Abstract
Background and Objectives
Left ventricular (LV) remodeling is a heterogeneous process, involving both infarcted and non-infarcted zones, which affects wall thickness and chamber size, shape and function.
Subjects and Methods
A total of 758 consecutive patients (62.8±12.0 years, 539 males) with acute myocardial infarction (AMI), who were examined by echocardiography at admission and after 6 months. An increase in LV end-diastolic volume index >10% was defined as a progressive LV dilation. They were divided into two groups according to the extent of progressive LV dilatation during 6 months. Group I with progressive LV dilatation (n=154, 61.4±11.0 years, 110 males) vs. group II without LV dilatation (n=604, 64.1±12.0 years, 429 males).
Results
The age and gender were no significant differences between two groups. The levels of glucose, creatinine, maximal creatine kinase (CK), CK-MB, troponin T and I were significantly increased in group I than in group II (p<0.05). Low ejection fraction (EF) and high wall motion score index (WMSI) were more common in group I than in group II (p<0.05). The presence of dyslipidemia {odds ratio (OR); 1.559, confidence interval (CI); 1.035-2.347, p=0.03}, low EF less than 45% (OR; 3.328, CI 2.099-5.276, p<0.01) and high WMSI above 1.5 (OR; 3.328, CI 2.099-5.276, p<0.01) were sig-nificant independent predictors of progressive LV dilatation by multivariate analysis.
Conclusion
Dyslipidemia, decreased systolic function and high WMSI were independent predictors of LV remodeling process in patients with AMI.
Keywords: Myocardial infarction, Heart failure, Prognosis
Introduction
Left ventricle (LV) remodeling following acute myocardial infarction (AMI) is a major predictor of morbidity and mortality for overt congestive heart failure (CHF) and life threatening arrhythmia.1) LV remodeling is a heterogeneous process, involving both infarcted and non-infarcted myocardium, which affects wall thickness and chamber size, shape and function. LV dilatation after MI procedures reduce exercise performance and it plays a role in the development of chronic he-art failure.2),3) Post-infarction remodeling has been arbitrarily divided into an early phase (within 72 hours), and a late phase (beyond 72 hours). The early phase involves expansion of the infarct zone, which may result in early ventricular rupture or aneurysm formation. Late ventricular remodeling involves the LV globally and is associated with time-dependent dilatation, distortion of ventricular shape, and mural hypertrophy.4) Patients who develop LV dilatation following AMI have significantly reduced survival.5),6) It has been reported that LV volume is the single most important predictor of survival in patients with coronary heart disease.7) LV remodeling after acute MI stimulates the interaction of a number of factors, such as loss of contractile elements, activation of circulating neurohormones and patency of the infarct-related artery (IRA), initial infarction size and LV size to normalize wall stress.8),9) It can begin very soon after AMI and, if not attenuated or reversed by intervention, has a poor prognosis.10)
The purpose of this study was to assess the associated factors with LV remodeling in the first 6 months following MI, and to define the clinical, biochemical, echocardiographic and angiographic predictors of LV dilatation after AMI.
Subjects and Methods
Study population
A total of 758 consecutive patients (62.8±12.0 years, 539 males) with AMI were examined by echocardiography at admission and after 6 months. An increase in left ventricular end-diastolic volume index (LVEDVI) more than 10% was defined as progressive LV dilation. They were classified into two groups according to the extent of progressive LV dilatation in 6 months. Group I with progressive LV dilatation (n=154, 61.4±11.0 years, 110 males 44 females) vs. group II without LV dilatation (n=604, 63.1±1.02 years, 429 males 175 females).
Definition of hypertension, diabetes, dyslipidemia and myocardial infarction
Subjects were considered to be hypertensive if their blood pressure was more than 140≥90 mmHg as Joint National Committee VII11) or if they were on treatment for hypertension. The American Diabetes Association criteria12) were used to define diabetes (DM). We considered a subject to have DM when the fasting plasma glucose levels were more than 126 mg/dL in 2 consecutive assessments, or if they were on treatment for DM. Dyslipidemia was diagnosed according to the 2004 update of the National Cholesterol Education Program guidelines.13) According to these guidelines, high level of low density lipoprotein-cholesterol (LDL-C) more than 160 mg/d, low high density lipoprotein-cholesterol (HDL-C) less than 40 mg/dL and high triglycerides more than 150 mg/dL were included.14)
The presence of ST-segment elevation MI was determined by more than 30 minutes of continuous chest pain, a new ST-segment elevation more than 2 mm on at least two contiguous electrocardiographic leads, creatine kinase (CK)-MB or troponin more than 3 times normal.15) The presence of non-ST-segment elevation MI was diagnosed by chest pain and a positive cardiac biomarker without new ST-segment elevation.16) Infarct-related arteries were identified using a combination of electrocardiographic findings, LV wall motion abnormalities on two-dimensional echocardiography and coronary angiography. Family history means early cardiovascular disease in direct relatives. Hospital records of patients were reviewed to obtain information on clinical demographics.
Measurement of serum biomarkers
Serum N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) was measured using the electrochemiluminescence sandwich immunoassay method for NT-pro-BNP with an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim, Germany). This method has high sensitivity and specificity, and large detection range. The analytic range of the NT-pro-BNP assay extends from 5 to 35,000 pg/mL. High sensitivity C-reactive protein (hs-CRP) was measured by immunoturbidime-tric CRP-Latex (II) high-sensitivity assay using an Olympus 5431 autoanalyzer.
Measurement of progressive left ventricular dilatation
Two-dimensional, M-mode echocardiography and Doppler ultrasound examination were performed with GE Vivid 7 Ultrasound (General Electronic Healthcare, Vingmed, Horten Norway) with a 2.5 MHz probe. Image-Point at the time of initial admission and at 6 months after MI. LV volume and ejection fraction (EF) were measured using the Simpson's formula.17) LV volume indices were obtained by dividing the volume by the body surface area. The mean values of three me-asurements of the technically best cardiac cycles were taken from each examination performed by two independent interobservers. Intraobserver and interobserver variabilities of the Simpson's method were 4±5% and 5±4% (absolute difference divided by mean value of measurement). In each patient the wall motion score index (WMSI) was derived. The LV was divided according to a 17-segment model.18) In each segment wall motion was scored from 1 (normal) to 4 (dyskine-tic). An increase in LVEDVI >10% between initial and 6 month follow up was considered as a progressive LV dilatation pattern.
Statistical analysis
The Statistical Package for Social Sciences for Windows, version 15.0 (Chicago, IL, USA) was used for all analysis. For each parameter mean, median and standard deviation were calculated. Statistical significance between means for different groups was calculated by the non-parametrical Wilcoxon signed rank test. Statistical significance between frequencies was calculated by the chi square test with Yates correction or, if the expected value was less than 5, by Fisher's exact test. Relative risk and confidence interval (CI) were also calculated. A p of less than 0.05 was required to reject the null hypothesis. The variables that were significant in univariate analysis were entered into the multivariate models.
Results
Baseline clinical characteristics
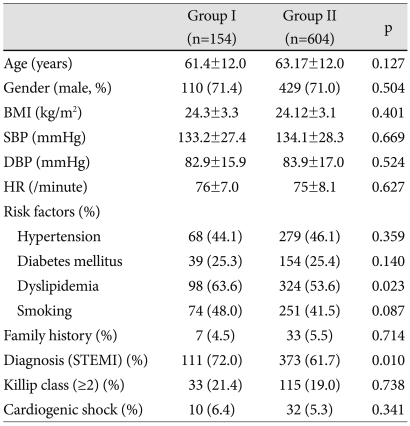
The baseline characteristics are summarized in Table 1. Age and gender exhibited no significant differences between two groups. The prevalence of dyslipidemia was higher in group I than group II (p<0.05). The proportion of ST-segment elevation MI was more frequent in group I than in group II (p<0.05). There was no difference between the groups with respect to the initial vital sign or Killip class.
Table 1.
Baseline clinical characteristics of the patients
BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, STEMI: ST-segment elevation myocardial infarction
Biochemical, echocardiographic and coronary angiographic parameter associated with progressive left ventricular dilatation
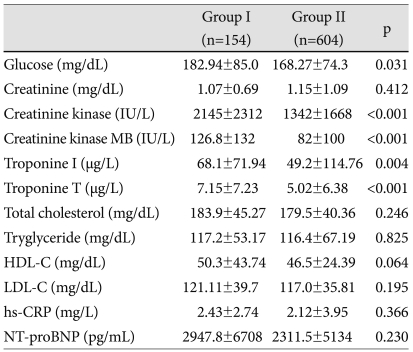
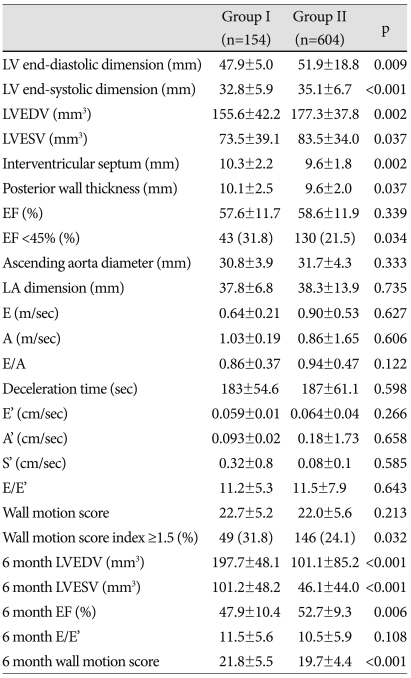
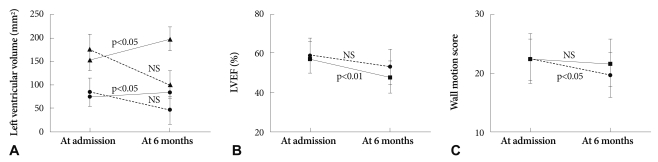
The levels of glucose, maximal CK, CK-MB, troponin-T and troponin-I were significantly increased in group I compared to group II (p<0.05) (Table 2). LV end-diastolic and systolic dimension, interventricular septal thickness, posterior wall thickness were increased in group II compared to group I at admission. However, 6-month follow up echocardiography showed reversal of LV size mentioned above. LV dimension and volume were more increased in group I compared to group II at 6 months (p<0.05). Although the mean valve of EF and total wall motion score (TWMS) at admission were not significantly different between the groups, the percentage of low EF (<45%) and high WMSI (≥1.5) were higher in group I than in group II (p<0.05). Six-month EF and wall motion score were deteriorated compared to the initial score (p<0.05) (Table 3). Changes in LV volume at admission and at 6 months were significantly increased in group I, but not in group II (Fig. 1A). Fig. 1B showed decreased EF in group I and no significant ch-ange in group II during the 6-month period. Serial changes of TWMS at admission and at 6 months are illustrated in Fig. 1C. It was decreased TWMS in group II and no significant change in group I.
Table 2.
Biochemical parameters of left ventricular dilatation in patients with acute myocardial infarction
HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, hs-CRP: high sensitivity C-reactive protein, NT-pro-BNP: N-terminal pro B natriuretic peptide
Table 3.
Echocardiographic parameters of left ventricular dilatations in the patients with acute myocardial infarction
LV: left ventricle, EF: ejection fraction, LA: left atrium; LVEDV: LV end diastolic volume, LVESV: LV end systolic volume
Fig. 1.
A: left ventricular end diastolic volume (LVEDV) and end systolic volume (LVESV) at admission and 6-month were significantly increased in group I, and not in group II (black line: group I; dot line: group II; triangle: LVEDV; circle: LVESV). B: serial changes of left ventricular ejection fraction (LVEF) at admission and 6-month showed decreased LVEF in group I and no significant change in group II (black line: group I; dot line: group II). C: serial changes of total wall motion score (TWMS) at admission and 6-month demonstrated decreased TWMS in group II and no significant change in group I (black line: group I; dot line: group II).
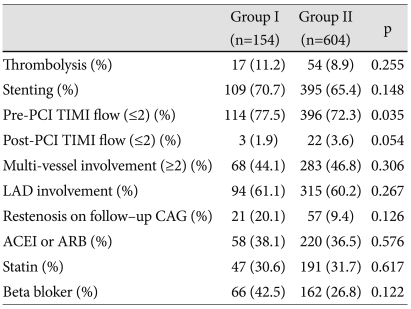
A total of 71 patients were treated with thrombolytic therapy in this patient group. Thrombolytic therapy did not affect progressive LV dilatation. There were no significant differences between groups in the stenting rate, involved vessel number, IRA, post-PCI thrombolysis in myocardial infarction (TIMI) flow and the percentage of restenosis on follow-up coronary angiography. The percentage of low TIMI flow (≤2) was higher in group I than in group II (p<0.5). Medication history of angiotensin converting enzyme inhibitor, angiotensin receptor blocker, statin, and beta blocker did not affect pro-gressive LV dilatation (Table 4).
Table 4.
Angiographic parameters of left ventricular dilatations and medication in patients with acute myocardial infarction
PCI: percutaneous coronary intervention, TIMI: thrombolysis in myocardiacl infarction, LAD: left anterior descending artery, CAG: coronary angiography, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor bloker
Independent predictors of progressive left ventricular dilatation
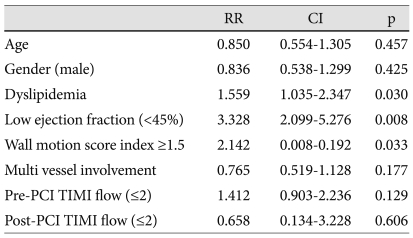
The presence of dyslipidemia {odd ratio (OR); 1.559, CI; 1.035-2.347, p=0.03}, low LVEF less than 45% (OR; 3.328, CI 2.099-5.276, p<0.01) and high WMSI above 1.5 (OR; 3.328, CI 2.099-5.276, p<0.01) were significant independent predictors of progressive LV dilatation by multivariate analysis (Table 5).
Table 5.
Predictors of progressive left ventricular dilatation by multivariate analysis
PCI: percutaneous coronary intervention, TIMI: thrombolysis in myocardiacl infarction
Discussion
The acute loss of myocardium in AMI results in an abrupt increase in loading conditions that induces a unique pattern of remodeling, involving the infarct border zone and remote non-infarcted myocardium.19) Myocyte necrosis and the resultant increase in load trigger a cascade of biochemical intracellular signaling processes that indicate and subsequently modulates reparative changes, including dilatation, hypertrophy, and the formation of a discrete collagen scar. Ventricular remodeling may continue for weeks or months until the distending forces are counterbalanced by the tensile strength of the collagen scar. Failure to normalize increased wall stresses results in progressive dilatation, recruitment of border zone myocardium into the scar, and deterioration in contractile function. This balance is determined by the size, location, and transmurality of the infarct, the extent of myocardial stunning, the patency of the IRA, and local tropic factors.20) Therefore, it may be important to identify patients at risk of LV remodeling to prevent LV dilation after AMI.
Dyslipidemia is a well-established risk factor for coronary artery disease, but few information is available on its effects on microvascular perfusion. Experimental studies showed that independent of coronary artery stenosis severity, dyslipidemia may reduce myocardial flow reserve and capillary density, and may increase capillary endothelial cell apoptosis following ischemia and reperfusion, thus contributing to reduced LV function after AMI.21),22) Among the clinical risk factor criteria, only the incidence of dyslipidemia was significantly higher in the LV dilatation group (p<0.05), whereas there were no statistical differences between groups in the other risk factors. Our data showed that dyslipidemia affected independent progressive LV dilation after 6 months in AMI patients. Treatment of dyslipidemia may reduce microvascular perfusion and myocardial salvage after AMI, and improve LV remodeling.
Studies have showed that inflammatory cytokines were involved in the process of LV remodeling after AMI, anti-inflammation treatment ameliorated LV remodeling and improved cardiac performance. Hydroxymethylglutary coenzyme A reductase inhibition (statins) could affect the expression of inflammatory cytokines.23),24) Therefore, treatment of dyslipidemia with statins may helpful to reduce progressive LV dilatation. If we recorded lipid levels one or two months after discharge, it would be more helpful to understand LV progression pattern. Unfortunatelly, we do not have sufficient data of the follow up lipid levels one or two months after AMI.
According to early reports, a major determinant of ventricular remodeling following AMI could be infarct size.25) Myocyte injury markers such as cardiac troponin I and T, CK and CK-MB appear to be useful in predicting late ventricular dilation. Anterior myocardial infarction, perfusion status of the culprit lesion, and CHF on admission are major predictors of LV dilation.26),27) Several studies showed an association between elevated blood glucose at admission and subsequent adverse events, such as CHF, cardiogenic shock, and death.28) Recently, hs-CRP, BNP and cardiac troponin I have been examined as potential predicting biomarkers of LV remodeling.29) High WMSI and markedly increased cardiac enzymes suggest large infarction. As mentioned above, our study showed high wall motion score and low EF affected progressive LV dilatation. The mean values of hs-CRP and BNP were increased in the LV dilatation group, consistent with the outcomes of previous studies. However, there was no statistical significance demonstrated.
Early reperfusion treatment improves survival by limiting infarct size and consequently preserving LV function. Early reperfusion therapy and patency of the IRA is crucial for reducing infarct expansion and LV enlargement. Some investigators have tested the hypothesis that LV remodeling occurs after percutaneous coronary intervention (PCI), despite persistent patency of the IRA, and may influence the prognosis.30)
Some reports showed Post-PCI TIMI grade was significantly related to the change in LVEDVI and left ventricular end-sistolic volume index after 9 month.30) But our data had no significance of post TIMI flow.
The limitation of our study was a lack of knowledge of long term patency of the IRA because we did not perform routine follow-up coronary angiography. We only performed follow-up coronary angiography on 365 patients (48.1%). AMI patients with dyslipidemia, low EF and high wall motion score at admission should be carefully monitored by clinical and serial echocardiographic examinations, which should serve helpful guidance to prevent or reverse LV remodeling.
Acknowledgments
This study was supported by a grant from the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084869).
This work was suppopted by a Grant-in-Aid for Strategy Technology Development programs (No. 10030039) from the Korea Ministry of Knowledge Economy.
Footnotes
The author has no financial conflicts of interest.
References
- 1.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 2.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling: concepts and clinical implications: a consensus paper from an international forum on cardiac remodelling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 3.Ko JS, Jeong MH, Lee MG, et al. Left ventricular dyssynchrony after acute myocardial infarction is a powerful indicator of left ventricular remodeling. Korean Circ J. 2009;39:236–242. doi: 10.4070/kcj.2009.39.6.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlebacher JA, Weiss JL, Weisfeldt ML, Bulkley BH. Early dilation of the infarcted segment in acute transmural myocardial infarction: role of infarct expansion in acute left ventricular enlargement. J Am Coll Cardiol. 1984;4:201–208. doi: 10.1016/s0735-1097(84)80203-x. [DOI] [PubMed] [Google Scholar]
- 5.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 6.Gaudron P, Kugler I, Hu K, Bauer W, Eiiles C, Ertl G. Time course of cardiac structural, functional and electrical changes in asymptomatic patients after myocardial infarction: their inter-relation and prog-nostic impact. J Am Coll Cardiol. 2001;38:33–40. doi: 10.1016/s0735-1097(01)01319-5. [DOI] [PubMed] [Google Scholar]
- 7.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end systolic volume as the major determinant of survival after recovery from myocardiol infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 8.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction: potential mechanisms and early predictors. Circulation. 1993;87:755–763. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 9.Giannuzzi P, Temporelli PL, Bosimini E, et al. Heterogenity of left ventricular remodeling after myocardial infarction: results of the Gruppo Italiano per lo Studio della Supravvivenza nell Infarcto Miocardico-3 Echo Substudy. Am Heart J. 2001;141:131–138. doi: 10.1067/mhj.2001.111260. [DOI] [PubMed] [Google Scholar]
- 10.Sim DS, Kim JH, Jeong MH. Differences in clinical outcomes between patients with ST-elevation versus non-ST-elevation acute myocardial infarction in Korea. Korean Circ J. 2009;39:297–303. doi: 10.4070/kcj.2009.39.8.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Expert Committee on the Diagnosis and the Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and the classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 14.Hatzitolios AI, Athyrosb VG, Karagiannisb A, et al. Implementation of strategy for the management of overt dyslipidemia. The IMPROVE-dyslipidemia study. Int J Cardiol. 2009;134:322–329. doi: 10.1016/j.ijcard.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation. 2004;110:e82–e293. [PubMed] [Google Scholar]
- 16.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guide-line update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2002;40:1366–1374. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 17.Simonson JS, Schiller NB. Descent of the base of the left ventricle: an echocardiographic index of the left ventricle. J Am Soc Echocardiogr. 1989;2:25–35. doi: 10.1016/s0894-7317(89)80026-4. [DOI] [PubMed] [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized for myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 19.Rouleau JL, de Champlain J, Klein M, et al. Activation of neurohumoral systems in postinfarction left ventricular dysfunction. J Am Coll Cardiol. 1993;22:390–398. doi: 10.1016/0735-1097(93)90042-y. [DOI] [PubMed] [Google Scholar]
- 20.Warren SE, Royal HD, Markis JE, Grossman W, McKay RG. Time course of left ventricular dilation after myocardial infarction: influence of infarct-related artery and success of coronary thrombolysis. J Am Coll Cardiol. 1988;11:12–19. doi: 10.1016/0735-1097(88)90159-3. [DOI] [PubMed] [Google Scholar]
- 21.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 22.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early athelosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 23.Eefting FD, Cramer MJ, Stella PR, Rensing BJ, Doevendans PA. A randomised trial with serial cardiac MRI follow-up testing the ability of atorvastatin to reduce reperfusion damage after primary PCI for acute MI. Neth Heart J. 2006;14:95–99. [PMC free article] [PubMed] [Google Scholar]
- 24.Hong YJ, Jeong MH, Hyun DW, et al. Prognostic significance of simvastatin therapy in patients with ischemic heart failure who underwent percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005;95:619–622. doi: 10.1016/j.amjcard.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Hochman JS, Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation. 1987;75:299–306. doi: 10.1161/01.cir.75.1.299. [DOI] [PubMed] [Google Scholar]
- 26.Yellon DM, Baxter GF. Protecting the ischaemic and reperfused myocardium in acute myocardial infarction: distant dream or near reality. Heart. 2000;83:381–387. doi: 10.1136/heart.83.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim SC, Rhee JA, Jeong MH, et al. Predictive factors for the recovery of left ventricular dysfunction in patients with acute myocardial infarction. Korean Circ J. 2007;37:113–118. [Google Scholar]
- 28.Bolk J, van der Ploeg TJ, Cornel JH, Arnold AE, Sepers J, Umans VA. Impaired glucose metabolism predicts mortality after a myocardial infarction. Int J Cardiol. 2001;79:207–214. doi: 10.1016/s0167-5273(01)00422-3. [DOI] [PubMed] [Google Scholar]
- 29.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angiography: patterns of left ventricular dilatation and long term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 30.Cerisano G, Bolognese L, Buonamici P, et al. Prognostic implications of restrictive left ventricular filling in reperfused anterior acute myocardial infarction. J Am Coll Cardiol. 2001;37:793–799. doi: 10.1016/s0735-1097(00)01203-1. [DOI] [PubMed] [Google Scholar]
- 31.Choi SY, Tahk SJ, Yoon MH, et al. Comparison of TIMI myocardial perfusion grade with coronary flow reserve for prediction of recovery of LV function and LV remodeling in acute myocardial infarction. Korean Circ J. 2004;34:247–257. [Google Scholar]