Abstract
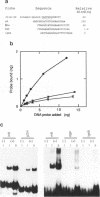
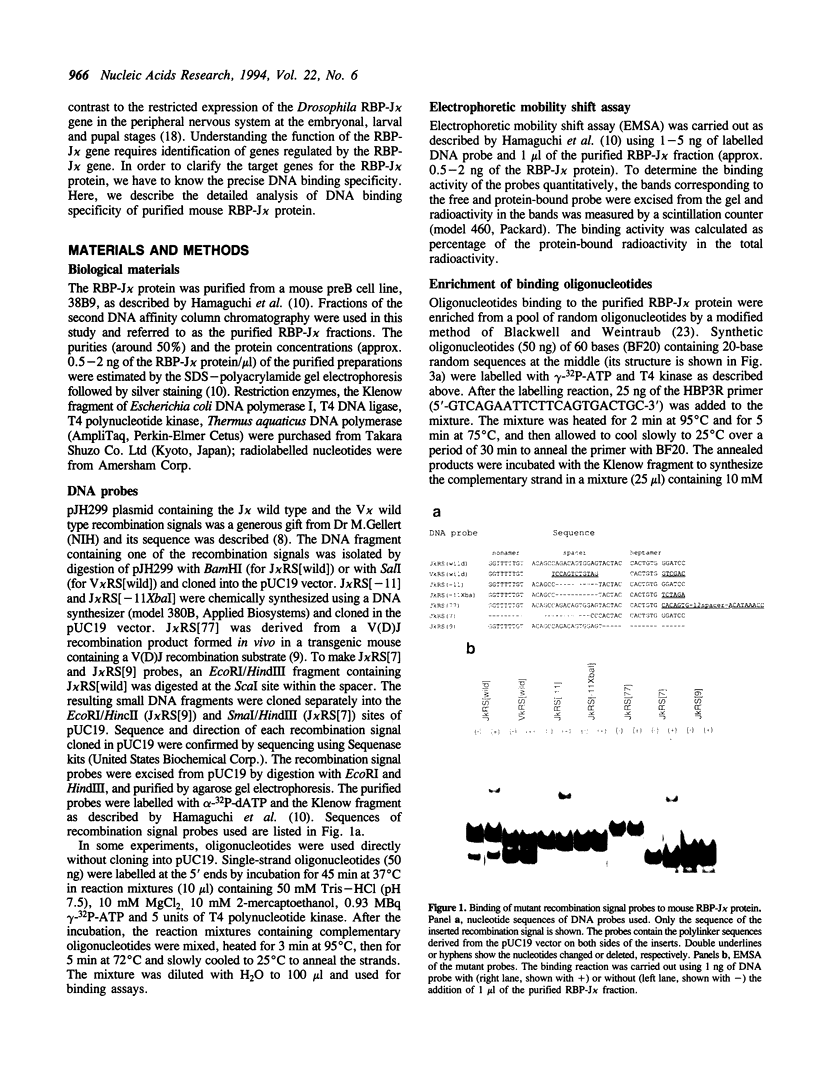
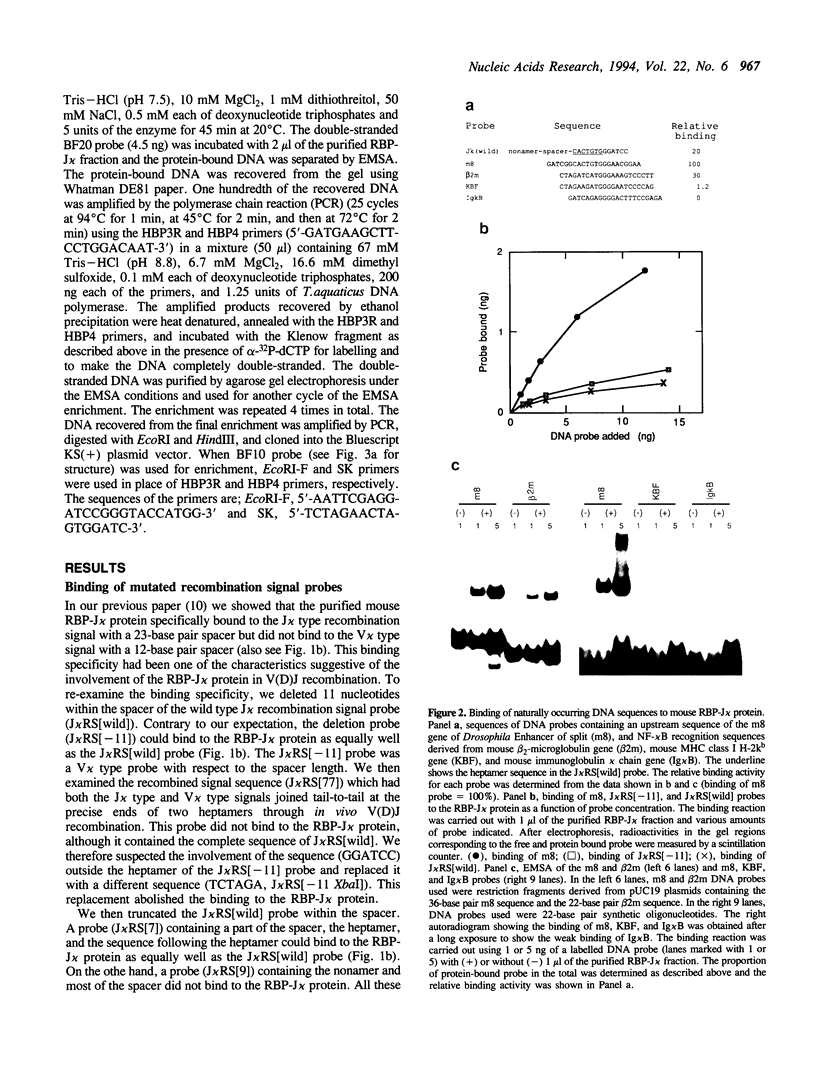
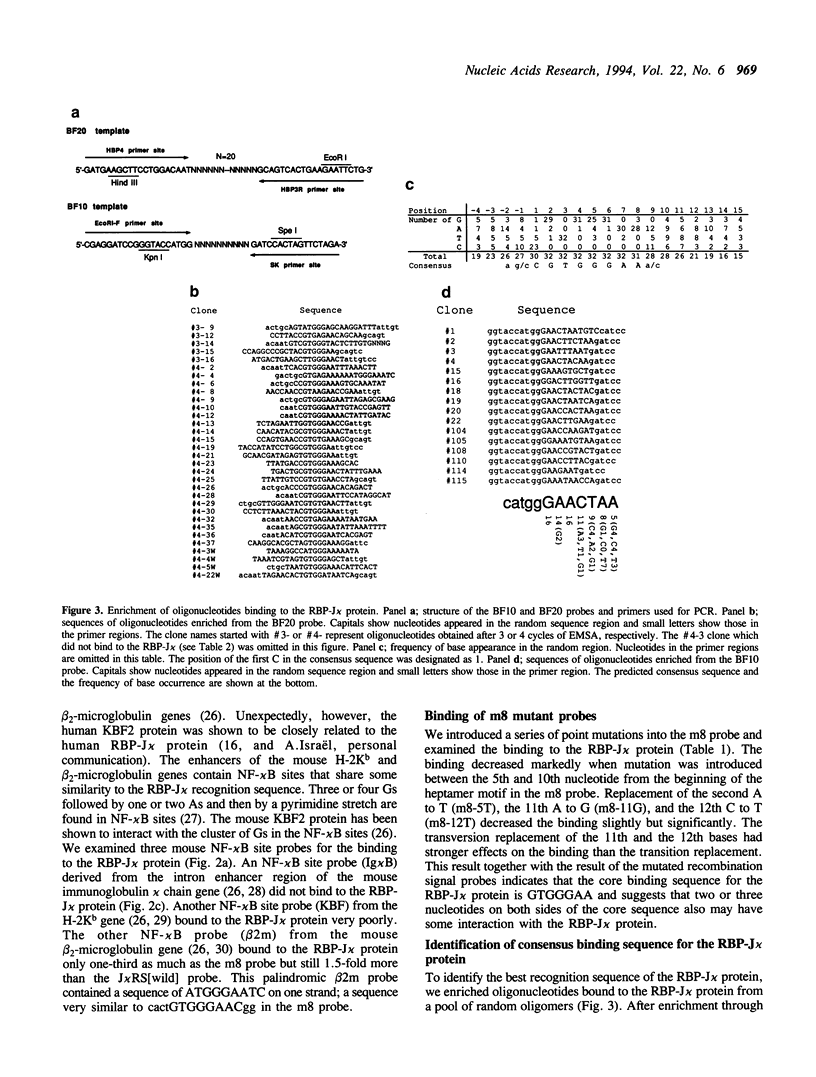
DNA binding specificity of the RBP-J kappa protein was extensively examined. The mouse RBP-J kappa protein was originally isolated as a nuclear protein binding to the J kappa type V(D)J recombination signal sequence which consisted of the conserved heptamer (CACTGTG) and nonamer (GGTTTTTGT) sequences separated by a 23-base pair spacer. Electrophoretic mobility shift assay using DNA probes with mutations in various parts of the J kappa recombination signal sequence showed that the RBP-J kappa protein recognized the sequence outside the recombination signal in addition to the heptamer but did not recognize the nonamer sequence and the spacer length at all. Database search identified the best naturally occurring binding motif (CACTGTGGGAACGG) for the RBP-J kappa protein in the promoter region of the m8 gene in the Enhancer of split gene cluster of Drosophila. The binding assay with a series of m8 motif mutants indicated that the protein recognized mostly the GTGGGAA sequence and also interacted weakly with ACT and CG sequences flanking this hepta-nucleotide. Oligonucleotides binding to the RBP-J kappa protein were enriched from a pool of synthetic oligonucleotides containing 20-base random sequences by the repeated electrophoretic mobility shift assay. The enriched oligomer shared a common sequence of CGTGGGAA. All these data indicate that the RBP-J kappa protein recognizes a unique core sequence of CGTGGGAA and does not bind to the V(D)J recombination signal without the flanking sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Amakawa R., Jing W., Ozawa K., Matsunami N., Hamaguchi Y., Matsuda F., Kawaichi M., Honjo T. Human Jk recombination signal binding protein gene (IGKJRB): comparison with its mouse homologue. Genomics. 1993 Aug;17(2):306–315. doi: 10.1006/geno.1993.1326. [DOI] [PubMed] [Google Scholar]
- Argos P., Landy A., Abremski K., Egan J. B., Haggard-Ljungquist E., Hoess R. H., Kahn M. L., Kalionis B., Narayana S. V., Pierson L. S., 3rd The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986 Feb;5(2):433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. III. Hypomorphic and hypermorphic mutations affecting the expression of hairless. Genetics. 1982 Jul-Aug;101(3-4):447–459. doi: 10.1093/genetics/101.3-4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Bang A. G., Hartenstein V., Posakony J. W. Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development. 1991 Jan;111(1):89–104. doi: 10.1242/dev.111.1.89. [DOI] [PubMed] [Google Scholar]
- Bang A. G., Posakony J. W. The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev. 1992 Sep;6(9):1752–1769. doi: 10.1101/gad.6.9.1752. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Kawaichi M., Matsunami N., Ryo H., Nishida Y., Honjo T. The Drosophila RBP-J kappa gene encodes the binding protein for the immunoglobulin J kappa recombination signal sequence. J Biol Chem. 1991 Dec 5;266(34):23334–23340. [PubMed] [Google Scholar]
- Furukawa T., Maruyama S., Kawaichi M., Honjo T. The Drosophila homolog of the immunoglobulin recombination signal-binding protein regulates peripheral nervous system development. Cell. 1992 Jun 26;69(7):1191–1197. doi: 10.1016/0092-8674(92)90640-x. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Matsunami N., Yamamoto Y., Honjo T. Purification and characterization of a protein that binds to the recombination signal sequence of the immunoglobulin J kappa segment. Nucleic Acids Res. 1989 Nov 25;17(22):9015–9026. doi: 10.1093/nar/17.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi Y., Yamamoto Y., Iwanari H., Maruyama S., Furukawa T., Matsunami N., Honjo T. Biochemical and immunological characterization of the DNA binding protein (RBP-J kappa) to mouse J kappa recombination signal sequence. J Biochem. 1992 Sep;112(3):314–320. doi: 10.1093/oxfordjournals.jbchem.a123898. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Israel A., Yano O., Logeat F., Kieran M., Kourilsky P. Two purified factors bind to the same sequence in the enhancer of mouse MHC class I genes: one of them is a positive regulator induced upon differentiation of teratocarcinoma cells. Nucleic Acids Res. 1989 Jul 11;17(13):5245–5257. doi: 10.1093/nar/17.13.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël A., Kimura A., Kieran M., Yano O., Kanellopoulos J., Le Bail O., Kourilsky P. A common positive trans-acting factor binds to enhancer sequences in the promoters of mouse H-2 and beta 2-microglobulin genes. Proc Natl Acad Sci U S A. 1987 May;84(9):2653–2657. doi: 10.1073/pnas.84.9.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaichi M., Oka C., Reeves R., Kinoshita M., Honjo T. Recombination of exogenous interleukin 2 receptor gene flanked by immunoglobulin recombination signal sequences in a pre-B cell line and transgenic mice. J Biol Chem. 1991 Sep 25;266(27):18387–18394. [PubMed] [Google Scholar]
- Kawaichi M., Oka C., Shibayama S., Koromilas A. E., Matsunami N., Hamaguchi Y., Honjo T. Genomic organization of mouse J kappa recombination signal binding protein (RBP-J kappa) gene. J Biol Chem. 1992 Feb 25;267(6):4016–4022. [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Knust E., Tietze K., Campos-Ortega J. A. Closely related transcripts encoded by the neurogenic gene complex enhancer of split of Drosophila melanogaster. EMBO J. 1989 Jan;8(1):203–210. doi: 10.1002/j.1460-2075.1989.tb03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Tietze K., Campos-Ortega J. A. Molecular analysis of the neurogenic locus Enhancer of split of Drosophila melanogaster. EMBO J. 1987 Dec 20;6(13):4113–4123. doi: 10.1002/j.1460-2075.1987.tb02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Litman G. W., Berger L., Murphy K., Litman R., Hinds K., Erickson B. W. Immunoglobulin VH gene structure and diversity in Heterodontus, a phylogenetically primitive shark. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2082–2086. doi: 10.1073/pnas.82.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman G. W., Murphy K., Berger L., Litman R., Hinds K., Erickson B. W. Complete nucleotide sequences of three VH genes in Caiman, a phylogenetically ancient reptile: evolutionary diversification in coding segments and variation in the structure and organization of recombination elements. Proc Natl Acad Sci U S A. 1985 Feb;82(3):844–848. doi: 10.1073/pnas.82.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami N., Hamaguchi Y., Yamamoto Y., Kuze K., Kangawa K., Matsuo H., Kawaichi M., Honjo T. A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature. 1989 Dec 21;342(6252):934–937. doi: 10.1038/342934a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Ozato K. Negative regulation of the major histocompatibility class I gene in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9537–9541. doi: 10.1073/pnas.83.24.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. Molecular characterization of two proteins involved in the excision of the conjugative transposon Tn1545: homologies with other site-specific recombinases. EMBO J. 1989 Aug;8(8):2425–2433. doi: 10.1002/j.1460-2075.1989.tb08373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J., Grossberger D., Du Pasquier L. Organization and rearrangement of immunoglobulin M genes in the amphibian Xenopus. EMBO J. 1988 Aug;7(8):2409–2415. doi: 10.1002/j.1460-2075.1988.tb03086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisguth F., Posakony J. W. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992 Jun 26;69(7):1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Vässin H., Vielmetter J., Campos-Ortega J. A. Genetic interactions in early neurogenesis of Drosophila melanogaster. J Neurogenet. 1985 Nov;2(5):291–308. doi: 10.3109/01677068509102325. [DOI] [PubMed] [Google Scholar]
- de-la-Concha A., Dietrich U., Weigel D., Campos-Ortega J. A. Functional interactions of neurogenic genes of Drosophila melanogaster. Genetics. 1988 Mar;118(3):499–508. doi: 10.1093/genetics/118.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]