Our findings suggest that, assuming a reasonable level of specificity, primary CT colonography may be more suitable than optical colonoscopy for the initial investigation of suspected colorectal cancer.
Abstract
Purpose:
To perform a systematic review and meta-analysis of published studies assessing the sensitivity of both computed tomographic (CT) colonography and optical colonoscopy (OC) for colorectal cancer detection.
Materials and Methods:
Analysis followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations. The primary data source was the results of a detailed PubMed search from 1994 to 2009. Diagnostic studies evaluating CT colonography detection of colorectal cancer were assessed by using predefined inclusion and exclusion criteria, in particular requiring both OC and histologic confirmation of disease. Studies that also included a mechanism to assess true-positive versus false-negative diagnoses at OC (eg, segmental unblinding) were used to calculate OC sensitivity. Assessment and data extraction were performed independently by two authors. Potential bias was ascertained by using Quality Assessment of Diagnostic Accuracy Studies guidelines. Specific CT colonography techniques were cataloged. Forest plots of per-patient sensitivity were produced on the basis of random-effect models. Potential bias across primary studies was assessed by using the I2 statistic. Original study authors were contacted for data clarification when necessary.
Results:
Forty-nine studies provided data on 11 151 patients with a cumulative colorectal cancer prevalence of 3.6% (414 cancers). The sensitivity of CT colonography for colorectal cancer was 96.1% (398 of 414; 95% confidence interval [CI]: 93.8%, 97.7%). No heterogeneity (I2 = 0%) was detected. No cancers were missed at CT colonography when both cathartic and tagging agents were combined in the bowel preparation. The sensitivity of OC for colorectal cancer, derived from a subset of 25 studies including 9223 patients, was 94.7% (178 of 188; 95% CI: 90.4%, 97.2%). A moderate degree of heterogeneity (I2 = 50%) was present.
Conclusion:
CT colonography is highly sensitive for colorectal cancer, especially when both cathartic and tagging agents are combined in the bowel preparation. Given the relatively low prevalence of colorectal cancer, primary CT colonography may be more suitable than OC for initial investigation of suspected colorectal cancer, assuming reasonable specificity.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11101887/-/DC1
Introduction
Colorectal cancer is a major cause of morbidity and mortality in Western countries (1,2). Early stages of colorectal cancer are associated with a relatively high 5-year survival rate, whereas late stages, characterized by nodal and distant metastasis, are associated with poor survival, despite the use of intensive and costly chemotherapeutic protocols (3,4). Despite its relatively low sensitivity for colorectal cancer detection, fecal occult blood test screening has nonetheless been shown to reduce colorectal cancer mortality in controlled trials, underlining the importance of cancer detection (5). Screening tests that aim to depict colorectal cancer directly, including both endoscopic and radiologic methods, would be expected to be more sensitive than the fecal test and therefore depict proportionately more early stage cancers, with a contingent beneficial effect on disease-specific mortality (6).
Computed tomographic (CT) colonography is a minimally invasive imaging examination of the colorectum that has been endorsed by several key medical organizations for colorectal cancer screening and diagnosis (7). Most published studies of CT colonography have focused primarily on detection of colorectal polyps, using optical colonoscopy (OC) as the reference standard test. Because of the low prevalence of invasive cancer, especially in a screening setting, test sensitivity for invasive cancer cannot be properly evaluated in single studies owing to the small numbers of cancers identified in the screening group. Furthermore, the two most recent systematic reviews of CT colonography diagnostic performance (8,9) dealt only with polyps and not cancer.
Owing to the lack of an independent reference standard, the sensitivity of OC for colorectal cancer has been mainly assessed indirectly by evaluating the rate of interval cancers diagnosed within a short interval after the index examination (10,11). Blinding of CT colonography results during subsequent OC, with either segmental or posttest unblinding of CT colonography results, allows for immediate retesting and thus creates an enhanced reference standard by which CT colonography and conventional OC can both be compared (12). Such a study design is primarily intended to minimize the likelihood of false-negative OC diagnoses masquerading as CT colonography false-positive diagnoses but has the added benefit of allowing a less biased estimate of OC sensitivity itself.
The aim of this systematic review and meta-analysis was to assess the sensitivity of both CT colonography and OC in the detection of colorectal cancer.
Materials and Methods
Methods for analysis and inclusion criteria were based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses, or PRISMA, recommendations for systematic reviews and meta-analyses (13) and were specified and documented in advance in a formal protocol.
Eligibility Criteria
We considered all studies for the period from January 1994 (the year CT colonography was first described) to December 2009 in which patients underwent CT colonography for the diagnosis of colorectal polyps and cancer with subsequent OC for verification of the CT colonography findings. For a study to be included in our review, its focus had to be detection of colorectal lesions (ie, polyps and masses), and it had to include a histologic reference standard to discriminate between benign and malignant lesions. Studies without sufficient details regarding lesion detection or without subsequent colonoscopic and histologic verification were excluded. Studies with excessively high cancer prevalence because of a priori patient selection (eg, known occlusive mass at previous endoscopy) were also excluded. Because our focus was on sensitivity for cancer, we also excluded studies in which no malignancies were found. Additional exclusion criteria included studies with fewer than 30 patients, studies based on data sets that were artificially enriched with positive cases, review articles, position papers, editorials, commentaries, and book chapters. If there was any suspicion of cohort overlap between studies, potential duplicate studies were excluded.
Information Sources
A literature search was performed for relevant publications in PubMed. The Medical Subject Headings, or MeSH, terms colonography, colography, CT colonography, CT colonoscopy, CT pneumocolon, virtual colonoscopy or virtual endoscopy were used, adopting “human studies” as the only limit. A preliminary search performed by using other search engines (eg, EMBASE and Scopus), failed to detect any additional references not already identified by PubMed, so we did not use these search engines further. The full articles of all potential studies that satisfied our inclusion criteria were retrieved, and additional manual searches of their reference lists were performed to identify any additional studies that may have been missed by using the above-mentioned procedure.
Study Selection
Potential studies were initially screened on the basis of article title and abstract by two researchers (P.J.P, an abdominal radiologist with nearly 10 years of experience with CT colonography, and C.H., a gastroenterologist with more than 15 years of experience in performing OC). The reviewers checked whether inclusion and exclusion criteria were met, and, for all articles with even a remote potential for study inclusion, the full text was retrieved and reviewed.
Data Extraction and List of Items
Data extraction was performed independently by the two reviewers, using predefined data extraction forms. From each primary study, reviewers independently abstracted the following: (a) the year of publication, (b) the country where the study was performed, (c) whether it was a single- or multicenter study, (d) the screening or nonscreening population characteristics, whether (e) cathartic preparation and/or (f) fecal tagging was performed, (g) whether intravenous contrast material was routinely administered, (h) whether prone and supine images were obtained, (i) the type of CT scanner (single- or multidetector) used, (j) the collimation used, (k) the reconstruction interval used, (l) the radiation dose, (m) the primary mode of CT colonography data interpretation (two-dimensional [2D] vs three-dimensional [3D]), (n) the number of study observers, (o) the number of observers per patient, (p) reader experience, (q) whether CT colonography results were initially blinded at OC to allow an enhanced reference standard against which conventional OC results could be compared, (r) the absolute number of cancers, (s) the relative prevalence of cancer, (t) the sensitivity of CT colonography for cancer, and (u) the sensitivity of OC for cancer (when assessable per item q). For the purpose of this analysis, histologically proved cancers were considered as true-positive results at CT colonography if they were detected prior to OC, surgical, and/or pathologic confirmation and as true-positive results at OC if they were identified at initial blinded endoscopy prior to the unblinding of CT colonography results. The sensitivity of OC could be assessed only for studies where criterion q applied. We also extracted lesion characteristics (location, size, morphology) for false-negative cancer diagnoses at CT colonography.
Multiple attempts were made to contact authors if data presentation was incomplete or if it was necessary to resolve an apparent conflict or inconsistency in the article. These details have been cataloged in Appendix E1 (online). The additional information most frequently required was specifically related to study performance for histologically proved malignant lesions or to further details about the cancers missed at CT colonography. Extraction was arbitrated by a third investigator (S.H., an abdominal radiologist with more than 10 years of experience in reading CT colonography images) in the event of any lack of agreement between the two researchers.
Potential Bias in Primary Studies
To assess the methodologic quality of the included primary studies and to detect potential bias, we used items from the Quality Assessment of Diagnostic Accuracy Studies, or QUADAS, tool that were relevant for our analysis (14). In particular, we tracked the following: (a) whether asymptomatic adults or patients at higher risk of colorectal neoplasia were included, (b) the time interval between the index and reference tests, (c) whether the reference standard was modified by CT colonography results, whether the technical methods for (d) CT colonography or (e) OC could be replicated on the basis of information provided in the included studies, whether data on (f) CT colonography failures or (g) incomplete OC examinations were provided, whether CT colonography lesions were matched at OC on the basis of (h) size and/or (i) segment location, and (j) how lesions were measured at OC.
Summary Measures
The primary end points of this systematic review were (a) the per-patient sensitivity of CT colonography for cancer and (b) the per-patient sensitivity of OC for cancer. The secondary end points of this systematic review were to address (a) the characteristics of cancers missed at CT colonography and (b) the clinical or technical characteristics associated with cancers missed at CT colonography.
Data and Statistical Analysis
The sensitivity of CT colonography for cancer was defined for each primary study as the proportion of true-positive per-patient diagnoses for CT colonography among the total number of per-patient cancers detected by using the ultimate reference standard. The sensitivity of OC for cancer was defined for each primary study that utilized segmental or posttest unblinding as the proportion of true-positive per-patient diagnoses for OC among the total number of per-patient cancers detected by using the ultimate reference standard (ie, final results after unblinding). Per-patient sensitivity values for CT colonography and OC for cancer detection were summarized by using a random-effects model. Heterogeneity between primary studies was assessed by using the I2 statistic, which provides an estimate of the degree of variance due to heterogeneity rather than chance and which is based on the traditional measure of variance, the Cochrane Q statistic (15). Values of I2 equal to 25%, 50%, and 75% were assumed to represent low, moderate, and high heterogeneity, respectively. When heterogeneity in sensitivty was present, meta regression analysis and Galbraith plots were used to determine characteristics that contributed the most to the heterogeneity. All collected variables were used in the meta regression. The meta-analysis was performed with software (Meta-DiSc; http://www.hrc.es/investigacion/metadisc_en.htm).
It is important to note and understand that the specificity of CT colonography (and OC) for cancer detection could not be assessed on the basis of these published trials because the number of false-positive and true-negative results for cancer assessment is not known because of the usual definition of CT colonography (and OC) test positivity according to lesion size and not morphology or likely histologic nature. Specifically, benign polyps are almost always included as a true-positive finding, especially when they are large, and this obscures the necessary cancer-specific false-positive versus true-negative data needed for calculation of specificity for cancer. Many relevant lesions, particularly large, advanced adenomas, would be included in the false-positive category for cancer but are rarely reported in this way. Any cancer detected at OC that was smaller than the lesion size threshold for CT colonography positivity was considered a false-negative finding at CT colonography.
Results
Study Selection
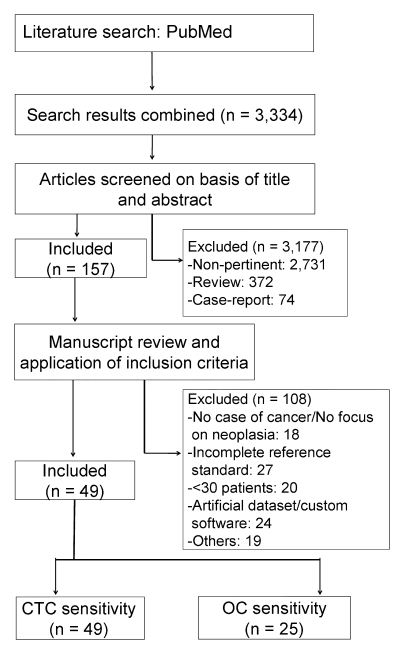
A flow diagram of this systematic review, with the number of articles retrieved, included, and excluded, as well as the reasons for exclusion, is shown in Figure 1. The last search was performed in December 2009. In summary, 3334 articles were identified in the PubMed search. After nonpertinent articles were excluded, 157 studies were considered for inclusion on the basis of a review of their abstracts. From among these 157 potential articles, an additional 108 articles were excluded, with the reasons for exclusion provided in Figure 1 (excluded studies are reported in Table E1 [online]). One study was excluded because of cohort duplication with another study. This left 49 studies for inclusion in the systematic review (16–64).
Figure 1:
Flowchart of meta-analysis. CTC = CT colonography.
Characteristics of the Included Studies
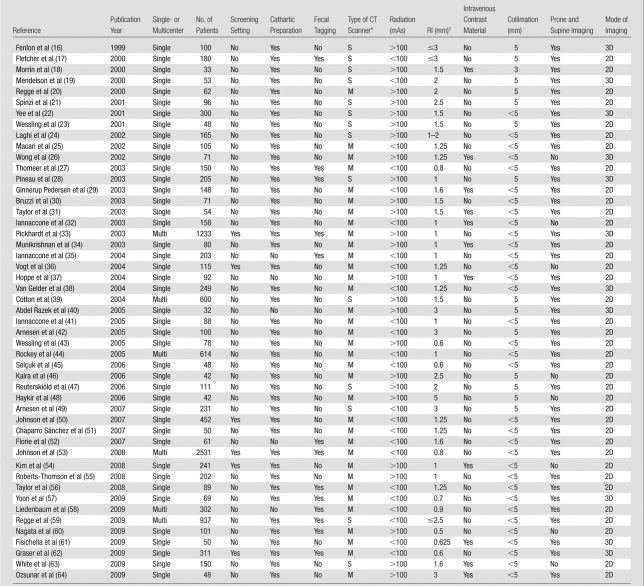
Overall, 29 (59%) of 49 studies were performed in Europe, 12 (24%) were performed in the United States, five were performed in Asia, two were performed in Australia, and one was performed in Africa. Only six (12%) of 49 studies were multicentric. A simultaneous evaluation of both CT colonography and OC cancer detection rates was possible for 25 studies, while lack of post-OC verification prevented assessment of OC sensitivity in the remaining 24 studies. To obtain further information, 16 authors were contacted, as detailed in Appendix E1 (online).
Patient Cohort
A total of 11 551 patients were enrolled in the selected studies (Table 1). The number of patients enrolled per study ranged from 32 to 2531, with a median of 101. Only six studies (with 4883 patients—42.3% of the total) included only asymptomatic subjects who were typically representative of individuals in a screening setting. The remaining 43 studies (with 6668 patients—57.7% of the total) examined a symptomatic and/or disease-enriched population. When we considered only the 25 series in which simultaneous evaluation of CT colonography sensitivity and OC sensitivity was permitted, 9223 patients were available for the analysis (79.8% of the total). Of note, five of the six screening studies allowed assessment of OC sensitivity for cancer.
Table 1.
Characteristics of the Included Studies
M = multidetector, S = single detector.
RI = reconstruction interval.
CT Colonography Technique
A cathartic bowel preparation for CT colonography, usually based on polyethylene glycol or sodium phosphate, was used in 44 (90%) of 49 studies, and a noncathartic preparation was used in the remaining five studies. Fecal tagging was used in all patients in 12 (24%) of 49 studies, was used in a subgroup of the study population in one series, and was not used in the remaining studies. A multidetector CT scanner was utilized in 35 (71%) of 49 studies, while a single-detector CT scanner was used in 14 series. Both prone and supine images were acquired in 41 (84%) of 49 studies, while in the remaining eight studies, a single position was used. Intravenous contrast material was routinely administered to all patients in only 11 (22%) of 49 studies. A primary 3D mode of lesion detection was chosen in 11 (22%) of 49 studies, while a primary 2D mode was used in 38 studies. The number of observers per study was greater than one in 36 (73%) of 49 studies, and the number of observers per patient was greater than one in 24 (49%) of 49 series. Further details on the technical characteristics of the CT colonography examinations are provided in Table 1. Colonoscopy was performed after cathartic preparation in all studies.
Colorectal Cancer Prevalence
Overall, 414 colorectal cancers were detected in the included studies in 11 551 patients, corresponding to a prevalence of less than 4% overall (Tables 2, 3). A total of 20 cancers were diagnosed in the screening studies in 4883 individuals, corresponding to a prevalence of less than 0.5%, and 394 cancers were found in the disease-enriched studies with 6668 patients, corresponding to a prevalence of nearly 6%. The number of cancers per study ranged from one to 41, with a median of six. When restricting our analysis to studies in which CT colonography findings were withheld for the initial OC evaluation (ie, those used to assess OC sensitivity)—studies that included 9223 patients—the overall number of cancers was 188, corresponding to a cancer prevalence of about 2%.
Table 2.
Distribution of Cases of Invasive Colorectal Cancer in All Included Studies

Note.—All data were calculated on the basis of a per-patient analysis.
Table 3.
Distribution of Cases of Invasive Colorectal Cancer in 25 Studies in which OC Sensitivity Could also Be Assessed
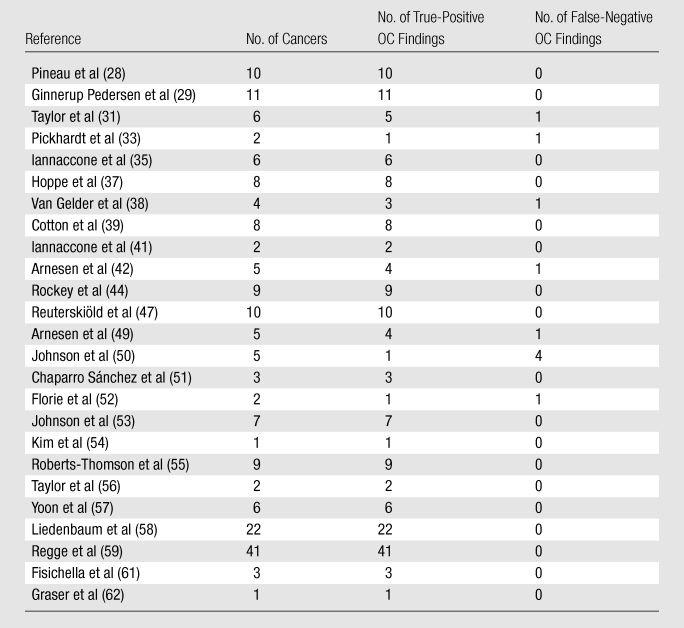
Note.—All data were calculated on the basis of a per-patient analysis.
Potential for within-Study Bias
Results of quality assessment of the individual studies are reported in Tables E2 and E3 (online). Radiologists were generally aware of the clinical indications for CT colonography in the included studies, so we may presume that a higher prevalence of disease was expected in the nonscreening studies, potentially leading to an overestimation of the sensitivity of CT colonography. A short interval between CT colonography and OC verification was noted in all studies, excluding the possibility of disease progression. Colonoscopy is an imperfect reference standard for detection of lesions, including cancer. For this reason, OC test characteristics may be enhanced by knowledge of the prior CT colonography results, whether the results are unblinded to the endoscopist prior to the initial OC examination, segmentally unblinded during the initial examination, or unblinded after the procedure—leading to the need for repeat OC in some cases. In detail, intraprocedural segmental unblinding was adopted in 18 studies, whereas post-OC unblinding with a second OC for potential misses was allowed for discordant lesions measuring greater than 5 mm and 10 mm or larger in two and four studies, respectively. Repeat OC was performed in all patients in one study. In the remaining 22 series, there was no provision for secondary evaluation of OC results. As mentioned, the unavoidable use of OC as the reference standard for both tests could lead to potentially underestimating the sensitivity of CT colonography and overestimating the sensitivity of OC, especially if the CT colonography results were not unblinded to the endoscopist. As shown in Tables E2 and E3 (online), the majority of the included studies reported data on technical failures for either CT colonography or OC, as well as ample information to allow for replication of the study by others.
To assess the accuracy of CT colonography on the basis of subsequent colonoscopy results, a lesion-matching algorithm between CT colonography and OC is required. Both size-based and location-based matching was clearly described in 36 studies, while either size or location was the only parameter listed in four series.
Synthesis of Results
The results of the included individual studies are provided in Table 4.
Table 4.
Clinical and Technical Characteristics of the 16 Cancers Missed at CT Colonography
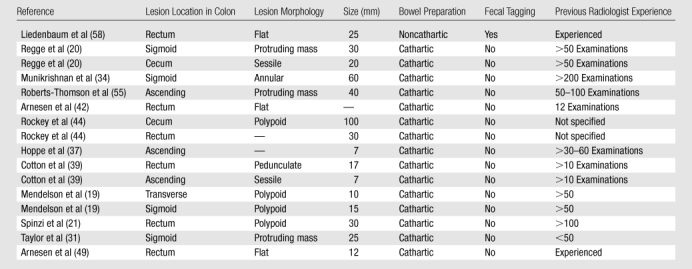
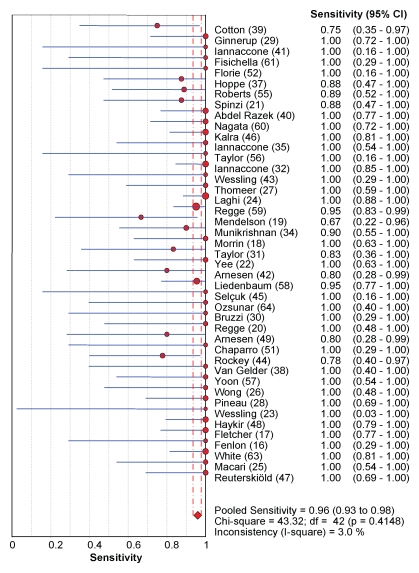
CT colonography sensitivity for cancer.—In the included studies, CT colonography depicted 398 (96.1%) of 414 histologically proved cancers (95% confidence interval [CI]: 93.8%, 97.7%), as shown in Figure 2. Interstudy heterogeneity (I2) was 0% (Fig 3). The main characteristics of the 16 cancers missed at CT colonography are listed in Table 4. Six (38%) of the 16 missed cancers were located proximal to the splenic flexure, and 10 were located in the rectosigmoid colon.
Figure 2a:
Forest plots of studies included in the meta-analysis show individual and pooled estimates for diagnostic sensitivity for colorectal cancers for (a) CT colonography and (b) OC.
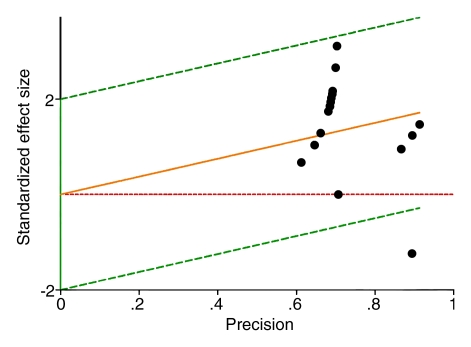
Figure 3a:
Galbraith plots of included studies show the sensitivity of (a) CT colonography and (b) OC for colorectal cancer detection. In b, the study by Johnson et al (50) (the data point at lower right, under the green line) appears to be mainly responsible for the heterogeneity detected in this estimate.
Figure 2b:
Forest plots of studies included in the meta-analysis show individual and pooled estimates for diagnostic sensitivity for colorectal cancers for (a) CT colonography and (b) OC.
Figure 3b:
Galbraith plots of included studies show the sensitivity of (a) CT colonography and (b) OC for colorectal cancer detection. In b, the study by Johnson et al (50) (the data point at lower right, under the green line) appears to be mainly responsible for the heterogeneity detected in this estimate.
OC sensitivity for cancer.—In the 25 included studies that allowed comparison between initial OC and OC subsequently enhanced by knowledge of prior CT colonography results, initial OC depicted 178 (94.7%) of 188 cancers (95% CI: 90.4%, 97.2%) (Fig 2). Interstudy heterogeneity (I2) was 50%. The moderate degree of heterogeneity when estimating OC sensitivity was mainly related to one study (50), in which a 20% OC sensitivity for cancer was calculated (Fig 3). When we excluded this study, a low degree of heterogeneity (I2 < 50%) remained. No variable at meta regression was found to explain the residual degree of heterogeneity.
False-negative results at CT colonography.—The lesion characteristics of all 16 cancers missed at CT colonography in the included studies are shown in Table 4. Ten (62%) of 16 missed cancers were located in the rectosigmoid colon, including six within the rectum, and six (38%) were located proximal to the splenic flexure. There were no cancers missed at CT colonography in the studies where both cathartic bowel preparation and fecal tagging were used.
Additional analyses.—As expected because of the lack of heterogeneity, the estimate of CT colonography sensitivity for colorectal cancer was robust to additional subanalysis. As an example, we report in Figure 4 the estimates of CT colonography sensitivity in screening and nonscreening series.
Figure 4a:
Forest plots of studies included in the meta-analysis show individual and pooled estimates for diagnostic sensitivity of CT colonography for colorectal cancer in (a) screening and (b) nonscreening studies.
Figure 4b:
Forest plots of studies included in the meta-analysis show individual and pooled estimates for diagnostic sensitivity of CT colonography for colorectal cancer in (a) screening and (b) nonscreening studies.
Discussion
Our meta-analysis shows that the pooled sensitivities of CT colonography and OC for colorectal cancer were about 96% and 95%, respectively. When one considers the large number of cancer cases included, the wide range in CT colonography techniques used, and the lack of heterogeneity in the CT colonography sensitivity estimate, our study results support the clinical equivalence between CT colonography and OC for the detection of invasive cancer. We purposely chose not to restrict our inclusion criteria to state-of-the-art techniques or technology, so that our estimates could be appropriately generalized to daily practice. This decision resulted in a wide variety of different technical combinations, including single- and multidetector CT scanners, variable use of fecal tagging and intravenous contrast material, and the use of standard supine and prone imaging versus single-position imaging. Despite this considerable variability in CT colonography techniques, there was very little heterogeneity in the sensitivity data, implying that CT colonography is a highly robust method for cancer detection, regardless of the specific technique used. In particular, it is important to note that the use of either a primary 2D mode or a primary 3D mode for lesion detection, which is a critical distinction for polyp sensitivity (65), had little impact on cancer detection, as most invasive cancers are readily detectable on 2D views. Interestingly, five continents were represented in our review, suggesting that CT colonography is now mature enough to be regarded as a universal procedure.
High sensitivity for colorectal cancer is crucial, whether dealing with symptomatic patients or asymptomatic screening. We found a 10-fold difference in cancer prevalence between asymptomatic screening and symptomatic study populations (<0.5% vs 6%). While it is often argued that diagnostic test performance may improve in line with presumed disease prevalence (owing perhaps to increased reader vigilange or clinical suspicion), we found that the sensitivity of CT colonography remained high regardless of disease prevalence.
Most cancers missed at CT colonography were located in the rectosigmoid colon, including six within the rectum, whereas only 38% (six of 16) were located proximal to the splenic flexure. The relative lack of missed right-sided cancers by CT colonography would seem to complement OC, where most missed cancers appear to be right sided (9,66). In general, the right colon is relatively easy to distend and therefore evaluate at CT colonography, whereas the increased distance in physical endoscopy makes right-sided evaluation more challenging. The relative increase in missed rectosigmoid cancers at CT colonography may relate to challenges with luminal distention and may possibly have been due in part to the prevalent anatomic distribution of cancer in the included studies.
We identified a subset of primary CT colonography studies that utilized a more optimal colonoscopic reference standard that incuded a provision for cancers initially missed at colonoscopy but detected at CT colonography, enabling us to compare the sensitivity of CT colonography and conventional OC (before unblinding of CT colonography results). OC sensitivity for cancer cannot be ascertained in previous tandem colonoscopy studies owing to the small sample sizes, the very low prevalence of cancer in these studies, and the suboptimal method of using colonoscopy as its own reference standard (67). The only available estimates of OC cancer sensitivity have previously been limited to the indirect observation of interval carcinomas in cohorts of patients after OC (9,68). According to our meta-analysis, conventional OC is a very sensitive test for detecting invasive cancer, supporting its use in patients at high risk for colorectal cancer, such as those with a positive fecal test or with a hereditary nonpolyposis colorectal cancer syndrome (7). There was, however, unexpected heterogeneity in our point estimate, suggesting the presence of significant interstudy variability. In particular, very poor performance for OC was reported in one CT colonography study, in which only one of five cancers was prospectively detected at OC (50), whereas all cancers were identified at CT colonography. In this study, all OC examinations were performed by a staff gastroenterologist or were supervised by one of approximately 50 experienced staff gastroenterologists and colorectal surgeons. In chemopreventive studies, in which OC was usually repeated 1 year after the index examination, an unexpectedly high rate of colorectal cancer has been reported (69), especially when compared with the very low rate of interval cancer reported in the National Polyp Study (70), suggesting pronounced interobserver variability for cancer detection. Similarly, recommendations for the first surveillance examination after colorectal cancer surgery have been changed from 3 years to 1 year owing to the unexpectedly high rate of interval cancers after a pre- or perioperative OC examination (71). When OC was performed by nongastroenterologists in cohort studies, a disappointingly low sensitivity of 88% was reported (68). Finally, a recent population study (66) showed that the risk of right-side colon cancer mortality within 6–24 months after a negative index OC examination was actually increased compared with that in the reference population, strongly suggesting the likelihood of missed right-sided cancer at OC. When one couples the heterogeneity found in our analysis for OC with the dire consequences of missing a cancer, OC sensitivity for cancer should become a major issue in the evaluation of OC quality. In particular, dedicated studies aimed at assessing interobserver variability in cancer detection at OC are needed.
There were limitations to our analysis. OC was the only reference standard used to assess CT colonography sensitivity in 24 studies, whereas an improved reference standard that included the CT colonography findings was applied in the remaining 25 studies. For this reason, our point estimates may underestimate the sensitivity of CT colonography, since it is possible that some lesions misclassified as false-positive CT colonography findings were actually cancers representing false-negative findings at OC. We present our sensitivity data for cancer with the implicit assumption that subsequent surgery would ultimately lead to a reduction in colorectal cancer mortality. However, detection of late-stage colorectal cancer does not improve 5-year survival (3). Unfortunately, none of the included studies reported cancer staging according to the TNM or Duke classification, so we could not estimate the proportion of early stage cancers detected at CT colonography. One recent study (72) of CT colonography screening in more than 10 000 adults showed that the detected asymptomatic cancers were of an earlier stage compared with symptomatic cancers. As described earlier, we could not include a specificity assessment in our review, because published studies generally report specificity for all lesions, including polyps, but not specifically for cancer. In a recent study (73) that included a CT colonography screening cohort of more than 5000 adults, the vast majority of colorectal cancers measured larger than 3 cm. Considering the relationship at CT colonography between diagnostic accuracy and lesion size (33,53), it is unlikely that the specificity of CT colonography for colorectal cancer would suffer disproportionately given the very high sensitivity for CT colonography reported in our analysis. Investigators in a recent study from New Zealand (74) evaluated the sensitivity of CT colonography for cancer by using their national cancer registry database as the reference standard in nearly 4000 adults and found a similar sensitivity of 95% (123 of 131). One final limitation of our study was that, by considering only invasive cancers and not advanced adenomas, we may inadvertently deemphasize the importance of cancer prevention, which is not our intent.
In conclusion, this systematic review and meta-analysis has provided point estimates of CT colonography and OC sensitivity for invasive cancer. Not only does the pooled sensitivity of CT colonography for colorectal cancer appear similar to that of OC, but its sensitivity was maintained despite wide variation in technique, which is important with regard to the generalizability and widespread implementation of CT colonography. In contrast, the presence of substantial heterogeneity in the data for OC suggests a need for greater understanding of the performance of this test. Given the relatively low prevalence of colorectal cancer, even among symptomatic cohorts, our findings suggest that, assuming a reasonable level of specificity, primary CT colonography may be more suitable than OC for the initial investigation of suspected colorectal cancer.
Advances in Knowledge.
CT colonography is highly sensitive for colorectal cancer detection across a broad spectrum of accepted indications, protocols, and techniques, with an overall sensitivity in published trials of 96.1% (398 of 414).
The sensitivity of CT colonography for colorectal cancer compares favorably with the sensitivity of optical colonoscopy (OC) (94.7%; 178 of 188).
Implications for Patient Care.
CT colonography and OC should be considered equivalent in terms of sesitivity in colorectal cancer detection and may be complementary.
The specific CT colonography reader paradigm (primary two-dimensional vs three-dimensional mode) is less important for cancer evaluation than the evaluation of benign polyps.
Disclosures of Potential Conflicts of Interest: P.J.P. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for Medicsight, Philips, Viatronix, and Check-Cap; is co-founder of VirtuoCTC, an educational website. Other relationships: none to disclose. C.H. No potential conflicts of interest to disclose. S.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for, institution received grants from, and owns stock in Medicsight. Other relationships: none to disclose. R.M. No potential conflicts of interest to disclose.
Received September 25, 2010; revision requested December 2; revision received December 13; accepted December 16; final version accepted January 6, 2011.
Funding: This research was supported by the National Institutes of Health (grant 1R01CA144835-01).
Abbreviations:
- CI
- confidence interval
- OC
- optical colonoscopy
- 3D
- three-dimensional
- 2D
- two-dimensional
References
- 1.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer 2000;88(10):2398–2424 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18(3):581–592 [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER): Fast Stats. http://seer.cancer.gov/faststats/. Accessed March 24, 2010
- 4.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JD, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst 2009;101(20):1412–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ 1998;317(7158):559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375(9726):1624–1633 [DOI] [PubMed] [Google Scholar]
- 7.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134(5):1570–1595 [DOI] [PubMed] [Google Scholar]
- 8.Chaparro M, Gisbert JP, Del Campo L, Cantero J, Maté J. Accuracy of computed tomographic colonography for the detection of polyps and colorectal tumors: a systematic review and meta-analysis. Digestion 2009;80(1):1–17 [DOI] [PubMed] [Google Scholar]
- 9.Rosman AS, Korsten MA. Meta-analysis comparing CT colonography, air contrast barium enema, and colonoscopy. Am J Med 2007;120(3):203–210, e4 [DOI] [PubMed] [Google Scholar]
- 10.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology 2004;127(2):452–456 [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology 1997;112(1):17–23 [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med 2004;141(5):352–359 [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151(4):W65–W94 [DOI] [PubMed] [Google Scholar]
- 14.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenlon HM, Nunes DP, Schroy PC, 3rd, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med 1999;341(20):1496–1503 [DOI] [PubMed] [Google Scholar]
- 17.Fletcher JG, Johnson CD, Welch TJ, et al. Optimization of CT colonography technique: prospective trial in 180 patients. Radiology 2000;216(3):704–711 [DOI] [PubMed] [Google Scholar]
- 18.Morrin MM, Farrell RJ, Kruskal JB, Reynolds K, McGee JB, Raptopoulos V. Utility of intravenously administered contrast material at CT colonography. Radiology 2000;217(3):765–771 [DOI] [PubMed] [Google Scholar]
- 19.Mendelson RM, Foster NM, Edwards JT, Wood CJ, Rosenberg MS, Forbes GM. Virtual colonoscopy compared with conventional colonoscopy: a developing technology. Med J Aust 2000;173(9):472–475 [DOI] [PubMed] [Google Scholar]
- 20.Regge D, Galatola G, Martincich L, et al. Use of virtual endoscopy with computerized tomography in the identification of colorectal neoplasms: prospective study with symptomatic patients. [in Italian]. Radiol Med (Torino) 2000;99(6):449–455 [PubMed] [Google Scholar]
- 21.Spinzi G, Belloni G, Martegani A, Sangiovanni A, Del Favero C, Minoli G. Computed tomographic colonography and conventional colonoscopy for colon diseases: a prospective, blinded study. Am J Gastroenterol 2001;96(2):394–400 [DOI] [PubMed] [Google Scholar]
- 22.Yee J, Akerkar GA, Hung RK, Steinauer-Gebauer AM, Wall SD, McQuaid KR. Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Radiology 2001;219(3):685–692 [DOI] [PubMed] [Google Scholar]
- 23.Wessling J, Fischbach R, Domagk D, Lügering N, Neumann E, Heindel W. Colorectal polyps: detection with multi-slice CT colonography. Rofo 2001;173(12):1069–1071 [DOI] [PubMed] [Google Scholar]
- 24.Laghi A, Iannaccone R, Carbone I, et al. Detection of colorectal lesions with virtual computed tomographic colonography. Am J Surg 2002;183(2):124–131 [DOI] [PubMed] [Google Scholar]
- 25.Macari M, Bini EJ, Xue X, et al. Colorectal neoplasms: prospective comparison of thin section low-dose multi-detector row CT colonography and conventional colonoscopy for detection. Radiology 2002;224(2):383–392 [DOI] [PubMed] [Google Scholar]
- 26.Wong BC, Wong WM, Chan JK, et al. Virtual colonoscopy for the detection of colorectal polyps and cancers in a Chinese population. J Gastroenterol Hepatol 2002;17(12):1323–1327 [DOI] [PubMed] [Google Scholar]
- 27.Thomeer M, Carbone I, Bosmans H, et al. Stool tagging applied in thin-slice multidetector computed tomography colonography. J Comput Assist Tomogr 2003;27(2):132–139 [DOI] [PubMed] [Google Scholar]
- 28.Pineau BC, Paskett ED, Chen GJ, et al. Virtual colonoscopy using oral contrast compared with colonoscopy for the detection of patients with colorectal polyps. Gastroenterology 2003;125(2):304–310 [DOI] [PubMed] [Google Scholar]
- 29.Ginnerup Pedersen B, Christiansen TE, Bjerregaard NC, Ljungmann K, Laurberg S. Colonoscopy and multidetector-array computed-tomographic colonography: detection rates and feasibility. Endoscopy 2003;35(9):736–742 [DOI] [PubMed] [Google Scholar]
- 30.Bruzzi JF, Moss AC, Brennan DD, MacMathuna P, Fenlon HM. Efficacy of IV Buscopan as a muscle relaxant in CT colonography. Eur Radiol 2003;13(10):2264–2270 [DOI] [PubMed] [Google Scholar]
- 31.Taylor SA, Halligan S, Saunders BP, et al. Use of multidetector-row CT colonography for detection of colorectal neoplasia in patients referred via the Department of Health “2-Week-Wait” initiative. Clin Radiol 2003;58(11):855–861 [DOI] [PubMed] [Google Scholar]
- 32.Iannaccone R, Laghi A, Catalano C, et al. Detection of colorectal lesions: lower-dose multi-detector row helical CT colonography compared with conventional colonoscopy. Radiology 2003;229(3):775–781 [DOI] [PubMed] [Google Scholar]
- 33.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003;349(23):2191–2200 [DOI] [PubMed] [Google Scholar]
- 34.Munikrishnan V, Gillams AR, Lees WR, Vaizey CJ, Boulos PB. Prospective study comparing multislice CT colonography with colonoscopy in the detection of colorectal cancer and polyps. Dis Colon Rectum 2003;46(10):1384–1390 [DOI] [PubMed] [Google Scholar]
- 35.Iannaccone R, Laghi A, Catalano C, et al. Computed tomographic colonography without cathartic preparation for the detection of colorectal polyps. Gastroenterology 2004;127(5):1300–1311 [DOI] [PubMed] [Google Scholar]
- 36.Vogt C, Cohnen M, Beck A, et al. Detection of colorectal polyps by multislice CT colonography with ultra-low-dose technique: comparison with high-resolution videocolonoscopy. Gastrointest Endosc 2004;60(2):201–209 [DOI] [PubMed] [Google Scholar]
- 37.Hoppe H, Netzer P, Spreng A, Quattropani C, Mattich J, Dinkel HP. Prospective comparison of contrast enhanced CT colonography and conventional colonoscopy for detection of colorectal neoplasms in a single institutional study using second-look colonoscopy with discrepant results. Am J Gastroenterol 2004;99(10):1924–1935 [DOI] [PubMed] [Google Scholar]
- 38.Van Gelder RE, Nio CY, Florie J, et al. Computed tomographic colonography compared with colonoscopy in patients at increased risk for colorectal cancer. Gastroenterology 2004;127(1):41–48 [DOI] [PubMed] [Google Scholar]
- 39.Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA 2004;291(14):1713–1719 [DOI] [PubMed] [Google Scholar]
- 40.Abdel Razek AA, Abu Zeid MM, Bilal M, Abdel Wahab NM. Virtual CT colonoscopy versus conventional colonoscopy: a prospective study. Hepatogastroenterology 2005;52(66):1698–1702 [PubMed] [Google Scholar]
- 41.Iannaccone R, Catalano C, Mangiapane F, et al. Colorectal polyps: detection with low-dose multi-detector row helical CT colonography versus two sequential colonoscopies. Radiology 2005;237(3):927–937 [DOI] [PubMed] [Google Scholar]
- 42.Arnesen RB, Adamsen S, Svendsen LB, Raaschou HO, von Benzon E, Hansen OH. Missed lesions and false-positive findings on computed-tomographic colonography: a controlled prospective analysis. Endoscopy 2005;37(10):937–944 [DOI] [PubMed] [Google Scholar]
- 43.Wessling J, Domagk D, Lugering N, et al. Virtual colonography: identification and differentiation of colorectal lesions using multi-detector computed tomography. Scand J Gastroenterol 2005;40(4):468–476 [DOI] [PubMed] [Google Scholar]
- 44.Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005;365(9456):305–311 [DOI] [PubMed] [Google Scholar]
- 45.Selçuk D, Demirel K, Ozer H, et al. Comparison of virtual colonoscopy with conventional colonoscopy in detection of colorectal polyps. Turk J Gastroenterol 2006;17(4):288–293 [PubMed] [Google Scholar]
- 46.Kalra N, Suri S, Bhasin DK, et al. Comparison of multidetector computed tomographic colonography and conventional colonoscopy for detection of colorectal polyps and cancer. Indian J Gastroenterol 2006;25(5):229–232 [PubMed] [Google Scholar]
- 47.Reuterskiöld MH, Lasson A, Svensson E, Kilander A, Stotzer PO, Hellström M. Diagnostic performance of computed tomography colonography in symptomatic patients and in patients with increased risk for colorectal disease. Acta Radiol 2006;47(9):888–898 [DOI] [PubMed] [Google Scholar]
- 48.Haykir R, Karakose S, Karabacakoglu A, Sahin M, Kayacetin E. Three-dimensional MR and axial CT colonography versus conventional colonoscopy for detection of colon pathologies. World J Gastroenterol 2006;12(15):2345–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnesen RB, von Benzon E, Adamsen S, Svendsen LB, Raaschou HO, Hansen OH. Diagnostic performance of computed tomography colonography and colonoscopy: a prospective and validated analysis of 231 paired examinations. Acta Radiol 2007;48(8):831–837 [DOI] [PubMed] [Google Scholar]
- 50.Johnson CD, Fletcher JG, MacCarty RL, et al. Effect of slice thickness and primary 2D versus 3D virtual dissection on colorectal lesion detection at CT colonography in 452 asymptomatic adults. AJR Am J Roentgenol 2007;189(3):672–680 [DOI] [PubMed] [Google Scholar]
- 51.Chaparro Sánchez M, del Campo Val L, Maté Jiménez J, et al. Computed tomography colonography compared with conventional colonoscopy for the detection of colorectal polyps. Gastroenterol Hepatol 2007;30(7):375–380 [DOI] [PubMed] [Google Scholar]
- 52.Florie J, van Gelder RE, Schutter MP, et al. Feasibility study of computed tomography colonography using limited bowel preparation at normal and low-dose levels study. Eur Radiol 2007;17(12):3112–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008;359(12):1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim YS, Kim N, Kim SH, et al. The efficacy of intravenous contrast-enhanced 16-raw multidetector CT colonography for detecting patients with colorectal polyps in an asymptomatic population in Korea. J Clin Gastroenterol 2008;42(7):791–798 [DOI] [PubMed] [Google Scholar]
- 55.Roberts-Thomson IC, Tucker GR, Hewett PJ, et al. Single-center study comparing computed tomography colonography with conventional colonoscopy. World J Gastroenterol 2008;14(3):469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor SA, Slater A, Burling DN, et al. CT colonography: optimisation, diagnostic performance and patient acceptability of reduced-laxative regimens using barium-based faecal tagging. Eur Radiol 2008;18(1):32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon SH, Kim SH, Kim SG, et al. Comparison study of different bowel preparation regimens and different fecal-tagging agents on tagging efficacy, patients’ compliance, and diagnostic performance of computed tomographic colonography: preliminary study. J Comput Assist Tomogr 2009;33(5):657–665 [DOI] [PubMed] [Google Scholar]
- 58.Liedenbaum MH, van Rijn AF, de Vries AH, et al. Using CT colonography as a triage technique after a positive faecal occult blood test in colorectal cancer screening. Gut 2009;58(9):1242–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Regge D, Laudi C, Galatola G, et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA 2009;301(23):2453–2461 [DOI] [PubMed] [Google Scholar]
- 60.Nagata K, Okawa T, Honma A, Endo S, Kudo SE, Yoshida H. Full-laxative versus minimum-laxative fecal-tagging CT colonography using 64-detector row CT: prospective blinded comparison of diagnostic performance, tagging quality, and patient acceptance. Acad Radiol 2009;16(7):780–789 [DOI] [PubMed] [Google Scholar]
- 61.Fisichella VA, Jäderling F, Horvath S, Stotzer PO, Kilander A, Hellström M. Primary three-dimensional analysis with perspective-filet view versus primary two-dimensional analysis: evaluation of lesion detection by inexperienced readers at computed tomographic colonography in symptomatic patients. Acta Radiol 2009;50(3):244–255 [DOI] [PubMed] [Google Scholar]
- 62.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 2009;58(2):241–248 [DOI] [PubMed] [Google Scholar]
- 63.White TJ, Avery GR, Kennan N, Syed AM, Hartley JE, Monson JR. Virtual colonoscopy vs conventional colonoscopy in patients at high risk of colorectal cancer: a prospective trial of 150 patients. Colorectal Dis 2009;11(2):138–145 [DOI] [PubMed] [Google Scholar]
- 64.Ozsunar Y, Coskun G, Delibaş N, Uz B, Yükselen V. Diagnostic accuracy and tolerability of contrast enhanced CT colonoscopy in symptomatic patients with increased risk for colorectal cancer. Eur J Radiol 2009;71(3):513–518 [DOI] [PubMed] [Google Scholar]
- 65.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT colonography. AJR Am J Roentgenol 2007;189(6):1451–1456 [DOI] [PubMed] [Google Scholar]
- 66.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150(1):1–8 [DOI] [PubMed] [Google Scholar]
- 67.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112(1):24–28 [DOI] [PubMed] [Google Scholar]
- 68.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol 2006;101(12):2866–2877 [DOI] [PubMed] [Google Scholar]
- 69.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136(3):832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993;328(13):901–906 [DOI] [PubMed] [Google Scholar]
- 71.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin 2006;56(3):160–167; quiz 185–186 [DOI] [PubMed] [Google Scholar]
- 72.Pickhardt PJ, Kim DH, Meiners RJ, et al. Colorectal and extracolonic cancers detected at screening CT colonography in 10,286 asymptomatic adults. Radiology 2010;255(1):83–88 [DOI] [PubMed] [Google Scholar]
- 73.Pickhardt PJ, Hain KS, Kim DH, Hassan C. Low rates of cancer or high-grade dysplasia in colorectal polyps collected from computed tomography colonography screening. Clin Gastroenterol Hepatol 2010;8(7):610–615 [DOI] [PubMed] [Google Scholar]
- 74.Sabanli M, Balasingam A, Bailey W, Eglinton T, Hider P, Frizelle FA. Computed tomographic colonography in the diagnosis of colorectal cancer. Br J Surg 2010;97(8):1291–1294 [DOI] [PubMed] [Google Scholar]