | 75 | 65 | 87 | 85 | 99 | 100 | 100 | 99 | 99 | 101 | 100 | 100 |
| t-test C vs. βB | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.80 | 0.75 | 0.75 | 0.56 | 1 | 1 |
| βB +STIM | 57±15 | 18±5 | 53±14 | 15±4 | 67±14 | 22±5 | 58±14 | 18±5 | 47±12 | 13±3 | 52±13 | 16±4 |
| % IVR vs. IVC | 99 | 101 | 112 | 116 | 129 | 140 | ||||||
| t-test IVR vs. IVC | 0.95 | 0.76 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| % C vs. βB +STIM | 96 | 91 | 114 | 114 | 126 | 135 | 127 | 134 | 134 | 152 | 131 | 145 |
| t-test C vs. βB +STIM | 0.04 | 0.10 | <0.001 | 0.02 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| % βB vs. βB +STIM | 128 | 140 | 131 | 134 | 127 | 140 | 128 | 135 | 135 | 149 | 131 | 145 |
| t-test βB vs. βB +STIM | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
C—Control; βB—Beta-blocked; βB +STIM— β-blocked with STIM; IVR—isovolumic relaxation period of the cardiac cycle; IVC—isovolumic contraction period of the cardiac cycle. Ecirc—circumferential stiffness (N/mm2); Erad—radial stiffness (N/mm2). See Figure 2 for locations of Annulus, Belly, Edge. p<0.025 significant.
Figure 3.
Group mean anterior leaflet circumferential (Ec) and radial (Er) stiffness values during isovolumic contraction (IVC, open symbols) and isovolumic relaxation (IVR, filled symbols) for the three leaflet regions (Annulus, Belly and Edge) for Control C, β-blockade (βB), and β-blockade plus STIM (βB+S).
The findings from C and βB from our previous study (Krishnamurthy et al., 2009b) were reproduced in the present study, demonstrating the robustness of those findings. AL anisotropy (Ecirc > Erad) was observed in all experimental runs. Control AL stiffness also demonstrated the same pattern of stiffening throughout the leaflet (Annular, Belly and Edge regions) with the onset of each ventricular systole (IVC). Relative to C, βB IVR values were unchanged (p=NS), Annular IVC stiffness was reduced to the IVR value (p=ns), leaflet belly IVC stiffness was slightly reduced (p<0.001), and the edge remained unaffected (p<0.001).
The new intervention in this study, application of sub-threshold saddlehorn electrical stimulation in the setting of beta-blockade (βB+STIM), resulted in an elevation in leaflet stiffness in all AL regions by an average of 35±7% (all p<0.001). BB+STIM did not, however, abolish the relationship between IVC and IVR stiffness resulting from βB alone: although total stiffness rose in all regions, IVC stiffness remained equal to IVR stiffness in the Annular region (p=0.94 circumferential; p=0.76 radial), while the difference between IVC and IVR stiffness was preserved from βB to βB+STIM in the Belly and Edge regions (p<0.001).
4. DISCUSSION
This study shows that the two contractile events shown previously by Itoh et al. (Itoh et al., 2009) are almost certainly due to separate contractile systems. When IVC stiffening, shown as IVC > IVR in the C run, is abolished with β-blockade (completely in the Annular region, partially in the Belly region), the increase in stiffness due to electrical stimulation (STIM) is unperturbed and increases both IVC and IVR AL stiffness equally. This study also demonstrates that these two contractile events use different pathways to alter leaflet stiffening; one (IVC stiffening) that is dependent on a β-adrenergic pathway and the other (STIM induced AL stiffening) that is not.
AL Annular and Belly IVC Stiffening
There is considerable evidence supporting AL cardiac myocytes as the basis for IVC annular stiffening. Histologic studies across species, including human and ovine, show cardiac myocytes extending into the AL from the annulus and terminating in the leaflet belly (Boucek et al., 1978). These cells share similar properties to left atrial myocytes (Sonnenblick et al., 1967; Fenoglio et al., 1972) and have been shown to be sensitive to β-agonists (Sonnenblick et al., 1967). Wit et al. showed that the cardiac conduction system leads to depolarization of the AL (Wit et al., 1973). AL cardiac myocytes are capable of responding to propagated depolarization. Fenoglio et al. (Fenoglio et al., 1972) showed that these cells are capable of stiffening briefly (~200ms) with the onset of each ventricular systole (IVC), thus they would be in a relaxed state by the end of systole (IVR).
The administration of systemic β-adrenergic blockade (βB) resulted in a loss of IVC annular stiffening and a moderate reduction in belly stiffness, as shown previously by Krishnamurthy et al. (Krishnamurthy et al., 2009b). This is consistent with the distribution of cardiac myocytes in the AL. It is interesting, however, to note that β-blockade resulted in complete abolishment of IVC Annular stiffening in this and previous studies, though at the same systemic dose it results in only a decrease in the inotropic state of the myocardium. Future in vitro studies may be able to elucidate why this may be.
AL Edge IVC Stiffening
Stiffening of the edge of the AL during IVC cannot be attributed to contraction of cardiac myocytes, as they are not present in this region. βB did not affect IVC stiffening in this region. Krishnamurthy et al. (Krishnamurthy et al., 2009b) has published evidence supporting the hypothesis that geometric stiffening results in the observed edge stiffening; viz. the edge of the anterior leaflet curls when the anterior and posterior leaflet just make contact during IVC, thus decreasing its radius of curvature and increasing its effective stiffness. Interestingly, however, sub-threshold STIM pulses applied to the β-blocked leaflet, greatly increased edge stiffness, demonstrating that the edge region, indeed, contains contractile tissues.
IVR Electrically Induced Stiffening
Itoh et al. (Itoh et al., 2009) used a homogenous finite-element model to show that sub-threshold electrical stimulation of the aorto-mitral continuity resulted in stiffening of the AL during IVC and IVR. This study prompted several questions: Is this electrically induced stiffening taking place throughout the leaflet or in a specific region? Is the stiffening occurring throughout the cardiac cycle in combination with IVC stiffening (two separate stiffening mechanism) or is it responsible for both IVC stiffening and the elevation in IVR leaflet tone (one single mechanism)? Does it share a common pathway with IVC stiffening that uses β-agonism to modulate leaflet stiffening?
The current study addresses these questions. STIM resulted in a mean elevation in stiffness during IVC and IVR of 37.5% that was quite consistent at both time points in the cardiac cycle where stiffness can be measured and occurs in all three regions of the leaflet in the presence of β-adrenergic blockade (Table 3). Therefore at least two separate stiffening mechanisms must be active. Further, the STIM stiffening pathway is not dependent on a β-adrenergic agonist and thus must use a different mechanism.
Valvular interstitial cell (VIC) contraction (Filip et al., 1986; Smith et al., 2007), perhaps in conjunction with leaflet smooth muscle cell (SMC) contraction (Wit et al., 1979; De Biasi et al., 1984), most likely accounts for the second contractile event observed in response to STIM in the presence of βB. The rise in IVR stiffness observed in the current study is consistent with the STIM findings of Itoh et al. (Itoh et al., 2009), where no βB was present. The results of the current study using a heterogeneous finite-element model of the AL further advance the findings of Itoh et al., revealing that STIM induces a rise in leaflet stiffness in all three regions. VICs are ubiquitous and present throughout the leaflet and could account for this leaflet stiffening.
Electrical stimulation of the neurally rich aorto-mitral continuity resulted in a nearly immediate rise in leaflet stiffness in all regions, independent of a β-agonist. Histologic examination of VICs has shown that motor neuron termini are present throughout the leaflet in close association with VICs (Tsumori and Domoto, 1994). Also, VICs have numerous cell-cell communication junctions that likely allow a contractile stimulus to be carried from cell-to-cell (Filip et al., 1986). Potentially, STIM results in direct neural stimulation of the network of VICs that extends throughout the leaflet and cell contraction. This, however, is conjecture and further studies will be needed to conclude this is in fact what is taking place at a cellular level. A recent study in our laboratory did, however, directly implicate VICs as force developing elements in anterior mitral valve leaflets (Stephens et al, 2010).
The findings of the current study are consistent with the Conceptual Leaflet Material Model proposed by Itoh et al. (Itoh et al., 2009) and advance it by providing a greater regional understanding of the leaflets’ contractile mechanisms as well as elucidating the stimulus pathways for AL contractile events. Figure 4 illustrates an updated conceptual model of the AL with potential stimulus pathways on the left. Figure 4B illustrates the effect of βB, where IVC Annular stiffening is abolished. Figure 4C shows the effect of adding electrical stimulation to the βB’d leaflet, whereupon the whole leaflet becomes stiffer during IVC and IVR; thereby illustrating a second β-independent contractile mechanism.
Figure 4.
A. Schematic of the anterior mitral valve leaflet with potential control inputs on the left. B. Schematic of the β-blockade experiment. C. Schematic of the β-blockade plus STIM experiment. Even though β-blockade has blocked the stiffness increase during IVC, STIM increases both IVC and IVR stiffness via an alternate pathway.
5. CONCLUSION
Cardiac myocytes are histologically present in the annular AL and their contraction is almost certainly responsible for normal, transient IVC stiffening. When this contraction is abolished with β-blockade, βB+STIM stiffens all regions of the AL during both IVC and IVR, perhaps via VIC, and/or SMC contraction. These findings strongly suggest that at least two contractile systems are present in the AL and that each of the events demonstrated herein employ different stimulus pathways to alter leaflet stiffening. These contractile systems, which may be adaptively controlled, could be important for proper coaptation between the mitral valve leaflets as well as maintenance of proper geometry of the left ventricular outflow tract.
Limitations
The finite element analysis in the present study employed an orthotropic material model characterized by elastic moduli in the radial and circumferential directions. This model, which allowed comparison of the derived material properties with those from ex vivo studies (May-Newman and Yin, 1995), was appropriate for the present study of the closed valve, as the mean difference between experimental and simulated displacements was found to be 0.4mm (Krishnamurthy et al., 2008). Studies of the open valve will require a more sophisticated approach, such as the use of a transversely isotropic leaflet material model.
The strut chordae were modeled using simple tension-only bar elements. Although sensitivity analysis with respect to chordae stiffness in our previous study (Krishnamurthy et al., 2008) showed that the influence of strut chordae on the derived material properties was minor, future analysis of the mitral valve throughout the cardiac cycle should include more elaborate modeling of the mechanics and insertion geometry of these strut chordae, as suggested in a recent report (Padala et al., 2010).
The leaflet surface was modeled from the 4-D positions of 16 radiopaque leaflet markers, the most that could currently be placed, taking into consideration the size of the leaflet, cardiopulmonary bypass time, and fluoroscopic reconstruction issues. Future studies utilizing more markers would be advantageous to more precisely define leaflet geometry and material stiffness values.
The present study clearly demonstrates the presence of at least two contractile systems in the anterior leaflet. It cannot be concluded from these experiments, however, that only two contractile systems are present in the leaflet. Further studies are needed to establish whether these contractile systems are present in other species and behave similarly in the normal heart in the absence of surgical interventions.
Acknowledgments
We thank Koji Arata and Paul Chang for acting as perfusionists in this study. We also thank Paul Chang, Lauren R. Davis, Eleazar P. Briones, Sigurd Hartnett and Kathy Vo for technical assistance in the operating room and catheterization laboratory, Maggie Brophy and Sigurd Hartnett for careful marker digitization, and George T. Daughters for extraction of 4D data from marker coordinates. Without the initial conceptualization of how to determine MV stiffness in vivo and the tireless assistance of Dr Ingels throughout this study, this work would not have been possible.
Funding
This work was supported in part by National Heart, Lung, and Blood Institute Grants RO1 HL-29589 and RO1 HL-67025, a fellowship from the Western States Affiliate of the American Heart Association to J.C. Swanson, a Medtronic Bio-X Graduate Student Fellowship to G. Krishnamurthy, and a Deutsche Herzstiftung (Frankfurt, Germany) Research Grant S/06/07 to W. Bothe. J-P. E. Kvitting was supported by the U.S.-Norway Fulbright Foundation and the Swedish Heart-Lung Foundation.
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boucek RJ, Bouckova B, Levy S. Anatomical arrangement of muscle tissue in the anterior mitral leaflet in man. Cardiovasc Res. 1978;12:675–680. doi: 10.1093/cvr/12.11.675. [DOI] [PubMed] [Google Scholar]
- Daughters GT, Sanders WJ, Miller DC, Schwarzkopf A, Mead CW, Ingels NBJ. A comparison of two analytical systems for 3-D reconstruction from biplane videoradiograms. IEEE Computers in Cardiology. 1989;15:79–82. [Google Scholar]
- De Biasi S, Vitellaro-Zuccarello L, Blum I. Histochemical and ultrastructural study on the innervation of human and porcine atrio-ventricular valves. Anat Embryol (Berl) 1984;169:159–165. doi: 10.1007/BF00303145. [DOI] [PubMed] [Google Scholar]
- Fenoglio J, Jr, Tuan DP, Wit AL, Bassett AL, Wagner BM. Canine mitral complex. Ultrastructure and electromechanical properties. Circ Res. 1972;31:417–430. doi: 10.1161/01.res.31.3.417. [DOI] [PubMed] [Google Scholar]
- Filip DA, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986;59:310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- Itoh A, Krishnamurthy G, Swanson JC, Ennis DB, Bothe W, Kuhl E, Karlsson M, Davis LR, Miller DC, Ingels NB., Jr Active stiffening of mitral valve leaflets in the beating heart. Am J Physiol Heart Circ Physiol. 2009;296:H1766–1773. doi: 10.1152/ajpheart.00120.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MO, Glasson JR, Bolger AF, Daughters GT, Komeda M, Foppiano LE, Miller DC, Ingels NB., Jr Mitral valve opening in the ovine heart. Am J Physiol. 1998;274:H552–563. doi: 10.1152/ajpheart.1998.274.2.H552. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy G, Ennis DB, Itoh A, Bothe W, Swanson JC, Karlsson M, Kuhl E, Miller DC, Ingels NB., Jr Material properties of the ovine mitral valve anterior leaflet in vivo from inverse finite element analysis. Am J Physiol Heart Circ Physiol. 2008;295:H1141–1149. doi: 10.1152/ajpheart.00284.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy G, Itoh A, Bothe W, Swanson JC, Kuhl E, Karlsson M, Craig Miller D, Ingels NB., Jr Stress-strain behavior of mitral valve leaflets in the beating ovine heart. J Biomech. 2009a;42:1909–1916. doi: 10.1016/j.jbiomech.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy G, Itoh A, Swanson JC, Bothe W, Karlsson M, Kuhl E, Craig Miller D, Ingels NB., Jr Regional stiffening of the mitral valve anterior leaflet in the beating ovine heart. J Biomech. 2009b;42:2697–2701. doi: 10.1016/j.jbiomech.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelman KS, Cochran RP. Stress/strain characteristics of porcine mitral valve tissue: parallel versus perpendicular collagen orientation. J Card Surg. 1992;7:71–78. doi: 10.1111/j.1540-8191.1992.tb00777.x. [DOI] [PubMed] [Google Scholar]
- May-Newman K, Yin FC. Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am J Physiol. 1995;269:H1319–1327. doi: 10.1152/ajpheart.1995.269.4.H1319. [DOI] [PubMed] [Google Scholar]
- Niczyporuk MA, Miller DC. Automatic tracking and digitization of multiple radiopaque myocardial markers. Computers and Biomedical Research. 1991;24:129–142. doi: 10.1016/0010-4809(91)90025-r. [DOI] [PubMed] [Google Scholar]
- Padala M, Sacks MS, Liou SW, Balachandran K, He Z, Yoganathan AP. Mechanics of the mitral valve strut chordae insertion region. J Biomech Eng. 2010;132:081004. doi: 10.1115/1.4001682. [DOI] [PubMed] [Google Scholar]
- Smith S, Taylor PM, Chester AH, Allen SP, Dreger SA, Eastwood M, Yacoub MH. Force generation of different human cardiac valve interstitial cells: relevance to individual valve function and tissue engineering. J Heart Valve Dis. 2007;16:440–446. [PubMed] [Google Scholar]
- Sonnenblick EH, Napolitano LM, Daggett WM, Cooper T. An intrinsic neuromuscular basis for mitral valve motion in the dog. Circ Res. 1967;21:9–15. doi: 10.1161/01.res.21.1.9. [DOI] [PubMed] [Google Scholar]
- Timek TA, Lai DT, Dagum P, Tibayan F, Daughters GT, Liang D, Berry GJ, Miller DC, Ingels NB., Jr Ablation of mitral annular and leaflet muscle: effects on annular and leaflet dynamics. Am J Physiol Heart Circ Physiol. 2003;285:H1668–1674. doi: 10.1152/ajpheart.00179.2003. [DOI] [PubMed] [Google Scholar]
- Tsumori T, Domoto T. Ultrastructural evidence for innervation of the endothelium and interstitial cells in the atrioventricular valves of the Japanese monkey. Anat Rec. 1994;240:157–166. doi: 10.1002/ar.1092400203. [DOI] [PubMed] [Google Scholar]
- Wit AL, Fenoglio J, Jr, Hordof AJ, Reemtsma K. Ultrastructure and transmembrane potentials of cardiac muscle in the human anterior mitral valve leaflet. Circulation. 1979;59:1284–1292. doi: 10.1161/01.cir.59.6.1284. [DOI] [PubMed] [Google Scholar]
- Wit AL, Fenoglio J, Jr, Wagner BM, Bassett AL. Electrophysiological properties of cardiac muscle in the anterior mitral valve leaflet and the adjacent atrium in the dog. Possible implications for the genesis of atrial dysrhythmias. Circ Res. 1973;32:731–745. doi: 10.1161/01.res.32.6.731. [DOI] [PubMed] [Google Scholar]





