Abstract
A 43-year-old male presenting with headache and dizziness underwent craniotomy and gross total resection of an extraaxial tumor was achieved via left occipital interhemispheric approach. The tumor was diagnosed as papillary meningioma arising from the left falcotentorium with such pathologic characteristics of bronchoalveolar adenocarcinoma. At postoperative day 40, he developed generalized tonic clonic seizure and then progressed to a status epilepticus pattern. Brain magnetic resonance imaging showed irregular leptomeningeal enhancement with a significant peritumoral area. Through a cerebrospinal fluid (CSF) study, we identified the meningioma cells of the papillary type from the CSF. At the postoperative day 60, he fell into semicomatose state, and the computed tomography imaging showed low density on both cerebral hemispheres, except the basal ganglia and cerebellum, with overall brain swelling and an increased intracranial pressure. He died on the following day. We experienced a rare case of a papillary meningioma with leptomeningeal seeding.
Keywords: Papillary meningioma, Leptomeningeal seeding, Leptomeningeal enhancement, Generalized tonic clonic seizure, Status epilepticus
INTRODUCTION
Meningioma accounts for 13-19%10) of all primary intracranial tumors, and most meningiomas are slowly growing and benign. The prognosis for this tumor has been reported to be good. But, papillary meningioma (PM) is an aggressive histological variant of meningioma, and it occurs so rarely that its incidence approximately corresponds to 1.0-2.5% of all intracranial meningiomas18). The tumor is pathologically identified when perivascular or pseudopapillary pattern in a component of meningioma is present which corresponds to grade III of the 2007 revision of the World Health Organization (WHO) classification. Local recurrence of PM has been 9% to 32% and extracranial metastases of general meningioma has been reported to be 0.1%17). We experienced a case of papillary falcotentorial meningioma with leptomeningeal seeding and metastasis to the spinal cord and the vertebral bodies.
CASE REPORT
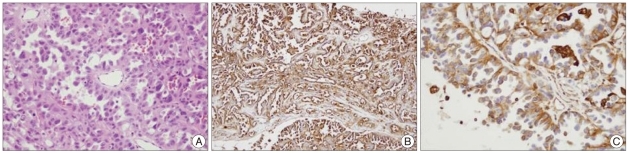
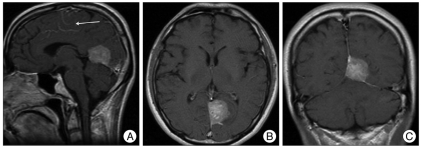
The patient was a 43-year-old male whose chief complaints were headache and dizziness. The preoperative brain magnetic resonance imaging (MRI) showed a 31×28×29 mm mass with a broad base on the falcotentorium and the tumor had a mass effect on the left occipital lobe with minimal edematous changes in the adjacent brain parenchyma. The mass revealed mixed isointensity and hypointensity on T1WI, heterogenous hyperintensity on T2WI and it was densely enhanced with Gadolinium enhancement. The mass was totally removed via the supratentorial interhemispheric approach. Microscopically, the tumor consisted of solid nests and papillary growth with some perivascular pseudorosette formation of rather round, uniform cells with variable eosinophilic cytoplasm and the some tumor cells showed epithelioid or rhambdoid in shape. Eight mitosis were observed in average from 10 high power field microscope. Necrosis was not seen (Fig. 4A). This finding was very similar to the bronchoalveolar adenocarcinoma of the lung. For the immunohistochemical staining, the tumor cells were positive for epithelial membrane antigen (EMA), vimentin and pancytokeratin. The immunostaining was negative for progesteron receptor, CK20, HMB45 and GFAP (Fig. 4B, C). The differential diagnosis of PM was considered. Clinically, there were no primary lesions in other organs including both lungs. This tumor showed the typical positive findings for EMA and vimentin. Bronchoalveolar carcinoma is restricted to the lung and it usually does not accompany metastasis. Also, bronchoalveolar carcinoma generally show positivity for progesterone receptors. It has been reported that these phenomena does not occur for grade III meningioma and particularly for male patients with grade III meningioma. According to the above criteria, the patient was diagnosed as PM based on histological and immunohistochemical findings.
Fig. 4.
This photomicrograph is pathologic finding of papillary meningioma. A : The tumor consisted of solid nests and papillary growth with some perivascular pseudorosettes formation of rather monotonous round, uniform cells that have variable eosinophilic cytoplasm with an epithelioid or rhambdoid shape (H&E, ×400). B and C : On the immunohistochemical staining, the tumor cells are positive for epithelial membrane antigen and vimentin (×200, ×400).
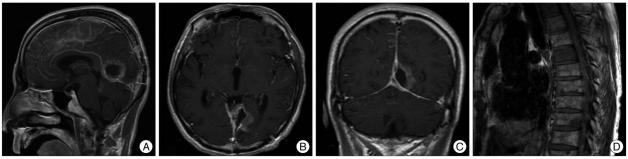
The patient received radiotherapy after the pathologic diagnosis was made. On postoperative day 27, he had complex partial seizure and was in confused state. Electroencephalographic measurement exhibited abnormal II-III with continuous slow, generalized and severe diffuse cerebral dysfunction. The patient was then given an additional anticonvulsant, and the seizure was temporally controlled. On postoperative day 40, he had another general tonic clonic type seizure. At the time, an electrolyte analysis showed Na 120 mmol/L, K 4.2 mmol/L and Cl 82 mmol/L. Brain MRI was performed again which revealed irregularly enhanced meningeal involvement with a significant peritumoral lesion and leptomeningeal seeding (Fig. 3A, B, C). Tumor cells were also identified from cerebrospinal fluid (CSF) studies. Thereafter, the patient complained of low extremity weakness and pain. Whole spine MRI was performed and there were findings suggestive of multiple, small spinal cord metastases and bony metastases on T2, T3, T6, T7, T8, T10 and L1 (Fig. 3D). We reviewed the initial MRI and found that leptomeningeal seeding had occurred before the operation (Fig. 1A : white arrow). He began to suffer from intractable seizure from postoperative day 40 onwards. On postoperative day 61, a brain computed tomography showed diffuse low densities over both hemispheres except for the basal ganglia and cerebellum. These findings were assumed to be from the changes due to the repeated damages caused by inadequate control of the status epilepticus. He died on the postoperative day 62. With this unexpected clinical course, we reviewed the MRI findings on admission. As a result, the patient was assumed to have had leptomeningeal spread before clinical presentation. So, we believe that the abrupt leptomeningeal seeding have caused the dissemination of the tumor during the surgery.
Fig. 3.
(A, B and C) The brain postoperative magnetic resonance imaging on day 40 shows severe leptomeningeal seeding and (D) the spinal MRI shows multiple small metastases on nearly the whole spinal cord and many bony metastases.
Fig. 1.
The preoperative brain magnetic resonance imaging showed a 31×28×29 mm mass with a broad base on the falcotentorium and the tumor had a mass effect on the left occipital lobe with minimal edematous changes in the adjacent brain parenchyma.
DISCUSSION
There have been reported cases of hematogenous or lymphatic dissemination of meningioma or spread through the CSF4,6). CSF dissemination is uncommon, but it can occur in 4% of these cases1,15). Dissemination of meningioma can be increased following a surgical procedure1,11).
According to the revised WHO classification, meningioma is classified into 15 different variants and it shows a wide range of histologic patterns. PM is characterized by a perivascular pseudopapillary pattern, atypical mitosis, necrosis and pleomorphism. PM was first reported by Cushing and Eisenhardt in 19385). Ludwin et al. analyzed 17 cases of PM. According to these authors, it frequently occurs in children (41%) showing frequent mitosis, local recurrence and brain invasion. Extracranial metastasis (23.5%) are commonly seen15). In cases of histologically malignant tumor, the incidence of metastasis was 43% and this is a relatively high value. But Stefanko and Mackay20) reported on 6 cases of PM and they had suggested that these papillary structures were no more than a secondary manifestation of tumor cell casotropism and poor cohesion between the cellular perivascular crowns. Extracranial metastases of PM are commonly seen in the lung9,13), and the 5-year survival rate for these cases has been reported to be approximately 40%.
According to a review of the literature, CSF dissemination has been rarely reported in cases of meningioma1,3,4,12,16,19). Particularly in recent years, rhabdoid papillary mixed meningioma with spinal cord invasion has been reported. Al-Habib et al.2) reported on cases in which rhabdoid papillary meningioma displayed leptomeningeal seeding and spinal cord metastasis. Eom KS et al.8) reported a cases of craniospinal metastases due to intraventricular rhabdoid papillary meningioma. Delgado-Lopez6) reported on cases of metastatic meningioma that had invaded to vertebral bodies. Lee TT et al.14) reported on cases of cauda equina metastases. Enam et al.7) reported on cases of spinal metastasis. In our case, the PM showed extensive leptomeningeal seeding to the spinal cord via CSF pathway as well as vertebral involvement. This phenomenon have been reported very rarely. From postoperative day 40, intractable seizure continued. We assume that the seizure lasted because of meningeal irritation by extensive tumor spread. The patient clinically deteriorated rapidly to such an extent that the patient expired within 67 days following the onset of symptoms and 62 days following total tumor removal. The presumptive causative factors include 1) the histologically malignant character of the tumor, 2) extensive leptomeningeal irritation and 3) the hypoxic damage due to intractable seizure.
To the best of our knowledge, this is a peculiar case of papillary falcotentorial meningioma in which CSF dissemination and metastasis of the spinal cord and vertebral bodies were seen. The pathologic features of the tumor were similar to those of metastatic adenocarcinoma.
CONCLUSION
We experienced a case of papillary falcotentorial meningioma with leptomeningeal seeding complicated by status epilepticus and fatal neurologic deterioration within two months of surgery. Leptomeningeal seeding of PM rarely occurs, but when it happen and accompanied by status epilepticus, we think that careful seizure control will be needed.
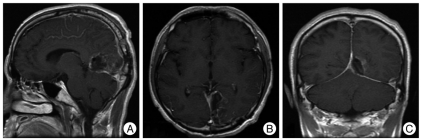
Fig. 2.
The postoperative brain magnetic resonance imaging shows total mass removal, but minimal leptomeningeal seeding is suspected.
References
- 1.Akimura T, Orita T, Hayashida O, Nishizaki T, Fudaba H. Malignant meningioma metastasizing through the cerebrospinal pathway. Acta Neurol Scand. 1992;85:368–371. doi: 10.1111/j.1600-0404.1992.tb04063.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Habib A, Lach B, Al Khani A. Intracerebral rhabdoid and papillary meningioma with leptomeningeal spread and rapid clinical progression. Clin Neuropathol. 2005;24:1–7. [PubMed] [Google Scholar]
- 3.Bigner SH, Johnston WW. The cytopathology of cerebrospinal fluid. II. Metastatic cancer, meningeal carcinomatosis and primary central nervous system neoplasms. Acta Cytol. 1981;25:461–479. [PubMed] [Google Scholar]
- 4.Chamberlain MC, Glantz MJ. Cerebrospinal fluid-disseminated meningioma. Cancer. 2005;103:1427–1430. doi: 10.1002/cncr.20926. [DOI] [PubMed] [Google Scholar]
- 5.Cushing H, Eisenhardt L. Meningiomas : Their classification, Regional behaviour, life history and surgical end results. Springfield: Charles C Thomas; 1938. [Google Scholar]
- 6.Delgado-López PD, Martín-Velasco V, Castilla-Diez JM, Fernandez-Arconada O, Corrales-García EM, Galacho-Harnero A, et al. Metastatic meningioma to the eleventh dorsal vertebral body : total en bloc spondylectomy. Case report and review of the literature. Neurocirugia (Astur) 2006;17:240–249. doi: 10.1016/s1130-1473(06)70346-3. [DOI] [PubMed] [Google Scholar]
- 7.Enam SA, Abdulrauf S, Mehta B, Malik GM, Mahmood A. Metastasis in meningioma. Acta Neurochir (Wien) 1996;138:1172–1177. doi: 10.1007/BF01809747. discussion 1177-1178. [DOI] [PubMed] [Google Scholar]
- 8.Eom KS, Kim DW, Kim TY. Diffuse craniospinal metastases of intraventricular rhabdoid papillary meningioma with glial fibrillary acidic protein expression: a case report. Clin Neurol Neurosurg. 2009;111:619–623. doi: 10.1016/j.clineuro.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima T, Tsugu H, Tomonaga M, Shirakusa T. Papillary meningioma with pulmonary metastasis. Case report. J Neurosurg. 1989;70:478–482. doi: 10.3171/jns.1989.70.3.0478. [DOI] [PubMed] [Google Scholar]
- 10.Kepes JJ. Cellular whorls in brain tumors other than meningiomas. Cancer. 1976;37:2232–2237. doi: 10.1002/1097-0142(197605)37:5<2232::aid-cncr2820370512>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Kepes JJ, Moral LA, Wilkinson SB, Abdullah A, Llena JF. Rhabdoid transformation of tumor cells in meningiomas : a histologic indication of increased proliferative activity : report of four cases. Am J Surg Pathol. 1998;22:231–238. doi: 10.1097/00000478-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Kleinschmidt-DeMasters BK, Avakian JJ. Wallenberg syndrome caused by CSF metastasis from malignant intraventricular meningioma. Clin Neuropathol. 1985;4:214–219. [PubMed] [Google Scholar]
- 13.Kros JM, Cella F, Bakker SL, Paz Y Geuze D, Egeler RM. Papillary meningioma with pleural metastasis : case report and literature review. Acta Neurol Scand. 2000;102:200–202. doi: 10.1034/j.1600-0404.2000.102003200.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee TT, Landy HJ. Spinal metastases of malignant intracranial meningioma. Surg Neurol. 1998;50:437–441. doi: 10.1016/s0090-3019(97)00348-0. [DOI] [PubMed] [Google Scholar]
- 15.Ludwin SK, Rubinstein LJ, Russell DS. Papillary meningioma : a malignant variant of meningioma. Cancer. 1975;36:1363–1373. doi: 10.1002/1097-0142(197510)36:4<1363::aid-cncr2820360427>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Noterman J, Depierreux M, Raftopoulos C, Brotchi J. [Metastases of meningioma. Apropos of 2 cases] Neurochirurgie. 1987;33:184–189. [PubMed] [Google Scholar]
- 17.Pasquier B, Gasnier F, Pasquier D, Keddari E, Morens A, Couderc P. Papillary meningioma. Clinicopathologic study of seven cases and review of the literature. Cancer. 1986;58:299–305. doi: 10.1002/1097-0142(19860715)58:2<299::aid-cncr2820580215>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Russell T, Moss T. Metastasizing meningioma. Neurosurgery. 1986;19:1028–1030. doi: 10.1227/00006123-198612000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Satoh T, Kageyama T, Yoshimoto Y, Kamata I, Date I, Motoi M. [Intrathecal dissemination of meningiomas; a case report] No Shinkei Geka. 1992;20:805–808. [PubMed] [Google Scholar]
- 20.Stefanko SZ, Mackay WM. Papillary meningioma. Acta Neuropathol Suppl. 1981;7:126–128. doi: 10.1007/978-3-642-81553-9_38. [DOI] [PubMed] [Google Scholar]