Abstract
The failure of large-scale drug trials targeting the amyloidogenic pathway in Alzheimer's disease (AD) is increasing the need to identify a novel pathogenic mechanism. Studies finding a relationship between sporadic AD and type-2 diabetes mellitus (T2DM) are now receiving more attention. The risk for developing both T2DM and sporadic AD increases exponentially with age, and having T2DM doubles the risk of developing AD. The postmortem brains of AD patients show altered activities of insulin receptors and downstream molecules, as well as reduced protein and mRNA levels of insulin. More-recent laboratory research using animal models has identified mechanisms that are shared by diabetes and AD. Exogenous application of streptozotocin, which disrupts systemic insulin secretion, results in insulin deficiency, increased tau phosphorylation, and cognitive impairments that can be reversed by exogenous insulin supplementation. However, AD pathology is more severe in T2DM animal models exhibiting hyperinsulinemia and insulin resistance, and this is not modulated by insulin. The symptoms of this AD pathology included increased tau phosphorylation at multiple sites, increased tau cleavage, and greater neuronal and synaptic damage, even with increased amyloid β protein production. It has therefore been suggested that hyperinsulinemia and insulin resistance represent major factors underlying AD in T2DM. A recent study involving cross-mating ob/ob and amyloid precursor protein transgenic mice provided evidence that T2DM and AD aggravate each other, and suggested that cerebral vessels constitute an important substrate that is commonly damaged by the two major disorders. Given the evidence provided by animal models, further investigation of the mechanisms underlying T2DM in AD should help to identify potential treatment targets in AD.
Keywords: Alzheimer's disease, animal model, diabetes, insulin resistance, mechanism
Introduction
Alzheimer's disease (AD), which is the most-prevalent neurodegenerative disease, results in progressive cognitive dysfunction and inability to perform complex daily activities. As a result of the incapacity experienced by those with AD, a huge burden is inevitably placed on caregivers, which makes AD a major social problem.1 There is strong evidence from genetic studies of familial AD that amyloid β proteins, which are produced by sequential cleavages of amyloid precursor proteins (APPs) by the β-site of the APP-cleaving enzyme (BACE), followed by γ-secretase, play central roles in AD.2 There have been repeated tests of therapeutic strategies directly targeting Aβ production, such as inhibiting or modulating BACE and γ-secretase, using aggregation blockers, clearance-enhancing, and vaccinating with Aβ peptides or antibodies against various Aβ epitopes.3 However, in contrast to the results obtained by studies on transgenic animals with familial AD mutation, no evidence of the efficacy of these approaches has been produced in human AD patients.4 Therefore, it is highly likely that familial and sporadic AD are characterized by different ty-pes of pathogenesis.
Several studies have produced epidemiological, clinical, and pathological evidence of the relationship between AD and type-2 diabetes mellitus (T2DM). Longitudinal epidemiological studies have revealed that the risk of developing late-onset AD is 1.4-4.3 higher among those with T2DM,5-10 and that AD patients showed elevated plasma but lower cerebrospinal fluid (CSF) insulin levels, disrupting the relationship between peripheral and central insulin.11 Furthermore, the postmortem
Studies in Diabetes Mellitus Models (Table 1)
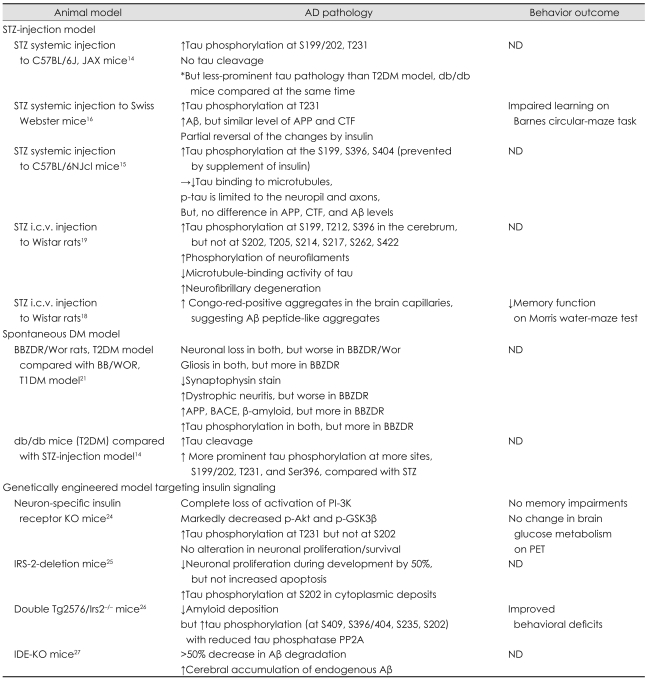
Table 1.
Animal studies using the diabetes mellitus model in the literature
*T2DM model was evaluated and compared at the same time.
Aβ: amyloid β protein, AD: Alzheimer's disease, APP: amyloid precursor protein, BACE: β-site of the APP-cleaving enzyme, BBZDR: bio-breeding zucker diabetic rat, CTF: c-terminal fragment, i.c.v.: intracerebroventricular, DM: diabetes mellitus, IDE: insulin-degrading enzyme, IRS-2: insulin receptor substrate 2, KO: knockout, ND: no test for cognition or behavior, PET: positron-emission tomography, p-GSK-3β: phosphorylated glycogen synthase kinase-3β, PI-3K: phosphatidylinositol 3-kinase, PP2A: protein phosphatase 2A, p-tau: phosphorylated tau, STZ: streptozotocin, T1DM: type-1 DM, T2DM: type-2 DM, Tg: transgenic.
Streptozotocin-injection model
Streptozotocin [STZ; 2-deoxy-2-(3-(methyl-3-nitrosoureido))-D-glucopyranose], a betacytotoxic drug, selectively destroys insulin-secreting pancreatic β cells and thereby causes type-1 diabetes mellitus (T1DM).13 Although the STZ-injection murine model has been the most-widely used to investigate the relationship between diabetes mellitus (DM) and AD, studies relying on this model have focused on T1DM, which has produced less evidence of a relationship with AD than has T2DM. Nonetheless, increased tau phosphorylation with decreased binding to microtubules has been noted consistently in research using the STZ-injection murine model;14-16 this result has probably been due to inhibited phosphatase activities rather than to enhanced kinase activities.14 This effect was substantially reversed by supplying insulin. However, contradictory results have emerged with respect to Aβ pathology.
STZ itself cannot pass through the blood-brain barrier due to the absence of the STZ transporter GLUT2. Therefore, the systemic-injection model can reveal only the effect of systemic hypoinsulinemia on the brain. Direct injection of STZ into the brain has thus been attempted.
Inside the mammalian brain, GLUT2 is distributed heterogeneously;17 therefore, intracerebroventricular (i.c.v.) injection of STZ can selectively decrease the level of insulin in the brain without disturbing systemic insulin and glucose levels. In addition, the expression levels of insulin and IGF-1 receptors were reduced, showing that insulin signaling was impaired,18,19 which is similar to the condition observed in the postmortem brains of AD patients with so-called type-3 diabetes,20 characterized by simultaneously decreased insulin production and resistance to insulin reception in the brain. Although increased phosphorylation of tau was noted consistently after i.c.v. injection of STZ, this phenomenon was present at more sites, inhibiting microtubule-binding activities. Overactivation of glycogen synthase kinase-3β (GSK3β) and decreased O-linked N-acetylglucosamine glycosylation (O-GlcNAcylation) resulting from impaired insulin signaling have been found to be the primary causes of hyperphosphorylation of tau. In relation to Aβ pathology, the change in brain parenchyma was subtle, but increased Congo-red-positive aggregates were observed in brain capillaries, suggesting that Aβ-related impairment of the microcirculation is present in this animal model.
In summary, increased tau phosphorylation and microtubule instability resulting from decreased phosphatase activity has been noted consistently in studies using the STZ-injection model, with these consequences being reversed by exogenous insulin supplementation. Significant Aβ pathology was not observed on the basis of this systemic insulin-deficient model, suggesting that insulin deficiency itself is not sufficient for the emergence of AD. On the other hand, i.c.v. STZ-injected subjects who experience problems with both insulin production and insulin signaling similar to those seen in the brains of animals with AD demonstrated more-extensive tau and Aβ-related pathology in microvessels. Thus, insulin resistance is thought to be an important contributor to the emergence of AD pathology in DM.
The spontaneous DM model
The spontaneous DM model may be more suitable for validating the relationship between DM and AD pathology since allowing a subject's experience to progress from the preclinical to the fully developed stage more closely replicates the progress of actual patients. Several different spontaneous DM models have been developed through specialized in-breeding. BB/Wor rats represent the T1DM model, in which autoimmune problems destroy insulin-secreting β cells in the pancreas. Cognitive dysfunction, along with typical neurodegenerative changes such as gliosis and neuronal and synaptic loss, have been noted in studies using this approach.21 In relation to AD pathology, increased tau phosphorylation as well as increased APP, BACE, and amyloid β proteins were present in this rodent model. All of this pathology was reversible by the application of insulinomimetic C-peptide, suggesting that insulin deficiency is the major mechanism underlying AD-related pathology in this model.
However, there was much less pathology in this model than in Bio-Breeding Zucker diabetic rat/Wor rats, which is a T2DM spontaneous model that was evaluated at the same time.21 AD pathology has been evaluated extensively in two widely available T2DM spontaneous models: Bio-Breeding Zucker diabetic rat/Wor rats and db/db mice.14,21 It was found that the neurodegenerative changes, consisting of neuronal loss, gliosis, and synaptic loss, were more prominent in these models. In addition, the diabetes-associated increase in tau phosphorylation was noted to be more widespread and more extensive than in the T1DM model. Interestingly, increased tau cleavage, which is known to be particularly toxic to neurons and to form a nucleation for neurofibrillary tangles,22 occurred exclusively with the T2DM model.14
From the greater AD pathology in the T2DM model it can be suggested that an important pathogenic factor intimately related to T2DM plays a critical role in AD pathology and that investigation into this factor will provide important clues as to the identity of the mechanism responsible for sporadic AD. T2DM is characterized by insulin resistance and consequent impaired glucose metabolism and utilization. The reduced ability of insulin receptors to respond to insulin characterizes the insulin resistance suffered by most AD patients.23 For this reason, insulin signaling has been the prime target of investigations into the factors linking T2DM and AD.
Genetically engineered model targeting insulin signaling
Studies performed using genetically modified animals and targeting insulin signaling have attempted to identify the steps involved in insulin signaling that have the greatest relevance for AD pathology.24-26
Neuron-specific insulin receptor knockout mice24
A mouse with selectively deficient insulin receptors in the brain shows complete inhibition of phosphatidylinositol 3-kinase (PI-3K) and dramatic inhibition of downstream Akt molecules. Blocking Akt activation is expected to decrease the phosphorylation of GSK3β, thereby inducing the phosphorylation of tau.24 However, the patterns of tau phosphorylation differ with the location of the phosphorylation characterized by the decrease at Thr231 and the increase at Ser201, even though both sites have been reported to be GSK3β substrates. Moreover, there is as yet no evidence of neuronal cell damage, cognitive dysfunction on the Morris water-maze test, or changes in glucose metabolism as detected on positron-emission tomography (PET) scans, which strongly suggests that the abolition of insulin receptors does not significantly affect AD pathology.
Insulin-receptor-substrate-2-knockout mice
Insulin receptor substrate (IRS) is the molecule connecting the receptors for insulin and IGF-1 to various downstream effectors including PI-3K, Akt, and extracellular signal-regulated kinase cascades. Consistent with other experiments conducted using DM models, studies of IRS-2-/-mice have shown highly increased phosphorylation of tau, but no disturbance of neuronal survival and gliosis.25 The questionable effect of IRS-2 on AD pathology was also demonstrated in a study of Tg2576/IRS-2-/-, which was conducted by cross-mating the IRS-2 deletion model with Tg2576, the APPswe-overexpressing AD model.26 Contrary to expectations, IRS-2 deletion significantly decreased the Aβ burden and improved cognitive functioning. However, increased tau phosphorylation at 396/404, 235, and 231 without the formation of tangles was also noted. The clear dissociation of tau pathology from Aβ pathology and cognition clearly shows that increased tau phosphorylation per se is not the critical factor linking T2DM and AD. Together the findings obtained using different spontaneous T2DM models show that specific ablation of individual steps of insulin signaling does not result in evident AD pathology, which suggests that simple impairment of the insulin signaling does not play an important role in AD pathology.
Insulin degradation enzyme knockout mice
Insulin degradation enzyme (IDE) is the major enzyme involved in insulin destruction through a direct interaction. It is also known to play a critical role in Aβ clearance.27 Its affinity for insulin is higher than for Aβ; therefore, more IDE tends to bind to insulin in the milieu of hyperinsulinemia, actually resulting in decreased Aβ degradation. A study using IDE-deficient mice clearly demonstrated the potent regulating effect of IDE on Aβ metabolism. Indeed, the level of cerebral Aβ increased by 10-65% in IDE-/- mice.27 Furthermore, IDE degraded primarily to soluble Aβ, the most-neurotoxic form of Aβ.28 Thus, the relative shortage of usable IDE as a result of the hyperinsulinemia associated with T2DM and the consequent increase in soluble Aβ may play central roles in the linkage between T2DM and AD.
Studies with Alzheimer's Disease Models (Table 2)
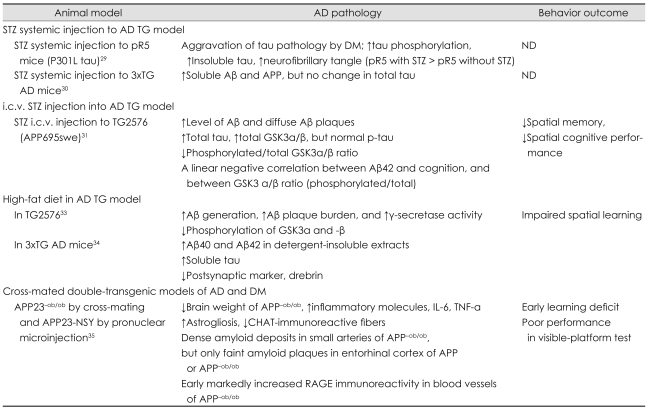
Table 2.
Animal studies using an AD transgenic animal model in the literature
Aβ: amyloid β protein, AD: Alzheimer's disease, APP: amyloid precursor protein, CHAT: choline acetyltransferase, DM: diabetes mellitus, i.c.v.: intracerebroventricular, IL: interleukin, ND: no test for cognition or behavior, p-tau: phosphorylated tau, RAGE: receptor for advanced glycation end products, STZ: streptozotocin, Tg: transgenic, TNF-a: tumor necrosis factor alpha, 3xTG: triple transgenic.
In addition to the aforementioned investigations involving on DM animal models, an increasing number of recent studies of the significance of the concurrent pathologies of DM and AD and their common mechanisms have relied on AD models that include diabetes. A review of recent well-designed studies using AD models enables understanding of the current status and future directions of research in this domain.
The effect of STZ injection in the transgenic mouse model of AD
The effects of DM as a result of systemic-STZ-induced insulin deficiency were evaluated in two transgenic models: pR5 mice with P301L tau and triple transgenic (3xTG) AD.29,30 Aggravation of tau pathology by concomitant DM was evident, with markedly increased phosphorylation and insoluble fraction of tau forming neurofibrillary tangles.29 The same effect of DM on APP and soluble Aβ was also noted, but not on the expression of total tau in the 3xTG AD model.30 Interestingly the amyloidogenic effect of DM was decreased by continuous supplementation of a glucagon-like peptide-1 agonist, one of the antidiabetic medications.
As described above, i.c.v. injection of STZ results in an insulin-resistant state in the brain.18,19 Therefore, this model is useful for evaluating the effect of insulin resistance. Reduced cognition as well as increased cerebral aggregated Aβ fragments, total tau proteins, and congophilic amyloid deposits were observed when Tg2576 mice injected with STZ at 3 months of age were evaluated at 9 months of age.31 Thus, both insulin deficiency and insulin resistance are thought to accelerate AD pathology.
AD model with a high-fat diet32-34
The ingestion of a high-fat diet over a long period leads to insulin resistance and obesity, which are core findings of T2DM. Functional abnormalities in insulin signaling, such as decreased insulin receptor β subunit autophosphorylation and reduced PI-3K-Akt signaling, are evident in this line of research. Feeding Tg2576 (APPswe-overexpressing) mice a high-fat diet for several months induced increased Aβ generation, Aβ plaque burden, and γ-secretase activity, with decreased IDE activities.32,33 Decreased phosphorylation of GSK-3α and GSK-3β, which increase tau phosphorylation, was found, and the extent of this decrease was strongly correlated with the levels of γ-C-terminal fragment. In addition, impaired behavioral performance accompanied this pathologic abnormality. The aggravating effect of T2DM on AD pathology via ingestion of a high-fat diet was reinforced by a study using 3xTG AD mice,34 which showed increases in Aβ40, Aβ42, and soluble tau.
Cross-mated double-transgenic models of AD and DM
Recent data obtained from cross-mated double-transgenic animals with APP23-ob/ob and APP23-NSY mice show the impact of T2DM on AD pathology, providing evidence of different pathogenic mechanisms.35 APP23 is a well-established AD model that overexpresses the Swedish mutation form of APP with a Thy-1 promoter. An ob/ob mouse is leptin deficient, and an NSY mouse is a polygenic T2DM model.36 Double-transgenic mice exhibited an earlier onset of diabetes and more-severe cognitive impairment. Interestingly, prominent microvascular changes, characterized by increased inflammation with up-regulated receptors for advanced glycation end products (RAGE) expression and amyloidosis, were prominent in these brains, and these signs correlated strongly with other neuropathological changes such as reactive gliosis and decreased cholinergic fibers. However, no increase in brain Aβ levels was observed. Consistent findings have been found in studies of APP23-NSY animals fed a high-fat diet; these include remarkable vascular inflammation with amyloidosis in the absence of an altered Aβ burden in the brain. RAGE are known mediate the pro-oxidant effects precipitated by Aβ acting on neuronal and cerebrovascular cells in the context of AD.37 Given the early and marked increase in RAGE expression combined with the Aβ found in microvessels, RAGE-Aβ interactions in small cerebral vessels probably play major roles in the pathogenesis linking T2DM and AD when both disorders are present.
Potential mechanisms linking T2DM and AD
Data from animal studies illustrating the intimate relationship between T2DM and AD provide insights into the mechanisms shared by these conditions. This section summarizes the mechanisms shared by T2DM and AD (Table 3), which were initially suspected to be epiphenomenal but are now considered to constitute the molecular mechanisms that link and mutually intensify the disorders.
Table 3.
Proposed common mechanisms linking AD and T2DM
Aβ: amyloid β protein, AD: Alzheimer's disease, IDE: insulin-degrading enzyme, O-GlcNAcylation: O-linked N-acetylglucosamine glycosylation, T2DM: type-2 DM.
Insulin resistance
Insulin resistance is characterized by reduced responsiveness of insulin receptors and decreased downstream signaling for the purpose of insulin stimulation. To compensate for these dysfunctions, the islet β-cells of the pancreas secrete more insulin, thereby creating a state of hyperinsulinemia. Insulin resistance with hyperinsulinemia constitutes the core feature of T2DM and is frequently also observed in AD patients.
Impaired insulin receptor signaling
There is evidence that Aβ oligomers can directly induce neuronal insulin resistance in the AD brain by inhibiting the insulin signaling targeting the insulin/Akt pathway38,39 and by removing insulin receptors after binding at the dendrites of synaptic sites.40,41 Impaired insulin signaling cannot efficiently inhibit GSK3; therefore, the activated GSKα triggers APP γ-secretase activity, while the activated GSK3β increases tau phosphorylation,42,43 which simultaneously aggravates the two major pathological substrates of AD. In addition, insulin signaling is important for the facilitation of memory via its regulation of synaptic structure and functioning and its promotion of neuronal survival.44 This effect is eliminated under conditions of impeded insulin functioning, resulting in neurodegeneration.
When prolonged systemic hyperinsulinemia is combined with insulin resistance, insulin-related activities in the brain become more impaired due to the decreased transport of insulin across the blood-brain barrier.45 Furthermore, hyperinsulinemia in the peripheral blood supply is thought to raise CSF Aβ42 levels.46 Therefore, a pathological feed-forward relationship between insulin resistance and AD pathology operates in the presence of comorbid T2DM.
Impairment of Aβ clearance via IDEs
AD and T2DM share their major catalytic enzyme, IDE. As demonstrated in IDE-/- animals,27 deficiency in IDE increases Aβ and aggravates AD pathology in the brain, and the enhancement of IDE activity reverses the exacerbated Aβ pathology in IDE-/- and APP double-transgenic mice.47 In states of hyperinsulinemia, increased insulin is more likely to efficiently bind to IDE than to Aβ; therefore, the level of native IDE that is available for binding to Aβ in the service of degradation markedly decreases, resulting in exacerbation of AD pathology. Postmortem analyses of the brains of individuals with late-onset AD show decreased levels of IDE expression in the hippocampus,48 suggesting that the decreased availability of IDE is a common pathophysiology linking T2DM and AD.
Impaired glucose metabolism
T2DM inevitably results in chronic hyperglycemia, which impairs glucose metabolism via ineffective glucose transport, depriving the neuronal cells of their major mechanism for metabolism and thereby producing serious complications that contribute to AD pathology. In addition, decreased glucose metabolism can be detected in AD even before the clinical onset of dementia, and is typically noted in fluorodeoxyglucose (FDG)-PET data. For this reason, FDG-PET can be a valuable neuroimaging marker for the early diagnosis of AD.49
Decreased O-GlcNAcylation
Diminished O-GlcNAcylation is considered to be a major molecular mechanism through which impaired glucose metabolism confounds T2DM and AD. A rat model produced by i.c.v. injection of STZ19 showed down-regulation of O-GlcNAcylation, impaired insulin signaling, and decreased glucose transporter activity. Decreased glucose metabolism in neurons lowers the level of UDP-GlcNAcylation, thereby decreasing tau O-GlcNAcylation. Decreased O-GlcNAcylation is also found in the brains of those with AD50 and is inversely related to the phosphorylation of tau. Therefore, diminished O-GlcNAcylation may result in the hyperphosphorylation of tau, leading to the tau-related pathology associated with AD.
Increased advanced glycation end products, inflammation, and oxidative stress
Chronic hyperglycemia induces the creation of advanced glycation end products (AGEs) via Maillard reactions, during which reducing sugars can react with the amino groups of proteins to produce cross-linked complexes and unstable compounds.51 Therefore, AGEs mediate various complications of diabetes by interacting with the receptors for AGE present in the vascular cells and microglia, which enhances various inflammatory processes and oxidative stress. Furthermore, increased AGEs can contribute to amyloidosis52 and tau phosphorylation in AD.53 Indeed, the immunoreactivity of AGEs was markedly increased in Aβ plaques and neurofibrillary tangles. Therefore, increased AGEs may be another important factor shared by T2DM and AD. On the other hand, increased inflammatory mediators such as tumor necrosis factor α, interleukin-1β, and interleukin-6 are induced not only by AGEs, but also by hyperinsulinemia itself.54 Specifically, the concentration of F2-isoprostane, a marker of lipid peroxidation, was increased in the CSF during insulin infusion in normal adults, and this was correlated with changes in CSF levels of Aβ42.54 Increased levels of inflammatory cytokines along with oxidative stress derived from different mechanisms would be expected to lead to AD pathology through a synergistic process.55
Enhanced glucocorticoid effect
In chronic uncontrolled DM, the concentration of circulating cortisol increases,56,57 which adversely affects cognitive functioning.58 In db/db mice (the well-known T2DM model), enhanced corticoid activity has been shown to result in impaired synaptic plasticity and decreased neurogenesis, thereby yielding learning and memory deficits59 and providing evidence that increased cortisol contributes to the neurodegenerative complications of T2DM. With respect to AD, elevated levels of cortisol have been correlated with the extent of hippocampal atrophy, decreased cognitive performance, and rapid decline.60,61 Together these findings indicate that enhanced glucocorticoid activity is a confounding factor in both T2DM and AD.
Cerebrovascular insufficiency
T2DM increases cerebrovascular disease via an atherosclerotic process,8,63 and data revealing the influence of cerebrovascular disease on the development and severity of AD have been reported.63 After excluding the influence of overt cerebrovascular disease, the intimate relationship between T2DM and AD persists, underpinned by the aforementioned common mechanisms. Recent data obtained from ob/ob or polygenic NSY mice and from APP23 transgenic mice indicate that another pathology in the cerebral microvessels leading to cerebrovascular insufficiency constitutes a major contributor to the cognitive decline linked to both T2DM and AD.35 Up-regulation of RAGE and inflammatory changes followed by increased amyloid deposition in the cerebral small vessels have been shown to be important in producing cognitive impairments in these models, but not parenchymal Aβ burdens. Therefore, cerebrovascular insufficiency caused by increased RAGE-Aβ interactions, leading to inflammation and amyloid angiopathy, can be considered as a shared mechanism of T2DM and AD in addition to the overt cerebrovascular disorder resulting from increased vascular risk factors related to T2DM.
Conclusion
Animal models provide strong evidence of a relationship between T2DM and AD, and they suggest several potential mechanisms linking the two disorders. It is strongly suggested that AD pathology in T2DM derives from various mechanisms operating synergistically rather than from a single mechanism operating independently. Pathogenic alterations in insulin signaling, Aβ clearance by IDE, glucose metabolism, O-GlcNAcylation, Aβ aggregation by AGEs, inflammation, oxidative stress, circulating cortisol, and cerebral vascular insufficiency are considered to contribute to both T2DM and AD. The incidence of comorbidity of T2DM and AD increases with age. Therefore, this common pathophysiology probably constitutes a major underpinning of late-onset sporadic AD, and a novel therapeutic approach targeting this pathological process could contribute to the development of a more efficient and effective treatment for AD.
Acknowledgements
This study is supported by a grant of the Korean Health Technology R & D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092004), and by the Korea Research Foundation (KRF) grant funded by the Korea government (MEST, 2009-0067850) The author has no conflict of interest.
Footnotes
The author has no financial conflicts of interest.
References
- 1.Suh GH, Knapp M, Kang CJ. The economic costs of dementia in Korea, 2002. Int J Geriatr Psychiatry. 2006;21:722–728. doi: 10.1002/gps.1552. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Rafii MS, Aisen PS. Recent developments in Alzheimer's disease therapeutics. BMC Med. 2009;7:7. doi: 10.1186/1741-7015-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 5.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 6.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 7.Grodstein F, Chen J, Wilson RS, Manson JE. Nurses' Health Study. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24:1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- 8.Peila R, Rodriguez BL, Launer LJ. Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 9.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 10.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 11.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 12.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 13.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 14.Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, et al. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer's disease and correction by insulin. J Neurosci Res. 2008;86:3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brant AM, Jess TJ, Milligan G, Brown CM, Gould GW. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun. 1993;192:1297–1302. doi: 10.1006/bbrc.1993.1557. [DOI] [PubMed] [Google Scholar]
- 18.Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem. 2006;96:1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- 19.Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: implication for Alzheimer's disease. Am J Pathol. 2009;175:2089–2098. doi: 10.2353/ajpath.2009.090157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 21.Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- 22.de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 24.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killick R, Scales G, Leroy K, Causevic M, Hooper C, Irvine EE, et al. Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice. Biochem Biophys Res Commun. 2009;386:257–262. doi: 10.1016/j.bbrc.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudoh S, Frosch MP, Wolf BA. Differential effects of proteases involved in intracellular degradation of amyloid beta-protein between detergent-soluble and -insoluble pools in CHO-695 cells. Biochemistry. 2002;41:1091–1099. doi: 10.1021/bi011193l. [DOI] [PubMed] [Google Scholar]
- 29.Ke YD, Delerue F, Gladbach A, Götz J, Ittner LM. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer's disease. PLoS One. 2009;4:e7917. doi: 10.1371/journal.pone.0007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaschke K, Kopitz J, Siegelin M, Schliebs R, Salkovic-Petrisic M, Riederer P, et al. Insulin-resistant brain state after intracerebroventricular streptozotocin injection exacerbates Alzheimer-like changes in Tg2576 AbetaPP-overexpressing mice. J Alzheimers Dis. 2010;19:691–704. doi: 10.3233/JAD-2010-1270. [DOI] [PubMed] [Google Scholar]
- 32.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 33.Kohjima M, Sun Y, Chan L. Increased food intake leads to obesity and insulin resistance in the tg2576 Alzheimer's disease mouse model. Endocrinology. 2010;151:1532–1540. doi: 10.1210/en.2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda H, Ikegami H, Kawaguchi Y, Fujisawa T, Nojima K, Babaya N, et al. Age-dependent changes in phenotypes and candidate gene analysis in a polygenic animal model of Type II diabetes mellitus; NSY mouse. Diabetologia. 2000;43:932–938. doi: 10.1007/s001250051472. [DOI] [PubMed] [Google Scholar]
- 37.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 38.Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 39.Lee HK, Kumar P, Fu Q, Rosen KM, Querfurth HW. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell. 2009;20:1533–1544. doi: 10.1091/mbc.E08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 41.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, et al. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 43.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 45.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 46.Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 47.Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- 48.Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, et al. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer's disease is associated with the apolipoprotein E-epsilon4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mistur R, Mosconi L, Santi SD, Guzman M, Li Y, Tsui W, et al. Current Challenges for the Early Detection of Alzheimer's Disease: Brain Imaging and CSF Studies. J Clin Neurol. 2009;5:153–166. doi: 10.3988/jcn.2009.5.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer's disease. Brain. 2009;132:1820–1832. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 52.Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Necula M, Kuret J. Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J Biol Chem. 2004;279:49694–49703. doi: 10.1074/jbc.M405527200. [DOI] [PubMed] [Google Scholar]
- 54.Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, et al. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005;62:1539–1544. doi: 10.1001/archneur.62.10.noc50112. [DOI] [PubMed] [Google Scholar]
- 55.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 56.Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol. 2004;10:36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- 57.Lee ZS, Chan JC, Yeung VT, Chow CC, Lau MS, Ko GT, et al. Plasma insulin, growth hormone, cortisol, and central obesity among young Chinese type 2 diabetic patients. Diabetes Care. 1999;22:1450–1457. doi: 10.2337/diacare.22.9.1450. [DOI] [PubMed] [Google Scholar]
- 58.Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, et al. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. J Clin Neurosci. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt R, Launer LJ, Nilsson LG, Pajak A, Sans S, Berger K, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53:687–692. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 63.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]