Abstract
Background and Purpose
It is particularly difficult to differentiate dementia with Lewy bodies (DLB) from the related dementias of Alzheimer's disease (AD) and Parkinson's disease dementia (PDD). Few studies have been designed to comparatively analyze detailed neuropsychological assessments of DLB patients and patients with AD and PDD.
Methods
Three groups of patients participated in this study: 10 with DLB, 76 with AD, and 17 with PDD, who had been diagnosed as probable DLB, AD, and PDD, respectively, according to the clinical criteria of the consortium on DLB, National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorder Association, and the clinical diagnostic criteria for PDD. All patients were evaluated by careful neurological examination with detailed neuropsychological testing.
Results
Significant differences among the three groups were found for attention, memory, and executive function, which included tasks of backward digit span, three-word recall, verbal delayed recall, and the Stroop test. Post hoc analysis revealed that the deficiencies of attention on the digit span task were greater in the DLB group than in the AD and PDD groups. The scores for episodic verbal memory tasks were significantly lower in the DLB and AD groups than in the PDD group. The performance in frontal executive function, as indicated by the Stroop test, was significantly worse in the DLB and PDD groups than in the AD group.
Conclusions
The results of the present study show that the pattern of cognitive dysfunction, in terms of attention, episodic memory, and executive functions, differ between patients with DLB and patients with AD and PDD.
Keywords: dementia with lewy bodies, Alzheimer's disease, Parkinson's disease dementia, cognition, neuropsychology
Introduction
Alzheimer's disease (AD) and idiopathic Parkinson's disease (PD) are the most common age-related neurodegenerative disorders. The parkinsonian feature is a relatively common sign in clinically diagnosed AD, and cognitive impairment is also quite common in PD.1,2 Dementia with Lewy bodies (DLB), which is characterized by progressive dementia and fluctuating cognition and is associated with visual hallucination and Parkinsonism, has recently emerged as one of the most common neurodegenerative dementias.3,4 DLB has now been identified as a separate disease, although it remains difficult to differentiate it from the related dementias of AD and PD dementia (PDD).5 DLB should be considered as a probable diagnosis in patients showing dementia in the early stage of Parkinsonism. Unlike PD, the clinical presentation of DLB demonstrates progressive cognitive decline, with significant deficits of visuospatial ability as well as loss of frontal executive function, accompanied by mild-to-moderate Parkinsonism.6,7 In attempts to differentiate DLB from other dementias, several studies have suggested the use of cognitive assessment to differentiate patients with DLB from those with AD or PDD.8-12
The importance of neuropsychological testing for DLB is being increasingly emphasized: careful cognitive assessments with clinical work-up are becoming the fundamental standards, providing basic information and guidelines for the appropriate treatment of patients with DLB. Few studies have been designed to comparatively analyze detailed neuropsychological assessments of DLB patients and AD and PDD.9-11
Some studies have found that DLB and PDD patients perform significantly worse on attentional functions and better on memory tests than AD patients,9,10 and that DLB patients exhibit lower scores than AD patients on visual memory and visuospatial tests. No significant differences have been noted between PDD and DLB subjects on any neuropsychological test. However, others found that DLB patients performed worse than PDD patients with regard to frontal executive function and visual recognition memory.13
In the present study we used neuropsychological evaluation to determine whether cognitive deficits differ between patients with DLB and those with either AD or PDD. The aims of this study were to determine the pattern of cognitive impairment in DLB and to differentiate DLB from AD and PDD using comprehensive neuropsychological testing.
Methods
Subjects
We consecutively enrolled 10 patients diagnosed with probable DLB, 76 patients with probable AD, and 17 patients with probable PDD. They were recruited sequentially between June 2006 and May 2009 from the dementia and PD clinics at a university hospital. They were diagnosed as probable DLB, AD, or PDD respectively according to the following three sets of clinical criteria: 1) the clinical criteria of the consortium on DLB,4 2) the diagnostic criteria for AD from the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorder Association,14 and 3) the clinical diagnostic criteria for PDD.15 The following exclusion criteria were applied:
Major depression, bipolar disorder, schizophrenia, or substance-use disorder.
Cerebrovascular disease, hydrocephalus, or brain tumor documented by structural imaging within the previous 12 months.
Deficiency of serum folate and vitamin B12, neurosyphilis, or clinically significant thyroid disease.
A history of traumatic brain injury.
Significant medical illnesses (e.g., poorly controlled diabetes or hypertension, cancer within the previous 5 years, clinically significant hepatic, renal, cardiac, or pulmonary disorders).
All patients were carefully evaluated by a neurologist and a neuropsychologist. Recruited subjects showing a severe state of dementia who were unable to participate in detailed neuropsychological evaluations were excluded. Patients with PDD who showed motor fluctuations were assessed in the "ON" phase. We avoided periods of any confusion or drowsiness during neuropsychological evaluation in DLB patients. Subjects were not taking cholinesterase inhibitors or memantine during the period of neuropsychological evaluation. Patients with DLB and PDD were evaluated while on their medications of levodopa or dopamine agonist.
The evaluation procedure comprised a detailed medical history, physical and neurological examinations, neuropsychological assessments, appropriate laboratory tests, and a brain MRI. Histories of medical and neurological problems were obtained from the patient and their family members or other caregivers. Informed consent to participate in this study was obtained from all patients, and the study was approved by the Institutional Review Board of the hospital.
Neuropsychological assessment
The general cognitive status and severity of dementia were evaluated using the Korean Mini-Mental State Examination (K-MMSE),16,17 the clinical dementia rating (CDR),18 and the sum of the box score of the CDR (CDRSB). The scores for physical activities of daily living were estimated using the Barthel activities of daily living (B-ADL) index.
All patients underwent the standardized neuropsychological test battery of the Seoul Neuropsychological Screening Battery,19 which includes the following tests:
Attention: forward and backward digit span and letter cancellation test.
Language and related functions: reading, writing, comprehension, repetition, and confrontational naming using the Korean version of the Boston Naming Test,20 finger naming, right-left orientation, body part identification, calculation, and ideomotor and buccofacial praxis.
Visuospatial function test: interlocking pentagon drawing and the Rey Complex Figure Test (RCFT).
Verbal memory test: three-word registration and recall, and Seoul Verbal Learning Test.
Visual memory test: the RCFT, immediate recall, 20-min delayed recall, and recognition.
Frontal executive function test: motor impersistence, contrasting program, go/no-go test, fist-edge-palm test, alternating hand movement, alternating square and triangle, Luria loop, semantic (animals and supermarket) and phonemic Controlled Oral Word Association Test, and the Stroop test (word and color reading of 112 items).
Statistical analysis
The clinical and baseline characteristics of the patients are summarized using descriptive statistics. A Fisher's exact chi-square test was used to compare categorical variables between groups, while an analysis of covariance was used to compare continuous variables. Response variables were adjusted for CDR, B-ADL, K-MMSE, and CDRSB, and adjusted means were compared between the groups with the Tukey-Kramer post hoc test. Plots of the mean±SD values are presented. The level of statistical significance was set at p<0.05. All statistical analyses were carried out using SAS version 9.1.3 and R 2.9.1 statistical software.
Results
Demographic characteristics
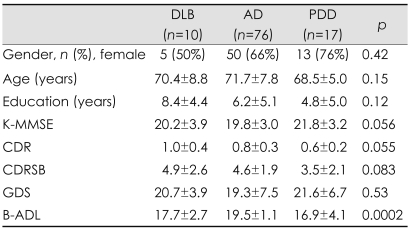
The demographic characteristics and cognitive functions, together with the daily functional and behavioral scales of the patients with DLB, AD, and PPD are presented in Table 1. There were no significant differences between the patient groups with regard to sex, age, educational level, or in general cognitive measures as indicated by K-MMSE, CDR, and CDRSB scores. The geriatric depression scales did not differ significantly between the three groups. However, with regard to the functional scales of Barthel activities of daily living (B-ADL), the post hoc comparisons indicated that the impairment in the basic activities of daily living was greater in the PDD group than in the AD group (p<0.05).
Table 1.
Demographic data and general cognitive functions with daily activities in the three patient groups
AD: alzheimer's disease, B-ADL: Barthel activities of daily living, CDR: Clinical Dementia Rating, CDRSB: Clinical Dementia Rating Sum of Boxes, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, K-MMSE: Korean version of the Mini-Mental State Examination, PDD: parkinson's disease dementia.
Neuropsychological findings
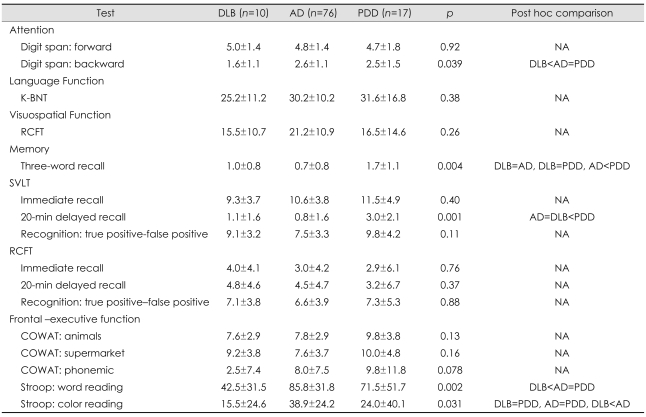
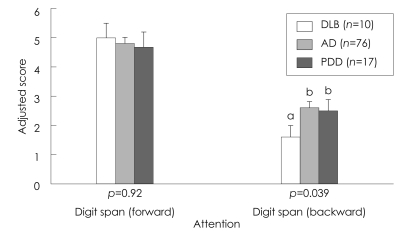
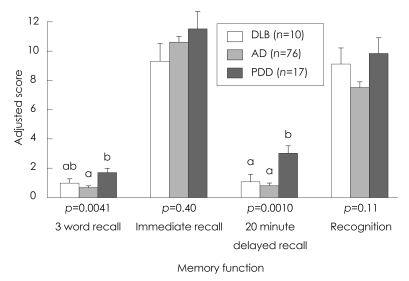
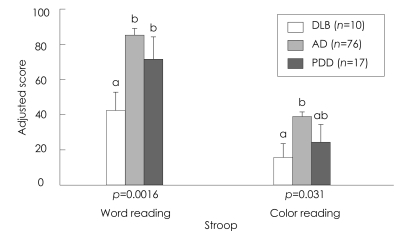
Comparison of the neuropsychological differences between patients with DLB, AD, and PDD is summarized in Table 2. analysis of covariance revealed significant differences among the three groups in the cognitive domains of attention, memory, and frontal executive function, which included tasks of backward digit span, three-word recall, verbal delayed recall, and the Stroop test. The post hoc analysis showed that the deficit in attention on the task of digit span was greater in the DLB group than in the AD and PDD groups (Table 2, Fig. 1). The DLB and AD groups achieved significantly lower scores than the PDD group in verbal memory function, on delayed recall of Seoul Verbal Learning Test, and on the task of three-word recall (Table 2, Fig. 2). Frontal executive function, as indicated by the word reading of the Stroop test, was significantly worse in the DLB group than in the AD and PDD groups (Table 2, Fig. 3). The score on the task of color reading of the Stroop test was significantly lower in the DLB group than in the AD group (Table 2, Fig. 3). Although the Controlled Oral Word Association Test scores did not differ significantly between the DLB, AD, and PDD groups, the score for the task of phonemic word fluency was lower in the DLB group than in the AD and PDD groups. Visuospatial function on the RCFT did not differ significantly between the three groups. Moreover, the scores for verbal and visual recognition did not differ significantly between patients with DLB and PDD.
Table 2.
Adjusted scores* of neuropsychological test in the three patient groups (mean±SD values)
*Scores were adjusted for CDR, B-ADL, K-MMSE, and CDRSB.
AD: Alzheimer's disease, COWAT: Controlled Oral Word Association Test, DLB: dementia with lewy bodies, K-BNT: Korean version of the Boston Naming Test, NA: Not applicable, PDD: Parkinson's disease dementia, RCFT: Rey Complex Figure Test, SVLT: Seoul Verbal Learning Test.
Fig. 1.
Neuropsychological comparison of the attention domain in the three patient groups. Values are mean and SE values; bars with different superscripts differ from each other within a specific category (p<0.05). Means with the same letter in their superscripts do not differ significantly from one another according to a Tukey-Kramer test with a 0.05 limit on the familywise error rate. AD: Alzheimer's disease, DLB: dementia with lewy bodies, PDD: Parkinson's disease dementia.
Fig. 2.
Neuropsychological comparison of the verbal memory domain in the three patient groups. Data are mean and SE values; bars with different superscripts differ from each other within a specific category (p<0.05). Means with the same letter in their superscripts do not differ significantly from one another according to a Tukey-Kramer test with a 0.05 limit on the familywise error rate. AD: Alzheimer's disease, DLB: dementia with lewy bodies, PDD: Parkinson's disease dementia.
Fig. 3.
Neuropsychological comparison of executive function domain in the three patient groups. Data are mean and SE values; bars with different superscripts differ from each other within a specific category (p<0.05). Means with the same letter in their superscripts do not differ significantly from one another according to a Tukey-Kramer test with a 0.05 limit on the familywise error rate. AD: Alzheimer's disease, DLB: dementia with lewy bodies, PDD: Parkinson's disease dementia.
Discussion
The present study investigated the characteristics of cognitive profiles of patients with DLB and attempted to differentiate DLB from AD and PDD by using comprehensive neuropsychological assessments, which covered a wide range of cognitive domains. It is very important to be able to differentiate between the cognitive profiles of DLB sufferers and those with AD and PDD. The distinctive patterns of neuropsychological dysfunction observed in these dementias probably represent a different distribution of pathological changes. The neuropathological substrate of AD affects predominantly the medial temporal cortex and the neocortical association areas, which explains the predominant dysfunction of episodic memory function. On the other hand, the neuropathological basis of DLB includes neuronal loss and the presence of Lewy bodies in the subcortical nucleus and in the frontal and parietal lobes, which explains the predominantly attentional, executive, and visuospatial dysfunctions.21 Recent clinical pathological data have demonstrated that DLB and PDD patients have the same underlying pathology of the Lewy body spectrum.22,23 Most PDD patients may indeed show clinical features and neuropsychological characteristics that are similar to those exhibited by DLB patients.
Our results showed that verbal memory function in DLB is comparable with that in AD. Unlike previous studies,9,10,24 the degree of impairment in the episodic memory tests was the same in our DLB and AD patients. The discrepancies between the results of our study and those of prior studies9,10,24 may be explained by several factors. First, the K-MMSE scores were lower in our patients than in those included in previous studies.9,10 Thus, the floor effect, in items of memory function, may have occurred in both DLB and AD patients. Second, both DLB and AD are often linked to degeneration in the medial temporal lobes, including the hippocampus and amygdala. Hamilton et al.11,25 suggested that the poor memory function in DLB patients is attributable to an encoding impairment rather than the consolidation defect. Third, despite our attempt to exclude mixed pathologies of DLB and AD, some patients with concomitant DLB and AD were included in our study sample.
Our findings are consistent with those of previous studies in showing that the frontal executive function is much worse in DLB than in AD.9-12 The findings of the current study reflect a different distribution of neuropathological changes in DLB, which include widespread neuronal loss with the presence of Lewy bodies in the subcortical nuclei, and the frontal subcortical association and dorsolateral areas of the frontal lobe. The Stroop test is widely used for evaluating executive function and is considered to measure impairments in the dorsolateral prefrontal system. The results of the Stroop test in the present study suggest that the circuit associated with color reading is frequently disrupted in patients with DLB, with these interruptions occurring in the dorsolateral prefrontal lobe.
Unlike the results of previous studies,9,10,26,27 our data revealed that visuospatial function on RCFT did not differ significantly between the DLB and PDD patients and the AD patients. The current results show that the patients with DLB and PDD had lower scores for K-MMSE and lower educational levels than the participants in previous studies,9,10 suggesting that these factors influenced their performance on the RCFT. The scores for visuospatial function on the RCFT tended to be lower scores in the DLB and PDD groups than in the AD group.
In the present study we found differences between DLB and PDD patients, which contrasts with the findings of previous studies.9,10,13,28 The deficits in the cognitive domains of attention, memory, and executive function were greater in our DLB patients than our PDD patients. However, visual recognition scores did not differ between these two groups.
In a comparison of the cognitive profiles of patients with PDD and those with DLB, the MMSE scores of whom indicated a mild stage of dementia, Aarsland et al.9 and Downes et al.28 demonstrated that executive function was impaired more in patients with DLB than in those with PDD. The K-MMSE scores of our patients indicated a mild stage of dementia, and the performance for attention, executive function, and verbal memory was worse in those with DLB than in those with PDD. These data suggest that although PDD and DLB share similar global cognitive patterns, the cognitive deficits in the frontal and medial temporal-related cognitive functions during the mild stages of dementia are greater in patients with DLB than in PDD patients. It is suggested that the distribution of Lewy body pathology differs between PDD and DLB patients in the early disease stage. There is also evidence that the presence of amyloid pathology differs between DLB and PDD.29,30 Demonstration of a significant amyloid pathology in [11C] Pittsburgh compound B positron-emission tomography might explain why the level of deterioration in memory and executive functions is higher in DLB than in PDD. A very recent study found that a subgroup of PDD patients not experiencing cognitive fluctuations showed significantly less attentional, executive, and memory deficits compared to those with DLB and PDD experiencing cognitive fluctuations.31 The differences in cognitive profiles between DLB and PDD in our data may be influenced by the discrepancy of distribution in the two subgroups of PDD.
There present study was subject to some shortcomings. First, selection bias may have been present in our sample because the patients were recruited either through a memory clinic or through a PD clinic. In addition, the results of our study are limited by the disproportionate numbers of AD patients and DLB and PDD patients. Second, we cannot be certain about the accuracy of our clinical diagnoses because of the lack of neuropathological confirmation. Although we attempted to exclude cases with suspected mixed pathology, some of the patients with DLB may have had concomitant AD. Third, the neuropsychological assessment tool utilized herein is not fully adequate to ascertain the detailed characteristics of several cognitive domains.
Acknowledgements
This paper was supported by the Dong-A University Research Fund in 2007.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lopez OL, Wisnieski SR, Becker JT, Boller F, DeKosky ST. Extrapyramidal signs in patients with probable Alzheimer disease. Arch Neurol. 1997;54:969–975. doi: 10.1001/archneur.1997.00550200033007. [DOI] [PubMed] [Google Scholar]
- 2.Marder K, Tang MX, Cote L, Stern Y, Mayeux R. The frequency and associated risk factors for dementia in patients with Parkinson's disease. Arch Neurol. 1995;52:695–701. doi: 10.1001/archneur.1995.00540310069018. [DOI] [PubMed] [Google Scholar]
- 3.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 5.Perry RH, Irving D, Blessed G, Perry EK, Fairbairn AF. Clinically and neuropathologically distinct form of dementia in the elderly. Lancet. 1989;1:166. doi: 10.1016/s0140-6736(89)91187-2. [DOI] [PubMed] [Google Scholar]
- 6.Stern Y, Tang MX, Jacobs DM, Sano M, Marder K, Bell K, et al. Prospective comparative study of the evolution of probable Alzheimer's disease and Parkinson's disease dementia. J Int Neuropsychol Soc. 1998;4:279–284. [PubMed] [Google Scholar]
- 7.McKeith IG, Ballard CG, Perry RH, Ince PG, O'Brien JT, Neill D, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54:1050–1058. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- 8.Ballard CG, Aarsland D, McKeith I, O'Brien J, Gray A, Cormack F, et al. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology. 2002;59:1714–1720. doi: 10.1212/01.wnl.0000036908.39696.fd. [DOI] [PubMed] [Google Scholar]
- 9.Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord. 2004;19:60–67. doi: 10.1002/mds.10633. [DOI] [PubMed] [Google Scholar]
- 11.Metzler-Baddeley C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer's disease and Parkinson's disease with dementia. Cortex. 2007;43:583–600. doi: 10.1016/s0010-9452(08)70489-1. [DOI] [PubMed] [Google Scholar]
- 12.Tröster AI. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson's disease with dementia: differentiation, early detection, and implications for "mild cognitive impairment" and biomarkers. Neuropsychol Rev. 2008;18:103–119. doi: 10.1007/s11065-008-9055-0. [DOI] [PubMed] [Google Scholar]
- 13.Mondon K, Gochard A, Marqué A, Armand A, Beauchamp D, Prunier C, et al. Visual recognition memory differentiates dementia with Lewy bodies and Parkinson's disease dementia. J Neurol Neurosurg Psychiatry. 2007;78:738–741. doi: 10.1136/jnnp.2006.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Kang Y, Na DL, Hahn S. A validity study on the korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y, Na DL. Seoul Neuropsychological Screening Battery. Incheon: Human Brain Research & Consulting Co; 2003. [Google Scholar]
- 20.Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J Clin Exp Neuropsychol. 1999;21:127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Isla T, Growdon WB, McNamara M, Newell K, Gómez-Tortosa E, Hedley-Whyte ET, et al. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53:2003–2009. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- 22.Aarsland D, Ballard CG, Halliday G. Are Parkinson's disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol. 2004;17:137–145. doi: 10.1177/0891988704267470. [DOI] [PubMed] [Google Scholar]
- 23.McKeith IG, Mosimann UP. Dementia with Lewy bodies and Parkinson's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S15–S18. doi: 10.1016/j.parkreldis.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Simard M, van Reekum R, Myran D, Panisset M, Cohen T, Freedman M, et al. Differential memory impairment in dementia with Lewy bodies and Alzheimer's disease. Brain Cogn. 2002;49:244–249. [PubMed] [Google Scholar]
- 25.Hamilton JM, Salmon DP, Galasko D, Delis DC, Hansen LA, Masliah E, et al. A comparison of episodic memory deficits in neuropathologically-confirmed Dementia with Lewy bodies and Alzheimer's disease. J Int Neuropsychol Soc. 2004;10:689–697. doi: 10.1017/S1355617704105043. [DOI] [PubMed] [Google Scholar]
- 26.Calderon J, Perry RJ, Erzinclioglu SW, Berrios GE, Dening TR, Hodges JR. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry. 2001;70:149–156. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downes JJ, Priestley NM, Doran M, Ferran J, Ghadiali E, Cooper P. Intellectual, mnemonic, and frontal functions in dementia with Lewy bodies: A comparison with early and advanced Parkinson's disease. Behav Neurol. 1998;11:173–183. [PubMed] [Google Scholar]
- 29.Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 30.Brooks DJ. Imaging amyloid in Parkinson's disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord. 2009;24(Suppl 2):S742–S747. doi: 10.1002/mds.22581. [DOI] [PubMed] [Google Scholar]
- 31.Varanese S, Perfetti B, Monaco D, Thomas A, Bonanni L, Tiraboschi P, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson's disease with dementia: comparison of dementia with Lewy bodies and Alzheimer's disease. J Neurol. 2010;257:1004–1011. doi: 10.1007/s00415-010-5453-3. [DOI] [PubMed] [Google Scholar]