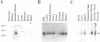Abstract
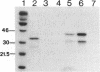
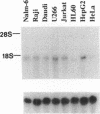
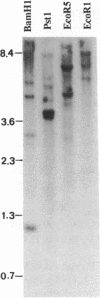
In human cells at least 20 different proteins or groups of proteins have been identified that are associated with hnRNAs. These proteins (designated A1-U) are highly abundant in the nucleus. In this study, we present the sequence of a novel cDNA clone, sub2.3, isolated from a human lymphocyte cDNA library. The predicted amino acid sequence shows homology to repeated domains in the human hnRNA binding protein K (hnRNP K), which are believed to be of functional importance. hnRNP K is among the major oligo(rC/dC) binding proteins in vertebrate cells and we show here that the protein product of sub2.3 also binds to oligo(dC). This is shown by a novel approach where we demonstrated specific binding of in vitro translated sub2.3 protein to biotinylated oligo(dC) which was immobilized on magnetic streptavidin-coated Dynabeads. Moreover we found that the sub2.3 transcript is expressed in a tissue dependent manner with the highest expression observed in several lymphoid tissues and skeletal muscle. The gene was also abundantly expressed in several lymphoid cell lines and the hepatoma cell line HepG2 while a low expression was observed in the HL60 myeloid cell line and in the HeLa cervical carcinoma cell line. In conclusion, this study presents the cDNA sequence of a novel transcript which shows tissue specific expression and encodes a protein with oligo(dC) binding specificity in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma T., Liu W. G., Vander Laan D. J., Bowcock A. M., Taggart R. T. Human gastric cathepsin E gene. Multiple transcripts result from alternative polyadenylation of the primary transcripts of a single gene locus at 1q31-q32. J Biol Chem. 1992 Jan 25;267(3):1609–1614. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Matunis M. J., Piñol-Roma S., Burd C. G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- Gabrielsen O. S., Huet J. Magnetic DNA affinity purification of yeast transcription factor. Methods Enzymol. 1993;218:508–525. doi: 10.1016/0076-6879(93)18038-e. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm K., Kim G., Turck C. W., Smale S. T. Isolation of a murine gene encoding a nucleic acid-binding protein with homology to hnRNP K. Nucleic Acids Res. 1993 Aug 11;21(16):3894–3894. doi: 10.1093/nar/21.16.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Johnson D., Raychaudhuri G., Beyer A. L. The Drosophila Hrb87F gene encodes a new member of the A and B hnRNP protein group. Nucleic Acids Res. 1991 Jan 11;19(1):25–31. doi: 10.1093/nar/19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hirose T., Sugita M., Sugiura M. cDNA structure, expression and nucleic acid-binding properties of three RNA-binding proteins in tobacco: occurrence of tissue-specific alternative splicing. Nucleic Acids Res. 1993 Aug 25;21(17):3981–3987. doi: 10.1093/nar/21.17.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Kim G. S., Prystowsky M. B., Lancki D. W., Sabath D. E., Pan J. L., Weissman S. M. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc Natl Acad Sci U S A. 1987 May;84(9):2896–2900. doi: 10.1073/pnas.84.9.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis M. J., Michael W. M., Dreyfuss G. Characterization and primary structure of the poly(C)-binding heterogeneous nuclear ribonucleoprotein complex K protein. Mol Cell Biol. 1992 Jan;12(1):164–171. doi: 10.1128/mcb.12.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K., Price S. R., Nightingale M. S., Kousvelari E., Moss J., Vaughan M. Regulation of ADP-ribosylation factor (ARF) expression. Cross-species conservation of the developmental and tissue-specific alternative polyadenylation of ARF 4 mRNA. J Biol Chem. 1992 Nov 25;267(33):24109–24116. [PubMed] [Google Scholar]
- Piñol-Roma S., Dreyfuss G. hnRNP proteins: localization and transport between the nucleus and the cytoplasm. Trends Cell Biol. 1993 May;3(5):151–155. doi: 10.1016/0962-8924(93)90135-n. [DOI] [PubMed] [Google Scholar]
- Rabbitts K. G., Morgan G. T. Alternative 3' processing of Xenopus alpha-tubulin mRNAs; efficient use of a CAUAAA polyadenylation signal. Nucleic Acids Res. 1992 Jun 25;20(12):2947–2953. doi: 10.1093/nar/20.12.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Siomi H., Matunis M. J., Michael W. M., Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993 Mar 11;21(5):1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto M., Tomonaga T., Matunis M., Avigan M., Krutzsch H., Dreyfuss G., Levens D. Specific binding of heterogeneous ribonucleoprotein particle protein K to the human c-myc promoter, in vitro. J Biol Chem. 1993 Aug 25;268(24):18249–18258. [PubMed] [Google Scholar]
- Thekkumkara T. J., Livingston W., 3rd, Kumar R. S., Sen G. C. Use of alternative polyadenylation sites for tissue-specific transcription of two angiotensin-converting enzyme mRNAs. Nucleic Acids Res. 1992 Feb 25;20(4):683–687. doi: 10.1093/nar/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Oltz E. M., Rathbun G., Berman J. E., Smith R. K., Lansford R. D., Rothman P., Okada A., Lee G., Morrow M. Isolation of coordinately regulated genes that are expressed in discrete stages of B-cell development. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5759–5763. doi: 10.1073/pnas.87.15.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]