Abstract
Objective
Minocycline, a second-generation tetracycline-class antibiotic, has been well established to exert a neuroprotective effect in animal models and neurodegenerative disease through the inhibition of microglia. Here, we investigated the effects of minocycline on motor recovery and neuropathic pain in a rat model of spinal cord injury.
Methods
To simulate spinal cord injury, the rats' spinal cords were hemisected at the 10th thoracic level (T10). Minocycline was injected intraperitoneally, and was administered 30 minutes prior surgery and every second postoperative day until sacrifice 28 days after surgery. Motor recovery was assessed via the Basso-Beattie-Bresnahan test. Mechanical hyperalgesia was measured throughout the 28-day post-operative course via the von Frey test. Microglial and astrocyte activation was assessed by immunohistochemical staining for ionized calcium binding adaptor molecule 1 (Iba1) and glial fibrillary acidic protein (GFAP) at two sites: at the level of hemisection and at the 5th lumbar level (L5).
Results
In rats, spinal cord hemisection reduced locomotor function and induced a mechanical hyperalgesia of the ipsilateral hind limb. The expression of Iba1 and GFAP was also increased in the dorsal and ventral horns of the spinal cord at the site of hemisection and at the L5 level. Intraperitoneal injection of minocycline facilitated overall motor recovery and attenuated mechanical hyperalgesia. The expression of Iba1 and GFAP in the spinal cord was also reduced in rats treated with minocycline.
Conclusion
By inhibiting microglia and astrocyte activation, minocycline may facilitate motor recovery and attenuate mechanical hyperalgesia in individuals with spinal cord injuries.
Keywords: Spinal cord injury, Minocycline, Astrocyte, Microglia
INTRODUCTION
Each year, many people suffer from spinal cord injury (SCI) : the estimated yearly incidence of SCI is 40 cases per million in the United States9) and 60,000 cases in China21). While costs associated with SCI exceed $7 billion annually in the United States alone9), such estimates do not account for the human suffering resulting from the multiple functional sequelae of SCI, including impaired ambulation, impaired bowel and bladder function, and sexual dysfunction.
To date, while many treatment regimens have been attempted in patients with SCI, none has demonstrated a sufficiently robust efficacy to be widely accepted in the clinical community3,12,17,18).
More recently, mounting evidence regarding the extensive inflammation after SCI has led to the clinical use of anti-inflammatory agents (such as methylprednisolone) in SCI patients5). However, despite the initial optimism, methylprednisolone did not live up to its clinical potential30). In addition, controversy currently exists regarding the innate immune response to injury in central nervous system (CNS). As the CNS is an immunologically privileged site, it remains unclear whether enhancing13) or suppressing16,20) the innate immune system of the CNS has a greater neuroprotective effect after SCI. Regardless, it is well accepted that post-traumatic inflammation contributes significantly to secondary injuries after SCI. Pro-inflammatory mediators-such as cytokines, proteases, and reactive oxygen species-are believed to promote the activation of cell death executioners (e.g., caspases) that are responsible for the neuronal loss and apoptosis that ultimately culminates in permanent neurological deficits14,29).
In cases of spinal cord injury, neuropathic pain and motor weakness often develop below the level of injury level. These phenomena result from microglia activation, which is believed to trigger nociceptive hypersensitivity8). Following SCI, microglia become activated19), often releasing neurotoxic molecules that further damage nearby neurons2). However, the exact role of microglial involvement in CNS injury and regeneration remains controversial28,36). Minocycline, a semi-synthetic second-generation tetracycline-class antibiotic, has been shown to exert anti-inflammatory effects that are mechanistically different its anti-microbial action1,11). Consequently, minocycline is now being investigated for clinical use in the treatment of SCI, with a phase I/II pilot study examining the efficacy of intravenously administered minocycline in patients with acute SCI currently underway in Calgary, Alberta32). In rat models of SCI, minocycline inhibits the release of cytochrome c from mitochondria, markedly enhancing long-term hindlimb locomotion33).
Here, we examined the ability of minocycline to promote motor recovery and reduce neuropathic pain in a rat model of SCI. Additionally, we also evaluated whether minocycline attenuates microglial and astrocyte activation post SCI.
MATERIALS AND METHODS
Animals
Fifteen male Sprague-Dawley rats (Orient Bio, Seoul, Korea) with body weights between 250-350 g were used. The rats were divided into three groups : sham control (n=5), hemisection control (n=5), and hemisection with minocycline (Hemi+Mino, n=5). All animals were provided with food and water ad libitum throughout the study.
Spinal cord injury model
Hemisection of the spinal cord was used to simulate SCI. The animals were anesthetized with a mixture of Tiletamine+Zolazepam (27.78 mg/kg) and Xylazine (0.647 mg/kg), before being mounted on a frame. A 3 cm midline incision was made on the back so the deep back muscles could be retracted to expose the lower thoracic vertebrae. The ligamentum flavum between T8 and T9 and dura mater was then carefully incised. After identifying the spinal cord midline, the right half was transected completely by microblade. Bleeding and cerebrospinal fluid (CSF) leakage were then controlled and the muscles and skin were sutured. After recovery from the anesthesia, the animals were housed individually in cages.
Administration of minocycline
Minocycline (Sigma, Saint Louis, MO, USA) was dissolved in normal saline (40 mg/mL of normal saline) and injected intraperitoneally (40 mg/kg) into animals in the hemisection and minocycline (Hemi+Mino) group. Injections were administered 30 minutes prior to surgery and on every second post-operative day until sacrifice on the 28th day.
Behavioral test
Motor recovery : Basso-Beattie-Bresnahan locomotor test
The locomotor activity of the rats was assessed via semiquantitative method both before the operation, and at 1, 2, 4, 7, 10, 14, 20 and 28 days after surgery. Testing was performed by two trained observers under blind conditions and using BBB scales. The BBB locomotor rating has previously been described qualitatively with a 21-point scale by Basso et al.4).
Mechanical hyperalgesia : von Frey test
Hindpaw mechanical allodynia was assessed by measuring withdrawal response to mechanical stimulation with von Frey filaments (North Coast Medical Inc., USA). Rats were placed in a clear plastic cage with a metal mesh floor and adapted for 15 minutes. Each hindpaw was stimulated to cause slight bending for 3-5 seconds with von Frey filaments (0.16, 0.4, 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0 and 26.0 g). Initially, the test was performed with a 0.16g probe, and probe size was increased incrementally until a filament consistently produced a withdrawal response to more than 3 of 5 stimuli. Fifty percent probability thresholds of mechanical paw withdrawal were calculated7). Statistical analysis was performed by one-way ANOVA using SPSS (12.0 program).
Morphological studies
Tissue preparation
On post-operative day 28, all animals were re-anesthetized with a mixture of Tiletamine+Zolazepam (27.78 mg/kg) and Xylazine (0.647 mg/kg) and then perfused with heparinized saline and 500 mL of 4% paraformaldehyde in phosphate buffer (PB, 0.1M, pH 7.4). Tissue specimens were obtained from the spinal cord at a lower thoracic level close to the hemisection incision and at a lower lumbar level. The tissue was then postfixed for 2 hours in the same fixative used for perfusion and cryoprotected overnight in 30% sucrose in PB. The specimens were cut into by cryostat into 40 µm sections, which were collected in cold PB. For morphological assessment of the SCI, the spinal cord tissue of one rat from each group were paraffin-embedded, cut by microtome into 8 mm sections, and processed with Luxol fast blue-Cresyl violet stain.
Luxol fast blue-Cresyl violet stain
For Luxol fast blue-Cresyl violet staining, the paraffin sections of the spinal cord were initially deparaffinized and hydrated. The tissue was then first stained with Luxol fast blue for 12 hours at 60℃, then with Cresyl violet for 10 minutes at 60℃, before being mounted.
Immunohistochemistry for ionized calcium binding adaptor molecule 1 and glial fibrillary acidic protein
For immunofluorescent staining, sections were blocked with 10% normal donkey serum (NDS, Jackson Immunolabs, USA) in phosphate-buffered saline (0.01M, pH 7.2) for 10 minutes and then incubated overnight with a mixture of a mouse anti-GFAP antibody (1 : 1,000, Chemicon, USA) and anti Iba1 antibody (1 : 1000, Wako, Japan). Next, sections were rinsed and incubated in 2% NDS for 10 minutes, and then in Cyanine (Cy) 3-conjugated donkey anti rabbit (1 : 200, Jackson) and Fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse (1 : 200, Jackson) for 3 hours. After several rinses, the sections were cover-slipped with Vectashield (Vector, USA) and examined by light microscopy (DMRE, Leica, Germany). All photos were obtained using a cooled charge coupled device (CCD) camera (F-View, SIS, Germany) that was attached to the microscope.
RESULTS
Behavioral test
BBB locomotor score
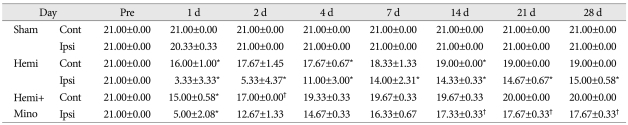
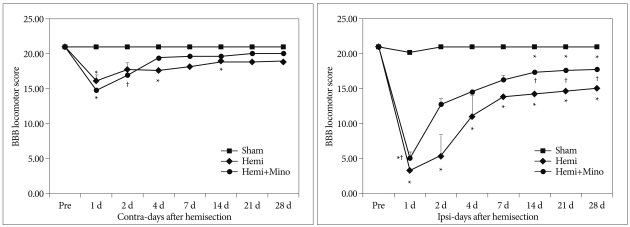
The BBB locomotor score for the side contralateral to the hemisection decreased slightly at post-operative day 1 and gradually improved until 21 days after surgery. On the ipsilateral side, the BBB score decreased maximally at post-operative day 1. For this side, gradual recovery was first observed on post-operative day 2, although the BBB score remained significantly lower than sham controls until 28 days after surgery (p<0.05). At post-operative day 2, the BBB score for the ipsilateral side was also higher in animals treated with minocycline than in the hemisection group, with this difference persisting until 28 days after surgery. A significant improvement from 14 to 28 days was observed (Table 1, Fig. 1).
Table 1.
Basso-Beattie-Bresnahan (BBB) locomotor scores from rats in the sham-control (Sham), hemisection (Hemi), and hemisection with minocycline injections (Hemi+Mino) groups. On the ipsilateral side (Ipsi), BBB score was maximally decreased at post-operative day 1, and gradually recovered beginning 2 days after surgery. BBB score was significantly lower than that of sham control until 28 days after surgery. In Hemi+Mino group, the ipsilateral BBB score was higher than that from hemisection group, beginning at postoperative day 2 and persisting until 28 days after surgery. Significant improvements in ipsilateral BBB score were observed from postoperative days 14 to 28
*p<0.05 versus sham control group, †p<0.05 versus hemisection group. Cont : contralateral side, Ipsi : ipsilateral side, Hemi : hemisection, Mino : minocycline
Fig. 1.
Basso-Beattie-Bresnahan (BBB) locomotor score for rats in the sham control (Sham), hemisection (Hemi) and hemisection with minocycline injection (Hemi+Mino) groups. After hemisection of the spinal cord, BBB score was more markedly decreased on the ipsilateral side (Ipsi) than the contralateral side (Contra), and gradually recovered until 28 days after surgery. Administration of minocycline attenuated the decrease in BBB score that was induced by hemisection. *p<0.05 vs. sham control group, †p<0.05 vs. hemisection group
Mechanical hyperalgesia
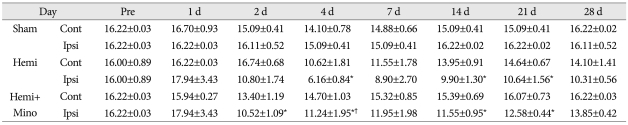
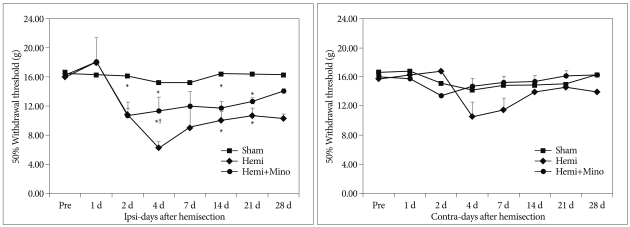
Compared with the sham control group, the mechanical withdrawal threshold in the hemisection group was significantly decreased on ipsilateral side, with the greatest difference occurring on post-operative day 4. These data indicate the development of mechanical hyperalgesia persisting until 28 days after surgery. The decrease of mechanical withdrawal threshold was markedly less in the animals treated with minocycline (hemisection+minocycline) when compared to the hemisection group, with significant differences first occurring 4 days after surgery (p<0.05) (Table 2, Fig. 2).
Table 2.
Von Frey test for mechanical hyperalgesia on the contralateral (Contra) and ipsilateral (Ipsi) hindpaws of rats in the sham control (Sham), spinal cord hemisection (Hemi) and hemisection with minocycline treatment (Hemi+Mino) groups. Versus the sham control group, the mechanical withdrawal threshold for the hemisection group was decreased on ipsilateral side, with a maximum value observed at 4 days after surgery. This result indicates the development of a mechanical hyperalgesia that persisted until 28 days after surgery. In the animals treated with minocycline (hemisection+minocycline), the threshold for mechanical withdrawal was attenuated when compared with that from hemisection group, with significant attenuation observed at postoperative day 4
*p<0.05 versus sham control group, †p<0.05 versus hemisection group. Cont : contralateral side, Ipsi : ipsilateral side, Hemi : hemisection, Mino : minocycline
Fig. 2.
Von Frey test for mechanical hyperalgesia on the contralateral (Contra) and ipsilateral (Ipsi) hindpaws in rats from the sham control (Sham), spinal cord hemisection (Hemi) and hemisection and minocycline treatment (Hemi+Mino) groups. The ipsilateral hindpaw withdrawal threshold was decreased from 2 days after surgery and persisted until postoperative day 28. Administration of minocycline attenuated the decrease of this threshold. *p<0.05 vs. sham control group, †p<0.05 vs. hemisection group.
Luxol fast blue-Cresyl violet staining
To confirm cord injury and for the general morphological assessment of the spinal cord after hemisection, tissue samples from the spinal cord were obtained from the injury site, 5 mm caudal to the injury, and at the L5 spinal level. These specimens were then stained with Luxol fast blue-Cresyl violet stain. At the level of hemisection, the right half of the spinal cord was completely destroyed, with necrotic tissue and glial cells observed in both the gray and white matter. In the specimens 5 mm caudal to the point of hemisection, a vacuolation and infiltration of inflammatory cells was noted in the dorsal column, however no neuronal necrosis was present in the gray matter (Fig. 3).
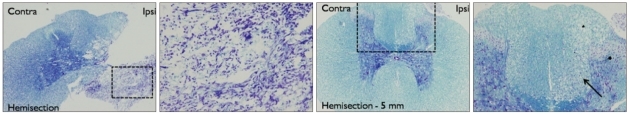
Fig. 3.
Luxol fast blue-Cresyl violet stain of spinal cord tissue samples obtained from the level of the hemisection (Hemisection) and at sites 5 mm caudal to the hemisection (Hemisection-5 mm). The right column shows tissue from the dotted areas in the left column at higher levels of magnification. At the level of hemisection, the ipsilateral half of the spinal cord was completely destroyed, with necrotic tissue and glial nuclei observed. At spinal cord sites 5 mm caudal to the hemisection location, a vacuolation and infiltration of inflammatory cells (arrow) was observed in both dorsal and lateral funiculi. No obvious findings of neuronal necrosis were noted in the gray matter.
In the tissues sections from spinal level L5, a substantial increase in glial nuclei was observed in the hemisection group in the ipsilateral dorsal columns and lateral funiculi. Moreover, the specimens from the L5 level in the hemisection group treated with minocycline showed decreased numbers of microglia and astrocytes. In the spinal cord gray matter, no neuronal necrosis was observed after hemisection (Fig. 4).
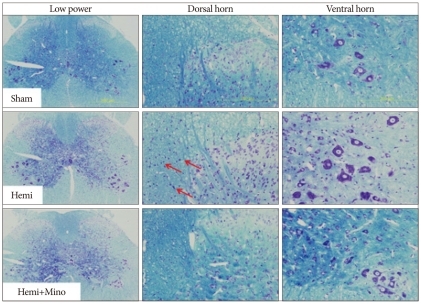
Fig. 4.
Luxol fast blue-Cresyl violet stain of L5 level spinal cord tissue from animals in the sham control (Sham), spinal cord hemisection (Hemi) and hemisection with minocycline treatment (Hemi+Mino) groups. In the Hemi group, increases of glial nuclei (arrows) are observed in the ipsilateral dorsal column and lateral funiculus. The total number of these nuclei is deceased in the hemisection with minocycline group. No obvious neuronal necrosis is observed in either the dorsal or ventral horn in the Hemi group.
GFAP and Iba1 immunohistochemistry
SCI site
In hemisection group lower thoracic level spinal cord specimens, the immunoreactivity for GFAP and Iba1 was markedly increased in the gray and white matter of both sides. In the spinal cord tissue from the hemisection group treated with minocycline, GFAP and Iba1 immunoreactivity only increased on the ipsilateral side, and mainly within the white matter. When compared with the untreated hemisection group, the immunoreactivity for both GFAP and Iba1 was decreased in the animals receiving minocycline in both ipsilateral and contralateral spinal cord specimens (Fig. 5).
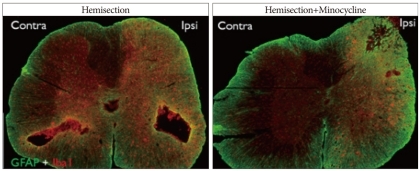
Fig. 5.
Double immunofluorescence for GFAP (green) and Iba1 (red), in the lower thoracic cord of the Hemi and Hemi+Mino groups. In Hemi group, levels of immunoreactivity for GFAP and Iba1 are increased in sides both contralateral and ipsilateral to the injury. In Hemi+Mino group, the increase in GFAP and Iba1 immunoreactivity is markedly attenuated and only observed in the side ipsilateral to the injury.
Iba1-immunoreactivity in L5 segment
In ipsilateral L5 spinal cord tissue specimens from the hemisection group, an increase in Iba1 immunoreactivity was observed in the dorsal column, superficial dorsal horn, lateral funiculus, ventral funiculus and ventral horn. Moreover, Iba1 immunoreactivity was attenuated in these same areas of ipsilateral spinal cord from the hemisection group treated with minocycline (Fig. 6, 7). Such increases in immunoreactivity were not observed in tissue obtained from the contralateral spinal cord of untreated, hemisected animals. Activated microglias with notably shorter processes were also especially abundant in lateral and ventral funiculus.
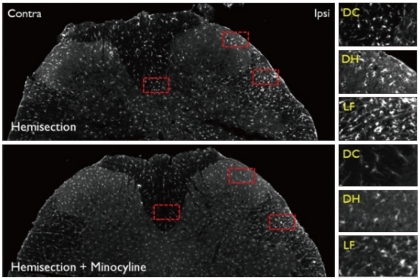
Fig. 6.
Iba1 Immunoreactivity in the L5 level spinal cord dorsal horn in the Hemi and Hemi+Mino groups. The dotted areas from the low power views are magnified in the right column. In the Hemi group, the amount of Iba1-immunoreactive activated microglia is increased in the dorsal column (DC), superficial laminae of the dorsal horn (DH) and lateral funiculus (LF). The number of activated microglia is decreased in spinal cord tissue from the Hemi+Mino group.
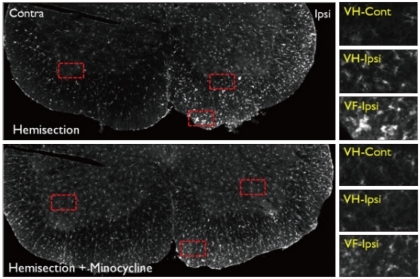
Fig. 7.
Iba1 immunoreactivity in the L5 level spinal cord ventral horn in the Hemi and Hemi+Mino groups. The dotted areas from the low power views are magnified in the right column. In the Hemi group, more iba1-positive microglias are observed in the ventral horn (VH) and ventral funiculus (VF) of the ipsilateral spinal cord, when compared with the contralateral side. The number of activated microglia is decreased in spinal cord tissue from the Hemi+Mino group.
GFAP-immunoreactivity in L5 segment
In L5 level spinal cord specimens obtained from animals in the hemisection group, GFAP immunoreactivity increased in the ipsilateral lateral funiculus and ventral horn, when compared with the contralateral spinal cord. Unlike Iba1 immunoreactivity, no obvious increases in GFAP immunoreactivity were observed in the ipsilateral dorsal horn or ventral funiculus. In spinal cord tissue from hemisected animals treated with minocycline, these increases in GFAP-immunoreactivity were markedly attenuated in the ipsilateral lateral funiculus and ventral horn (Fig. 8, 9).
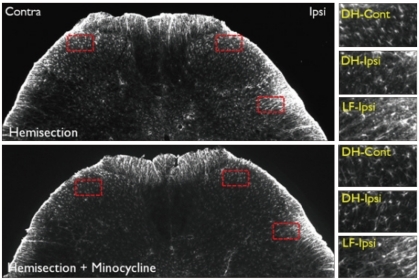
Fig. 8.
GFAP immunoreactivity in the L5 level spinal cord dorsal horn in the Hemi and Hemi+Mino groups. The dotted areas from the low power views are magnified in the right column. When compared with the contralateral side, an increase in GFAP-immunoreactivity is observed in the ipsilateral lateral funiculus (LF-ipsi), but not the ipsilateral dorsal horn (DH-ipsi). In the spinal cord tissue from the Hemi+Mino group, this lateral funiculus-specific increase in GFAP immunoreactivity is attenuated, when compared with the Hemi group.
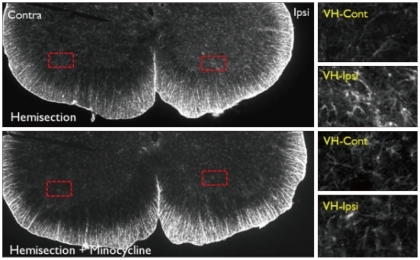
Fig. 9.
GFAP immunoreactivity in the L5 level spinal cord dorsal horn in the Hemi and Hemi+Mino groups. The dotted areas from the low power views are magnified in the right column. In the Hemi group, GFAP-immunoreactivity is increased in the ipsilateral ventral horn when compared with the contralateral side (VH-Cont). Such increases in GFAP-immunoreactivity are also attenuated in ventral horn tissue from the Hemi+Mino group.
DISCUSSION
Minocycline is a second-generation, semi-synthetic tetracycline analog, which is highly lipophilic and easily penetrates the blood-brain barrier12). Effective against both gram-positive and -negative infections, minocycline-like all tetracycline-class antibiotics-inhibits protein synthesis at the ribosomal level1). In addition to this antimicrobial activity, minocycline has also been reported to exert neuroprotective effects in various experimental models of neurologic injury, including cerebral ischemia, traumatic brain injury, amyotrophic lateral sclerosis, Parkinson's disease, kainic acid treatment, Huntington' disease, multiple sclerosis and Alzheimer's disease10,11,33). Very recently, minocycline has also been suggested for use in the treatment of SCIs. To date, many studies exist indicating that minocycline may reduce neuropathic pain after SCI. However, only few data exist regarding the ability of minocycline to promote motor recovery after SCI, leaving this indication controversial.
The distribution of microglia and astrocytes has been characterized throughout SCI. Peak microglial activation has been observed to occur within the lesion epicenter between three and seven days post-injury, preceding monocyte influx and macrophage activation. At sites rostral and caudal to the injury, a prolonged activation of inflammatory cells has also been reported to occur preferentially in white matter, consisting primarily of activated microglia and astrocytes15). Accordingly, microglia and astrocytes have both been implicated in the activation of resident and recruited immune cells, as well as the subsequent apoptosis of neuronal cells that occurs after SCIs15,35).
Here, we used Iba1 and GFAP as markers for microglial and astrocyte activation. Iba1 is both a sensitive and specific marker for the induction of a reactive profile in microglia. Physiologically, Iba1 has a functional role in intracellular calcium signaling, and is stimulated when local microglia react to a changes in homeostasis24,35). GFAP is a major constituent of astrocyte intermediate filaments and is widely used as a sensitive marker of the reactive gliosis that occurs after CNS injury. Increases in GFAP reactivity at post-injury day 7 imply that astrocytic proliferation, if present at all, would have been evident before or at day 725).
Activation of local astrocytes and microglia in the spinal cord may contribute to rat pain-related behaviors, as both cells express receptors for several neurotransmitters, possibly including glutamate, substance P and other factors. Continual excitation may also induce the activation of signal-regulated kinases (ERK), p38, and c-Jun N-terminal kinase (JUK) in glial cells8,13). Moreover, these signaling pathways lead to the increased synthesis of multiple proinflammatory factors, including interleukin 1ββ (IL-1ββ), tumour-necrosis factor (TNF α) and NO38). As a result of these cross-talk mechanisms between glia and neurons, the responsiveness of spinal nociceptive neurons to stimuli is believed to be further enhanced. In this way, spinal glia may represent novel targets for the effective treatment of pain syndromes secondary to SCI15).
Notably, the neuroprotective effects of minocycline on motor neurons remain controversial. In their study, Saganová et al.26) showed that both short- and long-term treatment with minocycline had a neuroprotective effect on the spinal cord rostral to the injury epicenter, although these effects were not observed at caudal sites and did not result in any overall improvement in motor outcome. Conversely, data from Teng et al. indicate that minocycline has a protective effect on white matter and motor neuron number at sites both rostral and caudal from the lesion epicenter33).
While the pharmacologic neuroprotective mechanism of minocycline remains unclear, minocycline has been shown to inhibit the activation of inflammatory microglia34), microglia/macrophages, and neutrophils37). Minocycline was also shown to improve functional recovery after SCI through the inhibiting the production of pro-nerve growth factor (NGF) by microglia, thereby reducing oligodendrocyte death and apoptosis after traumatic SCI38). Furthermore, these authors also demonstrated that minocycline both inhibited the expression of p75 neurotrophin receptors and the activation of Ras homolog gene family member A (RhoA) after SCI. More recently, Festoff et al. reported that minocycline also might exert a neuroprotective effect in SCI by reducing microgliosis and inhibiting caspase expression10). In combination, this emerging data suggests that minocycline may protect the spinal cord from secondary damage after SCI via several unique pharmacologic mechanisms. Regardless of the mechanism of primary insult, acute injury to the spinal cord typically involves several multifaceted, secondary degenerative processes that spread peripherally from the site of primary injury in the days to weeks following SCI27). In cases where these secondary injuries are exacerbated by delayed treatment, the cascade of neurodestructive events is likely more extensive6).
In the present study, we used an animal model of thoracic spinal cord injury to better characterize the effect of minocycline on neuroglial activation, both at the primary injury site and additional caudal levels. Consecutive rostral-caudal spreading of secondary lesion was commonly asymmetrical, with the extent of cavitation at post-injury day 28 greater caudally than rostrally10).
With regard to potential SCI therapies in humans, animals treated with minocycline exhibited an improved recovery of motor function after hemisection. This clinical observation appears to correlate with a significant reduction in injury lesion size, as the lesion size at 28 days post-SCI was significantly reduced in rats treated with minocycline, when compared to sham-treated controls. This sparing of spinal cord tissue was largely resulted from the preservation of neuronal profiles and a reduction in apoptosis. This result is especially significant, as functional recovery has not previously been supported by changes in tissue sparing22). Caspases, which are both initiators and executioners in the apoptotic cascade, are directly integrated into the proteolytic activities that regulate apoptosis as well as cell survival23,31).
Here, the attenuation of neuropathic pain behavior and motor recovery in treated animals correlated with reductions in microglia and astrocytes activation, respectively. After cord injury, a significant activation of the microglia was observed in the L5 level dorsal horn, which is believed to be directly responsible for mechanical hyperalgesia of the hindlimb. Such increases in microglia activation were attenuated in minocycline-treated animals. Conversely, the astroglial activation at the L5 level was most pronounced in the ventral horn. The attenuation of astroglial activation in ventral horn observed in minocycline-treated rats may be indirectly related to microglial inhibition and/or tissue sparing at the lesion site, as no direct effect of minocycline on astrocyte function has ever been described. Accordingly, further research regarding the effects of minocycline on reactive astrocytosis and motor improvement is indicated, and will surely yield both scientific and clinical value.
Our study results demonstrate that minocycline boosts motor recovery and lessens neuropathic pain after SCI in a rat model. Our results also show that minocycline decreases the activation of microglia and astrocytes, not only at the site of SCI but also a remote spinal cord segment (L5) responsible for hindlimb pain and motor behavior.
The most significant limitation of this study is the relatively small number of experimental rats. Moreover, although we did not quantify the resulting lesion size secondary to SCI, we expect these measurements to be included in any subsequent investigations that quantify the extent of minocycline's neuroprotective effect. Despite these limitations, our results strongly suggest that minocycline exerts a significant neuroprotective effect after SCI, and may also promote post-SCI motor recovery.
CONCLUSION
This study indicates that minocycline, a blood-brain barrier-penetrating tetracycline, possesses anti-inflammatory effects, which significantly improved the restoration of motor function and reduced spinal cord tissue injury volume after SCI in a rat model. Lower thoracic level SCI also was shown to induce microglia and astrocyte activation, both at the level of hemisection and a second site caudal to the injury, ultimately reducing hindlimb motor function. Minocycline inhibits microglia activation, and secondarily blocks astrocyte upregulation. We contend that, through these mechanisms, minocycline may facilitate motor recovery and reduce neuropathic pain after SCI.
References
- 1.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, et al. A novel mechanism of action of tetracyclines : effects on nitric oxide synthases. Proc Natl Acad Sci U S A. 1996;93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthes DL, Theriault E, Tator CH. Ultrastructural evidence for arteriolar vasospasm after spinal cord trauma. Neurosurgery. 1996;39:804–814. doi: 10.1097/00006123-199610000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 4.Basso DC, Beattie MG, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 5.Bracken MB. Treatment of acute spinal cord injury with methylprednisolone : results of a multicenter, randomized clinical trial. J Neurotrauma. 1991;8(Suppl 1):S47–S50. discussion S51-S52. [PubMed] [Google Scholar]
- 6.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury : 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 7.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 8.Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- 9.DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord. 1997;35:809–813. doi: 10.1038/sj.sc.3100501. [DOI] [PubMed] [Google Scholar]
- 10.Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- 11.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown : new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 12.Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg. 1992;76:13–22. doi: 10.3171/jns.1992.76.1.0013. [DOI] [PubMed] [Google Scholar]
- 13.Hauben E, Gothilf A, Cohen A, Butovsky O, Nevo U, Smirnov I, et al. Vaccination with dendritic cells pulsed with peptides of myelin basic protein promotes functional recovery from spinal cord injury. J Neurosci. 2003;23:8808–8819. doi: 10.1523/JNEUROSCI.23-25-08808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurlbert RJ. Methylprednisolone for acute spinal cord injury : an inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 15.Jiang F, Liu T, Cheng M, Pang XY, Bai ZT, Zhou JJ, et al. Spinal astrocyte and microglial activation contributes to rat pain-related behaviors induced by the venom of scorpion buthus martensi karch. Eur J Pharmacol. 2009;623:52–64. doi: 10.1016/j.ejphar.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, et al. Pathological cns autoimmune disease triggered by traumatic spinal cord injury : implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Lammertse DP. Update on pharmaceutical trials in acute spinal cord injury. J Spinal Cord Med. 2004;27:319–325. doi: 10.1080/10790268.2004.11753769. [DOI] [PubMed] [Google Scholar]
- 19.Lee YL, Shih K, Bao P, Ghirnikar RS, Eng LF. Cytokine chemokine expression in contused rat spinal cord. Neurochem Int. 2000;36:417–425. doi: 10.1016/s0197-0186(99)00133-3. [DOI] [PubMed] [Google Scholar]
- 20.Popovich PG. Immunological regulation of neuronal degeneration and regeneration in the injured spinal cord. Prog Brain Res. 2000;128:43–58. doi: 10.1016/S0079-6123(00)28006-0. [DOI] [PubMed] [Google Scholar]
- 21.Qiu J. China spinal cord injury network: Changes from within. Lancet Neurol. 2009;8:606–607. doi: 10.1016/S1474-4422(09)70162-0. [DOI] [PubMed] [Google Scholar]
- 22.Rabchevsky AG, Fugaccia I, Sullivan PG, Blades DA, Scheff SW. Efficacy of methylprednisolone therapy for the injured rat spinal cord. J Neurosci Res. 2002;68:7–18. doi: 10.1002/jnr.10187. [DOI] [PubMed] [Google Scholar]
- 23.Rideout HJ, Stefanis L. Caspase inhibition : a potential therapeutic strategy in neurological diseases. Histol Histopathol. 2001;16:895–908. doi: 10.14670/HH-16.895. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Sandoval A, Chai N, Nutile-McMenemy N, DeLeo JA. A comparison of spinal iba1 and gfap expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman SM, Winkelstein BA. Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res. 2007;1181:30–43. doi: 10.1016/j.brainres.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saganová K, Orendácova J, Cízková D, Vanický I. Limited minocycline neuroprotection after balloon-compression spinal cord injury in the rat. Neurosci Lett. 2008;433:246–249. doi: 10.1016/j.neulet.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, et al. Experimental strategies to promote spinal cord regeneration--an integrative perspective. Prog Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Schwab JM, Seid K, Schluesener HJ. Traumatic brain injury induces prolonged accumulation of cyclooxygenase-1 expressing microglia/brain macrophages in rats. J Neurotrauma. 2001;18:881–890. doi: 10.1089/089771501750451802. [DOI] [PubMed] [Google Scholar]
- 29.Shin MS, Chung BS, Kim YS. Effect of cyclosporin a in a rat spinal cord injury model. J Korean Neurosurg Soc. 1998;27:1361–1369. [Google Scholar]
- 30.Short DT, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury-a systematic review from a clinical perspective. Spinal Cord. 2000;38:273–286. doi: 10.1038/sj.sc.3100986. [DOI] [PubMed] [Google Scholar]
- 31.Smirnova IV, Citron BA, Arnold PM, Festoff BW. Neuroprotective signal transduction in model motor neurons exposed to thrombin : G-protein modulation effects on neurite outgrowth, Ca(2+) mobilization, and apoptosis. J Neurobiol. 2001;48:87–100. [PubMed] [Google Scholar]
- 32.Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- 33.Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Bernhardi R, Ramirez G. Microglia astrocyte interaction in alzheimer's disease : friends or foes for the nervous system? Biol Res. 2001;34:123–128. doi: 10.4067/s0716-97602001000200017. [DOI] [PubMed] [Google Scholar]
- 37.Wasserman JK, Schlichter LC. Neuron death and inflammation in a rat model of intracerebral hemorrhage : effects of delayed minocycline treatment. Brain Res. 2007;1136:208–218. doi: 10.1016/j.brainres.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 38.Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]