Abstract
Various effects of microgravity on prokaryotes have been recognized in recent years, with the focus on studies of pathogenic bacteria. No archaea have been investigated yet with respect to their responses to microgravity. For exposure experiments on spacecrafts or on the International Space Station, halophilic archaea (haloarchaea) are usually embedded in halite, where they accumulate in fluid inclusions. In a liquid environment, these cells will experience microgravity in space, which might influence their viability and survival. Two haloarchaeal strains, Haloferax mediterranei and Halococcus dombrowskii, were grown in simulated microgravity (SMG) with the rotary cell culture system (RCCS, Synthecon). Initially, salt precipitation and detachment of the porous aeration membranes in the RCCS were observed, but they were avoided in the remainder of the experiment by using disposable instead of reusable vessels. Several effects were detected, which were ascribed to growth in SMG: Hfx. mediterranei's resistance to the antibiotics bacitracin, erythromycin, and rifampicin increased markedly; differences in pigmentation and whole cell protein composition (proteome) of both strains were noted; cell aggregation of Hcc. dombrowskii was notably reduced. The results suggest profound effects of SMG on haloarchaeal physiology and cellular processes, some of which were easily observable and measurable. This is the first report of archaeal responses to SMG. The molecular mechanisms of the effects induced by SMG on prokaryotes are largely unknown; haloarchaea could be used as nonpathogenic model systems for their elucidation and in addition could provide information about survival during lithopanspermia (interplanetary transport of microbes inside meteorites). Key Words: Haloferax mediterranei—Halococcus dombrowskii—Simulated microgravity—Rotary cell culture system—Antibiotic resistance—Lithopanspermia. Astrobiology 11, 199–205.
1. Introduction
The effects of microgravity on plants and some eukaryotic microorganisms, such as Paramecium, Euglena, and Chlamydomonas, have been studied since the 19th century (see Häder et al., 2005, for historic examples). But the recognition of effects on prokaryotes has been delayed, possibly due to the influence of a theoretical paper by Pollard (1965), in which it was stated that gravity should be experienced by cells with a diameter larger than 10 μm, which is greater than that of most bacteria or archaea. The subject of microgravity and microorganisms attracted more attention after it was learned that intense growth of bacterial biofilms had blocked the water purification system on the Russian Mir space station (cited in Lynch et al., 2006). More recently, increased virulence of pathogenic microorganisms in response to microgravity has been reported (Rosenzweig et al., 2010), as have increased resistance to antibiotics (Nickerson et al., 2004), effects on the regulation of protein synthesis (Matin et al., 2006), an apparent increase in production of antibiotics (Benoit et al., 2006), and morphological changes (Zhou et al., 2006).
Spaceflight has provided opportunities for exposure of organisms to microgravity, but for ground experiments various devices have been developed. The rotary cell culture system (RCCS) manufactured by Synthecon (Houston, TX) was commissioned by NASA (Wolf and Schwarz, 1991) and has been described in detail (Lynch et al., 2004; Matin et al., 2006). In the RSSC, a vessel is rotated about a horizontal axis, and the cells reach a steady-state terminal velocity at which the gravitational force is mitigated by equal and opposite hydrodynamic forces, including shear, centrifugal, and Coriolis forces. This generates an overall time-averaged gravity of 10−2g on the cells in culture and is referred to as simulated microgravity or SMG (Lynch et al., 2004). During operation, the conditions should enable a suspension culture that is optimized for low shear, with careful adjustment of factors such as stir rate, viscosity of the medium, and gas exchange (Unsworth and Lelkes, 1998).
Haloarchaea are a very closely related prokaryotic group; besides their common requirement for high concentrations of NaCl, they cluster together in phylogenetic trees based on the sequences of 16S rDNAs, in contrast to many other archaea, which are set far apart (Kletzin, 2007). Haloarchaea have been selected for several spaceflight experiments. For example, Halorubrum chaoviator strain Halo-G* (Mancinelli et al., 2009) was flown on the Biopan facility, a small retrievable capsule developed by the European Space Agency for exposure of biological samples in low-Earth orbit (ESA, 2005), and survived exposure to outer space for 2 weeks (Mancinelli et al., 1998). Halococcus dombrowskii, an isolate from Permian salt sediments (Stan-Lotter et al., 2002), was included in the Adapt experiment on the International Space Station and was exposed to the space environment for 18 months (http://www.esa.int/esaHS/SEM9X9W0EZF_iss_0.html). For the exposure experiments, suspensions of haloarchaeal cells in high-salt medium or buffers are dried on quartz discs, which leads to the formation of fluid inclusions during crystallization. It was observed that cells accumulated preferentially within the fluid inclusions (Fendrihan and Stan-Lotter, 2004; Fendrihan et al., 2006, 2009); therefore, possible responses to microgravity by the cells, which are surrounded by liquids, should be considered. This report contains results from the examination of the effects of SMG on Hcc. dombrowskii; in addition, the haloarchaeal strain Haloferax mediterranei was investigated, since its susceptibility to antibiotics has been studied in detail (Bonelo et al., 1984). Some methodological adaptations of the use of RCCS with high-salt media are also described.
2. Materials and Methods
2.1. Growth of microorganisms
Cells of Halococcus dombrowskii DSM 14522T and Haloferax mediterranei DSM 1411T were grown, with shaking, in M2 medium (Tomlinson and Hochstein, 1976) in liquid culture as described previously (Stan-Lotter et al., 2002). Growth was followed by measuring optical density (OD) at 600 nm with a Novaspec II (Pharmacia).
2.2. Growth of haloarchaea in simulated microgravity
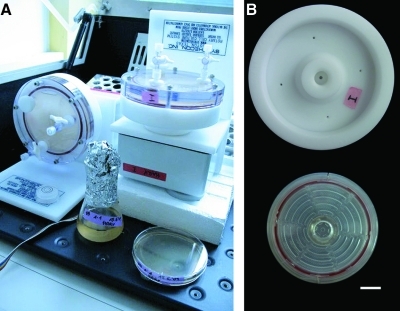
Two separate RCCS with high aspect ratio vessels (HARVs), each with a capacity of 50 mL (type HARV-50), from Synthecon, Inc. (Houston, TX) were used, which are sold in Europe by Cellon (Luxembourg). One HARV vessel rotates about a horizontal axis, providing SMG conditions, and a second HARV vessel rotates about a vertical axis, providing normal gravity (NG) control. Aeration is achieved through a semipermeable membrane at the back of the vessel. Cultures were usually grown in pairs, one in SMG and one in NG conditions. Both RCCS were placed in an Innova desktop incubator (Fig. 1, panel A), type 4080 (New Brunswick), and were connected by flat cables to power supplies, which were located outside the incubator. Temperature of incubation was 37°C, and rotary speed of the vessels was 20 rpm. In later experiments, as described in Results, the reusable HARV-50 vessels were replaced by disposable vessels type D-405 (Fig. 1, panel B).
FIG. 1.
Two RCCS in an incubator (A) for the generation of SMG, which uses a vertical vessel, and NG, which uses a horizontal vessel. (B) Back views of a standard reusable vessel, type HARV-50 (B, top) and a disposable vessel, type D-405 (B, bottom). Bar, 2 cm.
2.3. Assays for antibiotic resistance
Haloferax mediterranei DSM 1411T was grown in liquid medium at either NG or SMG to an OD600nm of about 1.0 and diluted with M2 medium to an OD600nm of 0.1. Five-milliliter aliquots of cell suspensions were then placed in glass tubes with antibiotic-containing solutions and incubated, with shaking, at 37°C, while growth was monitored by measuring the OD600 for several days. The following antibiotics (all from Sigma) and final concentrations during growth, which were 1/2, 1-fold, 2-fold, and 5-fold, respectively, of the minimal inhibitory concentrations as determined by Bonelo et al. (1984), were used: bacitracin, 0, 23.5, 47, 94, 235 μg/mL; erythromycin, 0, 187.5, 375, 750, 1875 μg/mL; novobiocin, 0, 1, 2, 4, 10 μg/mL; rifampicin, 0, 2, 4, 8, 20 μg/mL.
2.4. Analysis of whole cell protein patterns
Sodium dodecyl sulfate (SDS) gel electrophoresis of whole cell proteins was performed as described previously (Stan-Lotter et al., 1993, 2002). Briefly, haloarchaea were grown in NG or SMG to ODs of 0.6–0.8; approximately 50 mg of cells (wet weight) per milliliter were lysed by boiling them in SDS sample buffer (Laemmli, 1970) for 10 min, and then centrifuged at 10,000g for 1 min to remove any precipitates. For separation of proteins by one-dimensional gels, the system of Laemmli (1970) was used. Visualization of proteins was performed by staining with Coomassie Blue. In some experiments, the two-dimensional gel electrophoresis system by O'Farrell (1975) was used with modifications for Halococcus sp. as described by Legat (2009); proteins were stained with Sypro Ruby (Invitrogen, Austria), and patterns were visualized under UV light with a VersaDoc Imaging System Model 3000 (Bio-Rad). One-dimensional gels were repeated at least five times, two-dimensional gels at least three times.
2.5. Other methods
For assessment of pigmentation, 2 × 25 mL of cells, which had been grown for 96 h in SMG or NG, respectively, were pelleted by centrifugation in an SS34 rotor (Sorvall) at 6000 rpm for 15 min at room temperature. Staining with the BacLight LIVE/DEAD kit (Invitrogen, Austria) and fluorescence microscopy were carried out as described previously (Leuko et al., 2004; Fendrihan et al., 2009). For embedding experiments, cell suspensions were dried on glass slides for 2–3 days (Fendrihan et al., 2009). Unstained cells were observed with a Nikon Eclipse E200 microscope by using phase contrast. For statistics, the program Origin, version 6.0 (Originlab, Guangzhou, P.R. China), was used.
3. Results
3.1. Haloarchaea in fluid inclusions
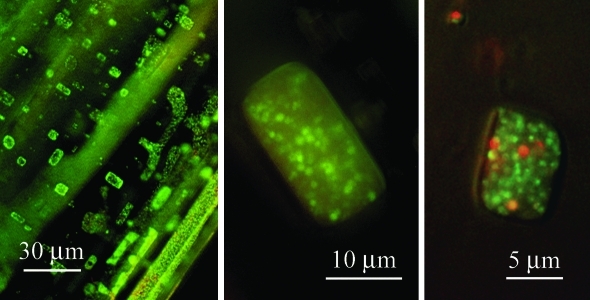
Haloarchaeal cells were pre-stained with the LIVE/DEAD kit before embedding in salt crystals (Fig. 2). At low magnification, the bright green fluorescence of stained haloarchaea outlined the morphology of the characteristic rectangular fluid inclusions of halite (Fig. 2, left panel). At higher magnifications, individual cells of Hfx. mediterranei DSM 1411T (Fig. 2, middle panel) and Hcc. dombrowskii DSM 14522T (Fig. 2, right panel), respectively, became visible. The data suggest that the localization of haloarchaeal cells in the salt was nearly exclusively in the fluid inclusions of artificial halite, which formed during desiccation. Such inclusions are present in natural halite as well (Roedder, 1984).
FIG. 2.
Accumulation of pre-stained haloarchaea in fluid inclusions. Cells were stained with the LIVE/DEAD BacLight kit prior to embedding in artificial halite. Viable cells show green fluorescence; nonviable cells are red. Low magnification (left panel): haloarchaeal cells. High magnification: cells of Haloferax mediterranei DSM 1411T (middle panel) and Halococcus dombrowskii DSM 14522T (right panel), respectively, trapped in fluid inclusions for about 3 days. Pictures were taken with a Leica fluorescence microscope type DM5000B.
3.2. Growth of haloarchaea in RCCS
Haloarchaea and Hcc. dombrowskii DSM 14522T in particular have much longer generation times (days instead of hours) than most microorganisms that have been grown so far in the RCCS vessels. Hfx. mediterranei DSM 1411T is one of the faster growing haloarchaea and grows also at higher temperatures (up to 45°C) and with a generation time of ca. 5–6 h. Hfx. mediterranei DSM 1411T was therefore used as the preferred model haloarchaeal organism for growth in the RCCS. Hcc. dombrowskii DSM 14522T was used in some experiments, since it had been exposed on the European Technology Exposure Facility on the International Space Station for 18 months. No halophilic microorganisms had yet been grown in the HARV vessel; therefore, several parameters needed to be assessed. The high salt concentration (≥20%, or 3.5–4 M NaCl), which is essential for growth of most haloarchaea, led to formation of crystals; together with the buildup of negative pressure, mechanical stress was exerted on the porous aeration membrane located on the back of the HARV vessel. After several runs of 1–2 weeks of duration, the membrane separated from its support and had to be re-attached with glue (General Electrics vulcanizing adhesive, obtained from Cellon, Luxembourg). When the membrane became visibly damaged and displayed uneven regions and even holes, new membrane sheets were cut to size and glued to the vessel. Evaporation during the runs also caused the formation of air bubbles within the medium in the vessel. The air was pushed out daily by careful addition of several milliliters of a 1:1 mixture of sterile water and medium with a syringe through the ports. Finally, disposable vessels (type D-405) were used for growth of haloarchaea in the RCCS (Fig. 1). They have the same designated capacity (50 mL) as type HARV-50 and are sterile; in practice, their capacity is about 55 mL. Figure 1 (panel B) shows both types of vessels for comparison; the reusable vessel HARV-50 has only a few holes on the back for aeration, but the disposable vessel type D-405 has wide slits, which, in our experience, is a much better design for the purpose of growing aerobic cells, especially in high-salt media. The disadvantage of not being able to reuse the vessels was greatly set off by the ease of handling and the elimination of the problems associated with evaporation, loss of volume, buildup of salt crystals, and detachment of membranes in high-salt buffers.
3.3. Antibiotic susceptibility
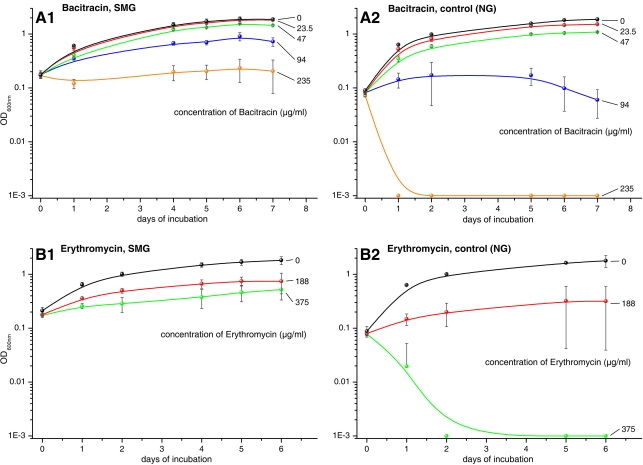
Cells of Hfx. mediterranei DSM 1411T were used to test the influence of microgravity on the response to antibiotics. Figure 3 shows the effect of prior growth in SMG on the resistance of Hfx. mediterranei DSM 1411T to bacitracin (Fig. 3, A1) and erythromycin (Fig. 3, B1), respectively. While concentrations of 235 μg/mL of bacitracin or 375 μg/mL of erythromycin inhibited cells grown in NG completely within about 48 or 72 h, respectively (Fig. 3, A2, B2), the same concentrations had much less effect on cultures that were grown at SMG (Fig. 3, A1, B1) and allowed growth to ODs of 0.2–0.3 within the period of 6 days of the experiment. A similar increase of resistance following growth in SMG was obtained with rifampicin (not shown), while the resistance to novobiocin was not influenced by growth in SMG (not shown).
FIG. 3.
Influence on the growth of Hfx. mediterranei DSM 1411T by the antibiotics bacitracin (A1, A2) and erythromycin (B1, B2). Cells grown in NG (A2, B2) were susceptible to a concentration of 235 μg/mL bacitracin or 375 μg/mL erythromycin, respectively, which killed cells in about 48–72 h (yellow curve in A2, green curve in B2, respectively). They were not susceptible to these concentrations when they had been grown in SMG (A1, B1). Error bars denote standard deviations based on six experiments each.
3.4. Proteome
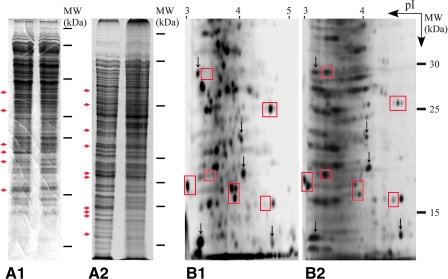
Cultures of Hfx. mediterranei DSM 1411T and Hcc. dombrowskii DSM 14522T were grown in both NG and SMG mode and harvested by centrifugation. Their whole cell proteins were separated on SDS polyacrylamide gels. One-dimensional protein patterns of cells grown in SMG displayed several differences, indicated by red arrows, with respect to intensity and location of bands to the patterns of cells grown in NG (Fig. 4, A1, A2). Two-dimensional gel electrophoresis of proteins, which involves separation by their isoelectric points (horizontal dimension) and molecular weights (vertical dimension), yielded characteristic arrangements of spots, which differed between cells grown in NG or SMG, as indicated by red boxes (Fig. 4, B1, B2). For orientation, several landmark spots, which are identical in both gels, are indicated (Fig. 4, B1, B2; black arrows). Spots represent mostly single proteins, some of which were missing in cells grown in NG but present in cells grown in SMG, or they were reduced or enhanced in intensity (Fig. 4, B1, B2). Proteins from such gels are identifiable with mass spectrometry (Rabilloud, 2002). The data suggests an influence of microgravity on the composition of the proteome of haloarchaeal strains, which was likely caused by up- or downregulation of certain protein-coding genes, as has been described for Salmonella bacteria grown in NG and SMG, respectively (Nickerson et al., 2000).
FIG. 4.
Whole cell proteins from haloarchaeal cells grown in NG or SMG, following separation by SDS polyacrylamide gel electrophoresis. (A1, A2) One-dimensional gels. Molecular mass markers are indicated to the right from top to bottom (in kDa) as follows: 200, 100, 50, 30, 25, 15, 10. Red arrows point to regions with significant protein differences between growth in NG (A1, A2, left lanes) or SMG (A1, A2, right lanes), respectively. (A1) Hfx. mediterranei DSM 1411T. (A2) Hcc. dombrowskii DSM 14522T. (B1, B2) Partial proteomes of Hcc. dombrowskii DSM 14522T grown in NG (B1) or SMG (B2), separated by two-dimensional gel electrophoresis. Isoelectric points (pI from 3 to 5) and molecular mass standards (30, 25, 15 kDa) are indicated. Several spots with identical locations in both gels are depicted by black arrows; significant differences between the two proteomes with respect to number and intensity of spots are highlighted with red boxes.
3.5. Pigmentation, cellular aggregation
The pigmentation of cultures of both strains grown in SMG was brownish red, whereas the pigmentation of cultures grown in NG was light red (not shown). Since haloarchaeal pigmentation is due to the presence of numerous carotenoids, especially C50 bacterioruberin and its derivatives (Oren, 2002), changes in pigmentation suggest differences in synthesis or incorporation of carotenoid molecules, or both.
Formation of aggregates of cells within areas of the RCCS vessel was quite pronounced with the NG cultures but did not occur with the SMG cultures. Microscopical data reveal growth of Hcc. dombrowskii DSM 14522T as very small aggregates (2–4 cells) in the SMG culture and larger aggregates (>4–8 cells) in the NG culture (not shown). Presumably, the surfaces of cells were altered, which increased the tendency toward clumping when grown in NG conditions; however, it is not known which molecules might be responsible for this effect. For Salmonella cells, which were grown aboard a space shuttle, cellular aggregation and clumping was demonstrated and attributed to the formation of an extracellular matrix (Wilson et al., 2007).
4. Discussion
Haloarchaea are generally insensitive to many antibiotics that are effective for bacteria, or they are inhibited only by high concentrations. Hfx. mediterranei DSM 1411T was inhibited by bacitracin, rifampicin, and novobiocin in comparatively low concentrations (2–47 μg/mL; Bonelo et al., 1984); erythromycin was effective in concentrations of at least 375 μg/mL. The haloarchaeal target sites for antibiotics are often not precisely known; for bacitracin, an interference with biosynthesis pathways of isoprenoid diether lipids has been suggested (Böck and Kandler, 1985). The marked increase in resistance to bacitracin, erythromycin, and rifampicin following growth in SMG was observable for at least 6 days after return of the cultures to NG (Fig. 3). This persistent response can be interpreted as a similar reaction of haloarchaea to microgravity as was described for the pathogenic bacterium Salmonella typhimurium, where one of the effects of microgravity—increased virulence toward mice infected with the Salmonella cells—was observable for more than 30 days post-exposure on a spaceflight (Wilson et al., 2007). The increased resistance to antibiotics occurred concomitantly with alterations in the proteome of Hfx. mediterranei DSM 1411T (Fig. 4, A1). Although no mechanisms for antibiotic resistance of haloarchaea are yet known, it is conceivable that some could be protein based and coded by many genes, as published for bacteria, for example, altered binding proteins, antibiotic-degrading enzymes, or efflux pumps (see Levy and Marshall, 2004, for a review). Effects of SMG on the proteome could also be demonstrated with Hcc. dombrowskii DSM 14522T (Fig. 4, A1, B1, B2), where differences in one- and two-dimensional protein patterns were apparent. Halococci possess acidic proteins with a rather narrow range of pIs between 4.2 and 5.2 (Stan-Lotter et al., 2002), which tend to cluster on two-dimensional gels. However, separation of spots on our two-dimensional gels was adequate (Fig. 4, B1, B2) and would be suitable for their excision and further analysis (Hunter et al., 2002).
Other haloarchaeal responses to SMG included aggregation of cells and changes in pigmentation, which suggests alterations in the cellular surfaces for the former and synthesis of carotenoids or their incorporation, respectively, for the latter phenomenon. Interestingly, an effect on the amount of the carotenoid staphyloxanthin has been reported with Staphylococcus aureus grown in SMG (Rosado et al., 2010). All the reactions observed here suggest a global response to SMG that involves perhaps a general regulator of transcription, as has been described for Salmonella typhimurium (Wilson et al., 2007) and Pseudomonas aeruginosa (Crabbé et al., 2010). However, archaeal transcription resembles more that of eukarya than bacteria (Bell and Jackson, 1998), and it remains to be explored which mechanisms are involved in the haloarchaeal response to microgravity. Haloarchaea are nonpathogenic; nevertheless, they could be useful models with which to study the response of possibly even eukaryotic microorganisms toward reduced gravity. In particular, long-term studies could be envisaged, since many haloarchaea remain viable in liquids for years (Arahal et al., 2000).
Several haloarchaea have been isolated from salt sediments that are believed to be millions of years old (Denner et al., 1994; Grant et al., 1998; Stan-Lotter et al., 1999, 2002; McGenity et al., 2000; Gruber et al., 2004); this along with evidence for the occurrence of halite on Mars (Treiman et al., 2000; Squyres et al., 2006) has led to speculation on the existence of halophilic life elsewhere in the Solar System that originated on Earth, or vice versa, but was transported via impact events. The transfer of microbes between planets could occur by way of meteoritic rocks within which viable microbes are embedded. Indeed, recent experimental evidence supports the idea that spores, bacteria, and lichens could survive space travel (Horneck et al., 2008; Nicholson, 2009). Ballistic studies have suggested that spores and coccoid bacteria, both of which possess sturdy cell walls, are the most likely “micronauts” with the capacity to survive transfer from one planet to another. Archaea, however, are not spore formers, and they do not contain the bacterial cell wall polymer peptidoglycan. Conceivably, archaea could endure space travel if contained within fluid inclusions of halite, which are apparently an old feature of the Universe. In this way, they could retain their brine state while traveling through space for billions of years (Zolensky et al., 1999). It thus remains to be determined which types of responses are elicited in archaea in general and haloarchaea in particular when exposed to reduced gravity over time.
Acknowledgments
This work was supported by the Austrian Science Foundation (FWF), project P18256-B06, and by the Austrian Research Promotion Agency (FFG), ASAP project 819674. We thank Anita Holzinger for expert technical assistance.
Disclosure Statement
No competing financial interests exist.
Abbreviations
HARV, high aspect ratio vessel; NG, normal gravity; OD, optical density; RCCS, rotary cell culture system; SDS, sodium dodecyl sulfate; SMG, simulated microgravity.
References
- Arahal D.R. Gutiérrez M.C. Volcani B.E. Ventosa A. Taxonomic analysis of extremely halophilic archaea isolated from 56-years-old dead sea brine samples. Syst Appl Microbiol. 2000;23:376–385. doi: 10.1016/S0723-2020(00)80068-5. [DOI] [PubMed] [Google Scholar]
- Bell S.D. Jackson S.P. Transcription and translation in Archaea: a mosaic of eukaryal and bacterial features. Trends Microbiol. 1998;6:222–228. doi: 10.1016/s0966-842x(98)01281-5. [DOI] [PubMed] [Google Scholar]
- Benoit M.R. Li W. Stodieck L.S. Lam K.S. Winther C.L. Roane T.M. Klaus D.M. Microbial antibiotic production aboard the International Space Station. Appl Microbiol Biotechnol. 2006;70:403–411. doi: 10.1007/s00253-005-0098-3. [DOI] [PubMed] [Google Scholar]
- Böck A. Kandler O. Woese C.R. Wolfe R.S. The Bacteria. A Treatise on Structure and Function. Vol. VIII—Archaebacteria. Academic Press; Orlando, FL: 1985. Antibiotic sensitivity of archaebacteria; pp. 525–544. [Google Scholar]
- Bonelo G. Ventosa A. Megias M. Ruiz-Berraquero F. The sensitivity of halobacteria to antibiotics. FEMS Microbiol Lett. 1984;21:341–345. [Google Scholar]
- Crabbé A. Pycke B. Van Houdt R. Monsieurs P. Nickerson C. Leys N. Cornelis P. Response of Pseudomonas aeruginosa PAO1 to low shear modelled microgravity involves AlgU regulation. Environ Microbiol. 2010;12:1545–1564. doi: 10.1111/j.1462-2920.2010.02184.x. [DOI] [PubMed] [Google Scholar]
- Denner E.B.M. McGenity T.J. Busse H.-J. Wanner G. Grant W.D. Stan-Lotter H. Halococcus salifodinae sp. nov., an archaeal isolate from an Austrian salt mine. Int J Syst Bacteriol. 1994;44:774–780. [Google Scholar]
- ESA. FOTON retrievable capsules. European Users Guide to Low Gravity Platforms, European Space Agency, Noordwijk, the Netherlands, chapter 6. 2005. http://www.spaceflight.esa.int/users/downloads/userguides/chapter_6_foton.pdf http://www.spaceflight.esa.int/users/downloads/userguides/chapter_6_foton.pdf
- Fendrihan S. Stan-Lotter H. Teodorescu H. Griebel H. Mars and Planetary Science and Technology. Performantica Press; Iasi, Romania: 2004. Survival of halobacteria in fluid inclusions as a model of possible biotic survival in martian halite; pp. 9–18. [Google Scholar]
- Fendrihan S. Legat A. Pfaffenhuemer M. Gruber C. Weidler G. Gerbl F. Stan-Lotter H. Extremely halophilic archaea and the issue of long-term microbial survival. Rev Environ Sci Biotechnol. 2006;5:203–218. doi: 10.1007/s11157-006-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrihan S. Berces A. Lammer H. Musso M. Ronto G. Polacsek T.K. Holzinger A. Kolb C. Stan-Lotter H. Investigating the effects of simulated martian ultraviolet radiation on Halococcus dombrowskii and other extremely halophilic archaebacteria. Astrobiology. 2009;9:104–112. doi: 10.1089/ast.2007.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W.D. Gemmell R.T. McGenity T.J. Halobacteria: the evidence for longevity. Extremophiles. 1998;2:279–287. doi: 10.1007/s007920050070. [DOI] [PubMed] [Google Scholar]
- Gruber C. Legat A. Pfaffenhuemer M. Radax C. Weidler G. Busse H.-J. Stan-Lotter H. Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum. Extremophiles. 2004;8:431–439. doi: 10.1007/s00792-004-0403-6. [DOI] [PubMed] [Google Scholar]
- Häder D.-P. Hemmersbach R. Lebert M. Gravity and the Behaviour of Unicellular Organisms. Cambridge University Press; New York: 2005. [Google Scholar]
- Horneck G. Stöffler D. Ott S. Hornemann U. Cockell C.S. Moeller R. Meyer C. de Vera J.P. Fritz J. Schade S. Artemieva N.A. Microbial rock inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology. 2008;8:17–44. doi: 10.1089/ast.2007.0134. [DOI] [PubMed] [Google Scholar]
- Hunter T.C. Andon N.L. Koller A. Yates J.R., III Haynes P.A. The functional proteomics toolbox: methods and applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;782:165–181. doi: 10.1016/s1570-0232(02)00570-6. [DOI] [PubMed] [Google Scholar]
- Kletzin A. Cavicchioli R. Archaea. Molecular and Cellular Biology. ASM Press; Washington DC: 2007. General characteristics and important model organisms; pp. 14–92. [Google Scholar]
- Laemmli U.K. Cleave of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legat A. Taxonomy, fluorescence microscopy and proteomics. University of Salzburg; Salzburg, Austria: 2009. Halobacteria from Permo-Triassic salt deposits. Ph.D. thesis, [Google Scholar]
- Leuko S. Legat A. Fendrihan S. Stan-Lotter H. Evaluation of the LIVE/DEAD BacLight kit for detection of extremophilic archaea and visualization of microorganisms in environmental hypersaline samples. Appl Environ Microbiol. 2004;70:6884–6886. doi: 10.1128/AEM.70.11.6884-6886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S.B. Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- Lynch S.V. Brodie E.L. Matin A. Role and regulation of sigma S in general resistance conferred by low-shear simulated microgravity in Escherichia coli. J Bacteriol. 2004;186:8207–8212. doi: 10.1128/JB.186.24.8207-8212.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S.V. Mukundakrishnan K. Benoit M.R. Ayyaswamy P.S. Matin A. Escherichia coli biofilms formed under low-shear modeled microgravity in a ground-based system. Appl Environ Microbiol. 2006;72:7701–7710. doi: 10.1128/AEM.01294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli R.L. White M.R. Rothschild L.J. Biopan survival I: exposure of the osmophiles Synechococcus sp. (Nägeli) and Haloarcula sp. to the space environment. Adv Space Res. 1998;22:327–334. [Google Scholar]
- Mancinelli R. Landheim R. Sánchez-Porro C. Dornmayr-Pfaffenhuemer M. Gruber C. Legat A. Ventosa A. Radax C. Ihara K. White M.R. Stan-Lotter H. Halorubrum chaoviator, sp. nov., a haloarchaeon isolated from sea salt in Baja California, Mexico, Western Australia and Naxos, Greece. Int J Syst Evol Microbiol. 2009;59:1908–1913. doi: 10.1099/ijs.0.000463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. Lynch S.V. Benoit M.R. Increased bacterial resistance and virulence in simulated microgravity and its molecular basis. Gravitational and Space Biology. 2006;19:31–42. [Google Scholar]
- McGenity T.J. Gemmell R.T. Grant W.D. Stan-Lotter H. Origins of halophilic microorganisms in ancient salt deposits (MiniReview) Environ Microbiol. 2000;2:243–250. doi: 10.1046/j.1462-2920.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- Nicholson W.L. Ancient micronauts: interplanetary transport of microbes by cosmic impacts. Trends Microbiol. 2009;17:243–250. doi: 10.1016/j.tim.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Nickerson C.A. Ott C.M. Mister S.J. Morrow B.J. Burns-Keliher L. Pierson D.L. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect Immun. 2000;68:3147–3152. doi: 10.1128/iai.68.6.3147-3152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson C.A. Ott C.M. Wilson J.W. Ramamurthy R. Pierson D.L. Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev. 2004;68:345–361. doi: 10.1128/MMBR.68.2.345-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oren A. Halophilic Microorganisms and Their Environments. Kluwer Academic Publishers; Dordrecht, the Netherlands: 2002. pp. 173–206. [Google Scholar]
- Pollard E.C. Theoretical studies on living systems in the absence of mechanical stress. J Theor Biol. 1965;8:113–123. doi: 10.1016/0022-5193(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Rabilloud T. Two-dimensional gel electrophoresis in proteomics: old, old fashioned, but still climbs up the mountains. Proteomics. 2002;2:3–10. [PubMed] [Google Scholar]
- Roedder E. The fluids in salt. American Mineralogist. 1984;69:413–439. [Google Scholar]
- Rosado H. Doyle M. Hinds J. Taylor P.W. Low-shear modelled microgravity alters expression of virulence determinants of Staphylococcus aureus. Acta Astronaut. 2010;66:408–413. [Google Scholar]
- Rosenzweig J.A. Abogunde O. Thomas K. Lawal A. Nguyen Y.U. Sodipe A. Jejelowo O. Spaceflight and modeled microgravity effects on microbial growth and virulence. Appl Microbiol Biotechnol. 2010;85:885–891. doi: 10.1007/s00253-009-2237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squyres S.W. Knoll A.H. Arvidson R.E. Clark B.C. Grotzinger J.P. Jolliff B.L. McLennan S.M. Tosca N. Bell J.F., 3rd. Calvin W.M. Farrand W.H. Glotch T.D. Golombek M.P. Herkenhoff K.E. Johnson J.R. Klingelhöfer G. McSween H.Y. Yen A.S. Two years at Meridiani Planum: results from the Opportunity Rover. Science. 2006;313:1403–1407. doi: 10.1126/science.1130890. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H. Sulzner M. Egelseer E. Norton C.F. Hochstein L.I. Comparison of membrane ATPases from extreme halophiles isolated from ancient salt deposits. Orig Life Evol Biosph. 1993;23:53–64. doi: 10.1007/BF01581990. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H. McGenity T.J. Legat A. Denner E.B.M. Glaser K. Stetter K.O. Wanner G. Very similar strains of Halococcus salifodinae are found in geographically separated Permo-Triassic salt deposits. Microbiology. 1999;145:3565–3574. doi: 10.1099/00221287-145-12-3565. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H. Pfaffenhuemer M. Legat A. Busse H.-J. Radax C. Gruber C. Halococcus dombrowskii sp. nov., an archaeal isolate from a Permo-Triassic alpine salt deposit. Int J Syst Evol Microbiol. 2002;52:1807–1814. doi: 10.1099/00207713-52-5-1807. [DOI] [PubMed] [Google Scholar]
- Tomlinson G.A. Hochstein L.I. Halobacterium saccharovorum sp. nov., a carbohydrate-metabolizing, extremely halophilic bacterium. Can J Microbiol. 1976;22:587–591. doi: 10.1139/m76-087. [DOI] [PubMed] [Google Scholar]
- Treiman A.H. Gleason J.D. Bogard D.D. The SNC meteorites are from Mars. Planet Space Sci. 2000;48:1213–1230. [Google Scholar]
- Unsworth B.R. Lelkes P.I. Growing tissues in microgravity. Nat Med. 1998;4:901–907. doi: 10.1038/nm0898-901. [DOI] [PubMed] [Google Scholar]
- Wilson J.W. Ott C.M. Höner zu Bentrup K. Ramamurthy R. Quick L. Porwollik S. Cheng P. McClelland M. Tsaprailis G. Radabaugh T. Hunt A. Fernandez D. Richter E. Shah M. Kilcoyne M. Joshi L. Nelman-Gonzalez M. Hing S. Parra M. Dumars P. Norwood K. Bober R. Devich J. Ruggles A. Goulart C. Rupert M. Stodieck L. Stafford P. Catella L. Schurr M.J. Buchanan K. Morici L. McCracken J. Allen P. Baker-Coleman C. Hammond T. Vogel J. Nelson R. Pierson D.L. Stefanyshyn-Piper H.M. Nickerson C.A. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc Natl Acad Sci USA. 2007;104:16299–16304. doi: 10.1073/pnas.0707155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D.A. Schwarz R.P. Analysis of gravity-induced particle motion and fluid perfusion flow in the NASA-designed rotating zero-head-space tissue culture vessel. NASA Technical Paper 3143. National Aeronautics and Space Administration; Washington, DC: 1991. Oct, 1991. [Google Scholar]
- Zhou J. Sun C. Wang N. Gao R. Bai S. Zheng H. You X. Li R. Preliminary report on the biological effects of space flight on the producing strain of a new immunosuppressant, Kanglemycin C. J Ind Microbiol Biotechnol. 2006;33:707–712. doi: 10.1007/s10295-006-0118-z. [DOI] [PubMed] [Google Scholar]
- Zolensky M.E. Bodnar R.J. Gibson E.K. Nyquist L.E. Reese Y. Shih C.Y. Wiesman H. Asteroidal water within fluid inclusion-bearing halite in an H5 chondrite, Monahans. Science. 1999;1998;285:1377–1379. doi: 10.1126/science.285.5432.1377. [DOI] [PubMed] [Google Scholar]