Abstract
Objective
We retrospectively evaluated the prognostic factors that can influence long-term survival in patients who suffered acute large cerebral infarction.
Methods
Between June 2003 and October 2008, a total of 178 patients were diagnosed with a large cerebral infarction, and, among them, 122 patients were alive one month after the onset of stroke. We investigated the multiple factors that might have influenced the life expectancies of these 122 patients.
Results
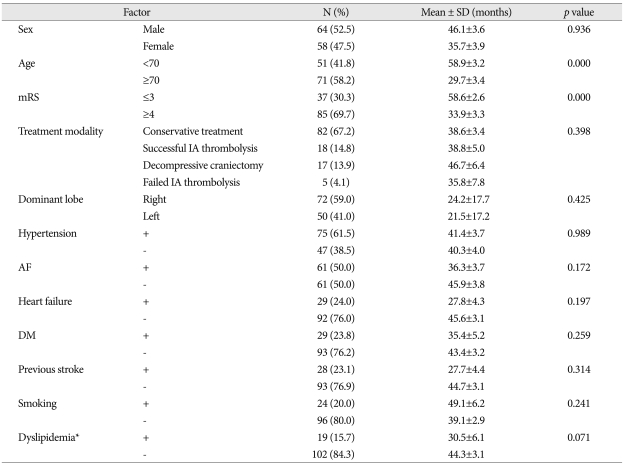
The mean age of the patients was 70±13.4 years and the mean survival was 41.7±2.8 months. The mean survival of the poor functional outcome group (mRS≥4) was 33.9±3.3 months, whereas that of the good functional outcome group (mRS≤3) was 58.6±2.6 months (p value =0.000). The mean survival of the older patients (≥70 years) was 29.7±3.4 months, whereas that of the younger patients (<70 years) was much better as 58.9±3.2 months (p value=0.000). Involvement of ACA or PCA territory in MCA infarction is also a poor prognostic factor (p value=0.021). But, other factors that are also known as significant predictors of poor survival (male gender, hypertension, heart failure, atrial fibrillation, diabetes mellitus, a previous history of stroke, smoking, and dyslipidemia) did not significantly influence the mean survival time in the current study.
Conclusion
Age (older versus younger than 70 years old) and functional outcome at one month could be critical prognostic factors for survival after acute large cerebral infarction. Involvement of ACA or PCA territory is also an important poor prognostic factor in patients with MCA territorial infarction.
Keywords: Age, Functional outcome, Large cerebral infarction, Prognostic factors
INTRODUCTION
Clinical outcome after stroke has been extensively investigated in other previous studies1,5,6,9,10). However, few studies have focused on large cerebral infarctions. Various prognostic factors, such as age, diabetes mellitus (DM), hypertension, physical inactivity, cardiac disease, recurrent stroke, stroke subtype, stroke severity, stroke unit care, and depression have been identified1,5,9,10). In particular, functional status is considered to be a critical factor that can predict long-term survival. Most previous studies have used the modified Rankin Scale (mRS) grade at three month or six month after stroke4,11). However, in most large stroke studies, data from both hemorrhagic and ischemic stroke were combined, and stroke of varying severity based on radiological findings were included1-7).
In addition, few studies have investigated the relationship between prognosis and clinical factors in the acute phase of stroke. Therefore, we investigated the prognostic factors for "large cerebral infarction" in the acute phase (=after one month of infarction) and retrospectively evaluated life expectancy after a large cerebral infarction.
MATERIALS AND METHODS
Between June 2003 and October 2008, 2,975 consecutive patients with stroke were managed at our hospital. Among them, 178 patients (5.98%) suffered a large cerebral infarction, which was defined as an infarction that involved one or more of the middle cerebral artery (MCA) division territories and/or the anterior or posterior cerebral artery (ACA or PCA). A total of 122 patients were included in the current study after excluding patients with the following characteristics : death within one month after the stroke (n=29); intracerebral hemorrhage or subarachnoid hemorrhage after intravenous and/or intra-arterial thrombolysis (n=11); reduced life expectancy because of a severe medical comorbidity such as cancer or a previous stroke (n=9); treatment refused by any relatives (n=4); other vascular disease, such as moyamoya disease (n=2); and lost follow-up after discharge (n=1).
All patient data were obtained from hospital charts and radiological studies and were collected in accordance with the case record form that was approved by the institutional review board. The level of function one month after stroke onset was assessed by the mRS grade. Prognostic factors, such as age, radiological findings, treatment modality, and stroke risk factors were collected. Any data that were missing from the medical records because a patient was lost to follow-up were obtained through a telephone interview with the patient or his or her relatives.
Statistical analysis
We measured the survival curves with the Kaplan-Meier product limit technique and the Log Rank test for univariate analysis. We also performed univariate and multivariate analyses of risk factors with Cox proportional hazard model. We used means ± SD rather than median values. Categorical variables were recorded using numbers and percentages. Overall survival was defined as the time interval between the date of stroke onset and the date of death or the most recent evaluation. Data from patients who were dead at one month after an infarction were not entered in this analysis to investigate the impact of functional status on subsequent survival. The results are presented as corresponding 95% confidence intervals and outcome with a p value<0.05. All statistical analyses were performed using SPSS®, version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
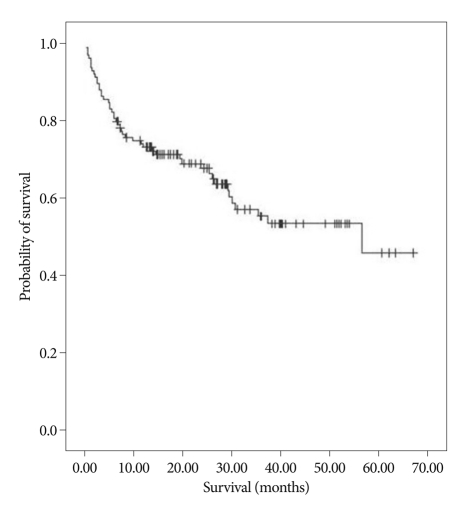
The mean survival of all 122 patients was 41.7 ± 2.8 months, and the survival curve is illustrated in Fig. 1 (Fig. 1).
Fig. 1.
Kaplan-Meier survival curve; a total of 122 patients.
Epidemiological factor
Of the 122 patients, 64 (52.5%) were male with a mean survival of 46.1±3.6 months, whereas 58 (47.5%) were female with a mean survival of 35.7±3.9 months; however, this difference was not statistically significant (p value=0.936).
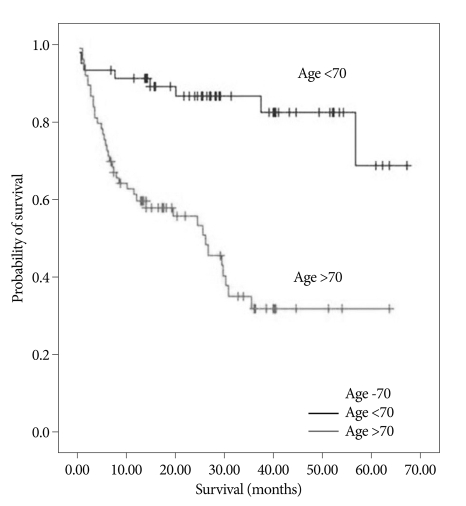
Of the 122 patients, 71 (58.2%) were older than 70 years old, and 51 (41.8%) were younger than 70 years old. The mean survival of the older patients (≥70 years) was 29.7±3.4 months, whereas that of the younger patients (<70 years) was significantly longer, as 58.9±3.2 months (p value=0.000). Life expectancy based on age is illustrated in Fig. 2 (Fig. 2).
Fig. 2.
Kaplan-Meier survival curve based on age (older and younger than 70 years) (p value=0.000).
Past medical history and comorbidity
The past medical history and comorbidities of the 122 patients were distributed as follows : 75 (61.5%) had a history of hypertension, 61 (50.0%) had atrial fibrillation (AF), 29 (23.8%) had heart failure, 29 (23.8%) had DM, 28 (23.0%) had previous stroke, 24 (19.7%) were smokers, and 19 (15.6%) had dyslipidemia. The mean survival based on these factors showed no statistically significant differences (Table 1).
Table 1.
The factors that are likely to affect the mean survival
*Total cholesterol >200 mg/dL. ACA : anterior cerebral artery, AF : atrial fibrillation, DM : diabetes mellitus, IA : intra-arterial, MCA : middle cerebral artery, mRS : modified Rankin Scale, PCA : posterior cerebral artery, SD : standard deviation
Functional status and survival
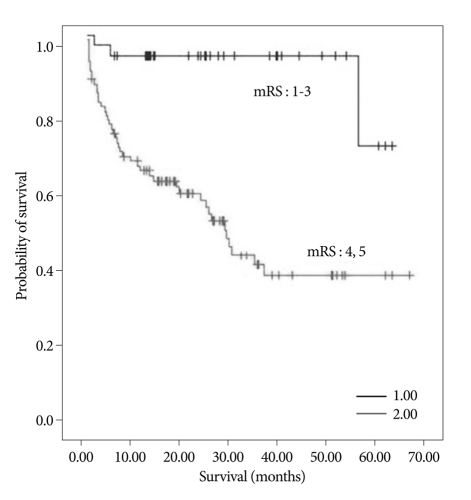
Of the 122 patients, 85 (69.7%) experienced a poor functional outcome (mRS≥4) at one month after the onset of stroke, whereas 37 (30.3%) experienced a good functional outcome (mRS≤3). The mean survival of the poor outcome group was 33.9±3.3 months, whereas that of the good outcome group was 58.6±2.6 months (p value=0.000). Life expectancy based on mRS is presented in Fig. 3 (Fig. 3).
Fig. 3.
mRS-based Kaplan-Meier survival curve one month after stroke (p value=0.000).
Treatment modality and survival
Table 1 shows relation between treatment modality and survival period. We classified treatment modalities into four categories; conservative treatment, successful intra-arterial thrombolysis, failed intra-arterial thrombolysis, and decompressive craniectomy. Intravenous injection of tissue plasminogen activator was included in conservative treatment. There were no overlapped patients among these four categories and no statistical difference was found between treatment modalities and survival period (p value=0.398). However, decompressive craniectomy group revealed the longest mean survival time (46.7 months) among four treatment modalities (Table 1).
Dominant hemisphere and survival
Right to left hemisphere ratio was 72 (59%) : 50 (41%), but there was also no statistical difference of survival period between two groups (p value=0.425) (Table 1).
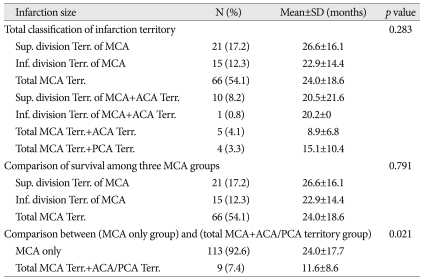
Infarction size and survival
We classified into seven categories by infarction size (Table 2). No statistical difference was found between each seven group and survival period (p value=0.283). We also compared survival period among three different MCA territorial groups; superior division, inferior division, and total MCA territory, but no statistical difference was found (p value=0.791) (Table 2). However, significant difference was detected between (MCA only group) and (total MCA territory+ACA/PCA territory group) (p value=0.021) (Table 2).
Table 2.
The relation between infarction size and survival
ACA : anterior cerebral artery, MCA : middle cerebral artery, PCA : posterior cerebral artery, SD : standard deviation, Sup. : superior, Inf. : inferior, Terr. : territory
Cause of death
Of the 122 patients, 47 patients (38.5%) were dead during follow-up period. The causes of death were as follows; cerebral infarction itself in four (8.5%), pneumonia in 35 (74.5%), sepsis due to bedsore in one (2.1%), sepsis due to urinary tract infection in one (2.1%), and others in six patients (12.8%, heart problem in four, liver disease in one, bowel perforation in one patient). Pneumonia was the most common cause of death among patients with a large cerebral infarction.
DISCUSSION
We performed the first study that investigated the early influence of disability level at one month after a large cerebral infarction. The idea to evaluate functional status at one month after stroke was primarily based on preventing follow-up loss of the patients and on the thinking that what would the result of functional status one month after stroke. Several studies have reported the correlation between functional outcome at three or six months after stroke and long-term survival4,11,13). Eriksson et al.4) reported that the functional outcome three months after stroke is an independent predictor of poor survival. Slot et al.11) also reported that functional status six months after an ischemic stroke correlated with long term survival. Erikson et al.4) and Vernino et al.12) proposed measuring functional outcome at three months after stroke because direct, stroke-mediated brain damage should be resolved at that point in time; however, we think that an unstable functional status one month after stroke also has some prognostic value in itself. Namely, if a patient is under unstable condition one month after stroke, the patient has more chance to have a high mRS grade, so long-term survival chance of the patient is likely to be relatively low on this unstable condition account.
Most large hospitals, such as ours, follow-up loss of patients is common after acute phase care of stroke and the proportion of large cerebral infarction patients who are lost to follow-up is higher than the proportion of patients with small cerebral infarction. At our hospital, most of the patients with large cerebral infarctions are transferred to rehabilitation department or another hospital for rehabilitation after acute-phase care, and those with poor functional status are transferred to long-term care facilities. Nevertheless, the data collected within one month of the cerebral infarction were preserved because, most of these patients were in the rehabilitation department of our hospital or visited outpatient clinic, which reduced the possibility of them being lost to follow-up. So, it is easier to obtain more accurate functional status of the patients at one month after stroke than at three or six months.
Early death after large cerebral infarction is a well-known and a common occurrence. Furthermore, approximately 60% of all deaths during the first year occur within the first month3,7). This concept is supported by our cause of death result, which demonstrates that pneumonia is the most common cause of death among patients with a large cerebral infarction after one month stroke. Thus, the special significance of life expectancy based on data after one month of stroke onset should be investigated.
Age has also been reported to be a significant prognostic factor that influences long-term survival after stroke. Boysen et al.2) reported that the 15 year-survival rate after stroke was 11% in the 65- to 72-year-old age group and was 28% in the <65 year-old age group. In the current study, there were significant differences in the mean survival times between the >70-year-old age group and the <70-year-old age group (Fig. 2). But, there are some weak points in this result. If ignoring disease and following 70 months, by age standard, it is likely to be possible for over 70-year-old adults to have low survival rate than under 70-year-old adults. So, age is not thought to be a fresh prognostic factor carrying weight.
In addition, other factors that are also significant predictors of poor survival (male gender, hypertension, heart failure, AF, DM, a previous history of stroke, smoking, and dyslipidemia) did not significantly influence the mean survival time in the current study. We assume that this was caused by the relatively small number of patients (n=122) that were included in this study, in comparison to the larger numbers that have been included in other stroke reports.
Treatment modality and dominance of hemisphere did not also significantly influence the survival period in the current study (p value=0.398, 0.425), but decompressive craniectomy group revealed the longest mean survival time (46.7 months) among four treatment modalities (Table 1). There are several reports regarding the effectiveness of decompressive craniectomy in acute cerebral infarction8,14); therefore, early and active interventions that reduce dependency one month after a large cerebral infarction might positively impact long-term survival.
Table 2 shows the relation between infarction size and survival. This result demonstrates that involvement of ACA or PCA territory is an important poor prognostic factor in MCA territorial infarction patients (p value=0.021). But, no statistical difference was found between one division of MCA territorial infarction and total MCA territorial infarction in survival period (p value=0.791).
In conclusion, age and functional outcome after one month could be a critical prognostic factor for survival. Life expectancy after a large cerebral infarction is short and is similar to that of malignant diseases, such as cancer. Furthermore, a multi-center-based investigation of mRS grade at one, three, and six months after a large cerebral infarction with a large study population might reveal the correlations between the mRS grade at each point in time and the long-term survival.
CONCLUSION
Age (older and younger than 70 years old) and functional outcome at one month after stroke could be critical prognostic factors for survival in large cerebral infarction patients. MCA territorial infarction with ACA, PCA territorial infarction is a poor prognostic factor. Therefore, early and active interventions that reduce dependency one month after a large cerebral infarction might have positive impact on long-term survival.
Acknowledgements
This study was partially supported by a grant from the Korea Health 21 R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (grant no : A06-0171-B51004-06N1-00040B).
References
- 1.Acciarresi M, Caso V, Venti M, Milia P, Silvestrelli G, Nardi K, et al. First-ever stroke and outcome in patients admitted to Perugia Stroke Unit: predictors for death, dependency, and recurrence of stroke within the first three months. Clin Exp Hypertens. 2006;28:287–294. doi: 10.1080/10641960600549249. [DOI] [PubMed] [Google Scholar]
- 2.Boysen G, Marott JL, Grønbaek M, Hassanpour H, Truelsen T. Long-term survival after stroke : 30 years of follow-up in a cohort, the Copenhagen City Heart Study. Neuroepidemiology. 2009;33:254–260. doi: 10.1159/000229780. [DOI] [PubMed] [Google Scholar]
- 3.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson M, Norrving B, Terént A, Stegmayr B. Functional outcome 3 months after stroke predicts long-term survival. Cerebrovasc Dis. 2008;25:423–429. doi: 10.1159/000121343. [DOI] [PubMed] [Google Scholar]
- 5.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989-1990. Stroke. 2002;33:1034–1040. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- 6.Hannerz H, Nielsen ML. Life expectancies among survivors of acute cerebrovascular disease. Stroke. 2001;32:1739–1744. doi: 10.1161/01.str.32.8.1739. [DOI] [PubMed] [Google Scholar]
- 7.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year survival after first-ever stroke in the Perth Community Stroke Study. Stroke. 2003;34:1842–1846. doi: 10.1161/01.STR.0000082382.42061.EE. [DOI] [PubMed] [Google Scholar]
- 8.Huh JS, Shin HS, Shin JJ, Kim TH, Hwang YS, Park SK. Surgical management of massive cerebral infarction. J Korean Neurosurg Soc. 2007;42:331–336. doi: 10.3340/jkns.2007.42.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes : a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 10.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 11.Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock P. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ. 2008;336:376–379. doi: 10.1136/bmj.39456.688333.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernino S, Brown RD, Jr, Sejvar JJ, Sicks JD, Petty GW, O'Fallon WM. Cause-specific mortality after first cerebral infarction : a population-based study. Stroke. 2003;34:1828–1832. doi: 10.1161/01.STR.0000080534.98416.A0. [DOI] [PubMed] [Google Scholar]
- 13.Weimar C, Ziegler A, König IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. J Neurol. 2002;249:888–895. doi: 10.1007/s00415-002-0755-8. [DOI] [PubMed] [Google Scholar]
- 14.You SH, Lee JI, Kim JS, Hong SC. Decompressive craniectomy in acute cerebral infarction : treatment results and prognostic factors. J Korean Neurosurg Soc. 2004;35:551–554. [Google Scholar]