Abstract
The aim of this study was to evaluate the long-term effect of localized growth factor delivery on sciatic nerve regeneration in a critical-size (>1 cm) peripheral nerve defect. Previous work has demonstrated that bioactive proteins can be encapsulated within double-walled, poly(lactic-co-glycolic acid)/poly(lactide) microspheres and embedded within walls of biodegradable polymer nerve guides composed of poly(caprolactone). Within this study, nerve guides containing glial cell line-derived neurotrophic factor (GDNF) were used to bridge a 1.5-cm defect in the male Lewis rat for a 16-week period. Nerve repair was evaluated through functional assessment of joint angle range of motion using video gait kinematics, gastrocnemius twitch force, and gastrocnemius wet weight. Histological evaluation of nerve repair included assessment of Schwann cell and neurofilament location with immunohistochemistry, evaluation of tissue integration and organization throughout the lumen of the regenerated nerve with Masson's trichrome stain, and quantification of axon fiber density and g-ratio. Results from this study showed that the measured gastrocnemius twitch force in animals treated with GDNF was significantly higher than negative controls and was not significantly different from the isograft-positive control group. Histological assessment of explanted conduits after 16 weeks showed improved tissue integration within GDNF releasing nerve guides compared to negative controls. Nerve fibers were present across the entire length of GDNF releasing guides, whereas nerve fibers were not detectable beyond the middle region of negative control guides. Therefore, our results support the use of GDNF for improved functional recovery above negative controls following large axonal defects in the peripheral nervous system.
Introduction
Biodegradable nerve conduits have been used clinically to repair small (<3 cm) nerve defects in the human peripheral nervous system.1 However, nerve autografts are routinely used in the surgical arena when repairing nerve injuries despite known drawbacks instead of synthetic conduits because evaluation of nerve regeneration consistently shows that autografts produce superior repair results.2 Nerve autografts serve as an ideal bridge for nerve gaps because the extracellular matrix scaffold composition and cellular populations closely match that of the nerve being replaced; however, there are disadvantages of using autografts. These include the additional surgery of harvesting the nerve graft, and the decreased function obtained due to the use of a sensory nerve to replace a motor nerve. For nerve repair, nerve guides rely on self-regulatory biological mechanisms by containing growth factors secreted by the surviving nerve stumps and existing Schwann cells to the injured area. However, by exploiting the natural regenerative capacity of the peripheral nerves by developing conduits that promote and direct axonal outgrowth, it may be possible to overcome the current barriers for synthetic nerve grafts and provide surgeons with a more appealing tool for repairing nerve defects. One method for improving the design of nerve guides has been to deliver protein growth factors from the nerve guides to the area of nerve injury, thereby targeting axonal survival or outgrowth from the proximal to distal nerve stump.3
In this study, we hypothesized that a sustained delivery of glial cell line-derived neurotrophic factor (GDNF) applied directly to the site of injury will result in an improved regeneration of lost nerve tissue. This hypothesis is based on the following observations. First, nerve injury results in rapid upregulation of GDNF by Schwann cells in distal segments of lesioned sciatic nerve.4 Second, GDNF has been shown to prevent avulsion-induced motorneuron death after complete nerve transection, whereas nerve growth factor, brain-derived neurotrophic factor, and insulin-like growth factor all failed to enhance cell survival or cell size.5 Third, GDNF treatment with guidance channels after spinal cord injury resulted in a reduction of reactive astrocytosis and macrophage accumulation.6 Finally, histological comparison of nerve guides implanted with and without added GDNF in preclinical animal studies has shown that nerve guides releasing GDNF increase nerve fiber number, fiber diameter, and improved myelin thickness, indications of improved nerve regeneration.7–10
To evaluate this hypothesis, poly(caprolactone) (PCL) nerve guides were fabricated as previously described with double-walled microspheres encapsulating GDNF.11 To assess the long-term efficacy of the nerve guide design, a 16-week functional and histological assessment of nerve regeneration across a 1.5-cm-long gap defect in the adult male Lewis rat was evaluated after GDNF therapy. Nerve repair was evaluated through functional assessment of joint angle range of motion (RoM) during locomotion, gastrocnemius twitch force, and gastrocnemius wet weight. Histological evaluation of nerve repair included assessment of Schwann cell and neurofilament location with immunohistochemistry (IHC), evaluation of tissue integration and organization throughout the lumen of the regenerated nerve with Masson's trichrome (MTC) stain, and quantification of axon fiber density and g-ratio.
This study demonstrates that localized GDNF delivery to an injured nerve would improve nerve regeneration in terms of both functional recovery and nerve histomorphometry. Recordings of gastrocnemius twitch force of anesthetized rats showed an increase in twitch force of animals treated with GDNF above the negative control guides. In addition, tissue architecture from within PCL guides further confirmed that nerve fiber density was greater in the middle and distal regions of GDNF releasing guides. Finally, observation of Schwann cells and neurofilament proteins with immunofluorescence revealed a higher degree of nerve tissue organization within nerve guides releasing GDNF, with tissue located in the center of the explanted conduit as opposed to negative control guides, which produced nerve tissue only along the surface of the conduit. The distribution of nerve fibers within the guides may reflect the overall improvement in tissue in growth with GDNF treatment.
Materials and Methods
Reagents
All chemicals were analytical grade or purer and were purchased from commercial suppliers. Poly(vinyl alcohol) (PVA; average Mw 9000–10,000, 80% hydrolyzed), poly(DL-lactide-co-glycolide) (lactide:glycolide (50:50), Mw 40,000–75,000 units), PCL (Mw 65,000), dichloromethane, ethyl acetate, xylene, monoclonal anti-S100 and anti-Neurofilament, and phosphate-buffered saline (PBS) were all purchased from Sigma-Aldrich. Poly-L-lactide (0.90–1.20 dL/g) was purchased from Durect Corporation (Pelham, AL). The Masson's trichrome kit was purchased from American MasterTech. The GDNF Emax ImmunoAssay Systems Kit was purchased from Promega. Recombinant human GDNF produced in Escherichia coli was purchased from Leinco Technologies.
Fabrication of nerve guides with double-walled microspheres
Double-walled microspheres were prepared as previously described.11,12 Briefly, a 17.5% poly(lactic-co-glycolic acid) (PLGA) solution was created with 150 mg PLGA in dichloromethane. In a separate glass scintillation vial, a 10% solution of poly(lactide) of equal polymer mass was prepared. A solution of 40 μL (0.1 mg/mL) of GDNF and human serum albumin in a 1:10 molar ratio was prepared in 0.5 mL sterile water over ice (formulation adapted from ref.13). After mixing well, the solution was frozen and lyophilized. The protein/surfactant mixture was then added to PLGA already dissolved in dichloromethane and vortexed for ∼30 s to achieve a homogenous mixture. The PLGA solution was combined with the poly(lactic acid) (PLA) solution and vortexed for an additional 60 s. This oil-in-oil emulsion was added drop-wise through a Pasteur pipette to 200 mL of aqueous 0.5% PVA solution stirring at 900 rpm for 3 h. Then, the polymer microspheres were collected through centrifugation (1500 g for 10 min) and washed three times. Finally, the microspheres were lyophilized using a Labconco freeze dry system (without a cryoprotectant) and stored in a desiccant at −20°C.
Twenty PCL nerve guides were fabricated using a modification of previously reported methods.14 Glass capillary mandrels were coated with a 17% w/v% aqueous solution of PVA, air-dried, and then immersed into the polymer slurry creating NaCl/PCL mandrel coatings. Microspheres (15 mg) were evenly spread onto a drawn grid on parchment paper (illustrated in ref.11). After the first immersion of the glass mandrel into the PCL slurry, the ethyl acetate was allowed to evaporate for 30 s leaving a semi-hardened polymer layer on the mandrel. This was then smoothly rolled across the microspheres on parchment paper. The PCL with embedded microspheres was allowed to dry for 10 min and then repeatedly coated with additional layers of polymer as done in nerve guides without microspheres.
In vitro release of GDNF from nerve guides with double-walled microspheres
After fabrication, the guide was allowed to dry completely and NaCl leaching was performed by immersing the guides in sterile deionized (DI) water for 5 h. Nerve guides were removed from glass mandrels used during initial preparation and cut to 1.7-cm lengths. From this group of nerve guides, four were randomly selected for measuring in vitro GDNF release kinetics. The remaining 16 were implanted into the experimental group of animals.
Long-term release studies were performed to approximate the amount of GDNF released during the duration of the in vivo studies. Nerve guides were individually added to clean eppendorf tubes. To each tube, 0.6 mL of PBS was added and the guides were incubated at 37°C for specified time increments. To collect the releasate, the guides were removed from each eppendorf tube using clean forceps and added to clean tubes with fresh PBS. The amount of soluble GDNF from the collected samples was analyzed using an enzyme linked immunosorbent assay (ELISA) per manufacturer's instructions. The optical density was recorded at 450 nm in an ELISA plate reader (Tecan). The GDNF concentrations were calculated against a six-point standard curve, and then adjusted to picograms of GDNF per milligram of microspheres.
Surgical methods
Following the guidelines of the University of Pittsburgh Institutional of Animal Care and Use Committee, 48 male Lewis rats (250–300 g, Harlan Labs) were used to evaluate the initial efficacy of GDNF released from PCL nerve guides for improved nerve regeneration. Each rat was anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). The sciatic nerve was exposed with a muscle splitting incision of the gluteal muscle. The nerve was sharply transected ∼0.5 cm from the proximal bifurcation and 0.5 cm of tissue was excised. After the proximal and distal nerve stumps were allowed to retract, the exposed fascicles were trimmed and sutured with 10-0 prolene epineurial mattress stitch 1 mm into each end of a 1.7-cm nerve guide, creating a 1.5-cm defect. The gluteal muscle and skin were then closed with 4-0 vicryl suture. The animals were randomly divided evenly amongst three groups, one of which received conduits with microspheres encapsulating GDNF and the other group received identical conduits with empty microspheres as a negative control. The third group of rats consisted of those which received a nerve isograft as a positive control; that is, nerve grafts from sacrificial rats of the same strain were used such that one donor animal produced two nerve grafts.
Functional assessment of nerve regeneration
Functional reinnervation of the lower limb muscles was assessed through three methods: video gait kinematics, gastrocnemius muscle twitch force, and gastrocnemius wet weight.
Video gait kinematics
A high-speed (100 full frames per second) digital video camera (Basler A602f) was used to record motion of the hindlimb during walking along a 10-cm-wide runway (Fig. 1A). Black ink marks of contrast (3 mm diameter) or reflective markers (Fig. 1B) were positioned over the iliac crest, greater trochanter, knee, ankle, and fifth metatarsal-phalangelal joints. During each test, the rat was positioned at one end and encouraged to walk the length of the 60-cm runway. This procedure is repeated until 15–20 steps were recorded with the rat walking straight forward (Fig. 1C). Figure 2 shows an image taken from the high-speed video of a rat walking on the runway. A custom Matlab program was used to automatically track the location of each mark of contrast in all frames of the video. The marker locations were then used to construct line segments connecting each pair of adjacent markers. Intersegmental joint angles for the hip, knee, and ankle joints were calculated using Equation 1, which results from the dot product rule.
 |
(1) |
FIG. 1.
(A) Photo of high-speed camera with equipment for data acquisition used for recording videos of animal gait. (B) Photo of anatomical placement of reflective markers for calculating joint angles during gait. (C) Photo of rat traversing walkway while being video recorded for gait analysis.
FIG. 2.
Image of rat captured during plantarflexion immediately before initiatin the swing phase. Line segments connect each of the five reflective markers. Representative vectors indicated with A and B are used to calculate the intersegmental joint angle θ. Color images available online at www.liebertonline.com/tea
In this equation, the vectors A and B denote the line segments proximal and distal to the joint. The intersegmental angle (θ) was computed as the inverse cosine of the dot product of vectors A and B, normalized by their respective lengths. The joint angle trajectories were computed from the measured marker positions. The foot touchdown and liftoff times were identified by visual inspection of the video and used to parse the swing (liftoff to touchdown) and stance (touchdown to liftoff) phases of the step cycle. The range of angular motion (maximum–minimum angles) for each joint was calculated during the swing and stance phases. These measures were then used for comparing gait kinematics across time points and treatment groups.
Gastrocnemius twitch force
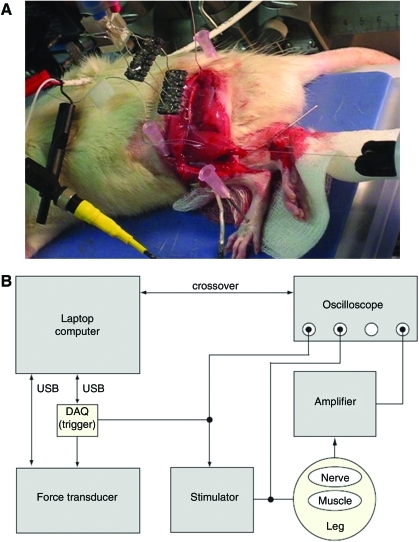
Immediately before sacrifice, animals were anesthetized with sodium pentobarbitol and the injured sciatic nerve was exposed with a muscle splitting incision made following the scar line remaining from graft or guide implantation. After freeing the isograft or regenerated nerve from the superficial and underlying muscle tissues, a bipolar nerve cuff electrode (1.5 mm diameter, Microprobe, Cat # NC (1.5)24) was placed around the nerve. Because the implanted nerve conduit enclosed the regenerated nerve, the PCL material had to be carefully removed such that sufficient nerve material was available for contact with the nerve cuff. The lower gastrocnemius was then completely isolated from the anterior and posterior tibialis muscles and the Achilles tendon was cut and fastened to a force transducer using a transfixation stitch and 4-0 silk sutures (Fig. 3). Finally, to completely stabilize the femur position, the animal's foot, knee, and back were immobilized on the data acquisition board using 16-0 gauge needles.
FIG. 3.
(A) Photo and (B) schematic of rat prepared on data acquisition board for muscle contraction force recordings. Color images available online at www.liebertonline.com/tea
Gastrocnemius twitch force was measured during stimulation of the sciatic nerve via the bipolar nerve cuff electrode. Tension in the suture connecting the gastrocnemius muscle to the force transducer was adjusted to obtain the maximum force. Stimulation pulses (200 μs wide; Model A320R; WPI) were applied at supramaximal intensity and the resulting twitch force was recorded at a sampling rate of 3 s (Model USB6009; National Instruments). The peak twitch force (relative to baseline) that was measured for five consecutive stimulation pulses was then calculated and optimized for the strongest stimulation points of contact between the nerve cuff and the sciatic nerve.
Gastrocnemius muscle weight
After muscle force measurements, the animals were euthanized with an overdose of sodium pentobarbital (100 mg/kg intraperitoneal [IP]). A longitudinal incision in the lower leg parallel to the extension of the Achilles tendon and gastrocnemius (gastroc) muscle was made followed by a dissection of the skin which adequately exposed the gastroc muscle. The two proximal tendonous insertions of the gastroc in the femoral area were identification and sectioned. The distal gastroc insertion in the heel through the Achilles tendon was then severed for the extraction of the gastroc muscle. The muscle was then immediately weighed. The wet muscle mass of the unoperated contralateral control was compared to the muscle mass of the gastroc from the injured leg.
Histological analysis
After the animals were sacrificed the implanted guides or isografts were harvested and immediately immersed in a fixative. Nerves prepared for paraffin embedding for MTC stain and IHC were fixed in 4% paraformaldehyde. Samples prepared for histomorphometry analysis were fixed in 2.5% glutaraldehyde. After the tissue samples were treated with fixative for at least 24 h, the samples were washed with PBS and postfixed in 1% osmium tetroxide for either 2 h in sample preparation for MTC and IHC analysis or 24 h for those samples prepared for histomorphometry. Nerve specimens were then dehydrated with increasing concentrations of ethanol (30%–100%) and sectioned with a sharp razor blade at the proximal nerve stump, the proximal, middle, and distal regions of the nerve conduit, and at the distal nerve stump as described previously.11 The sections were then embedded in paraffin or epoxy in descending order and sectioned at thicknesses of 3 μm (IHC, MTC) or 0.5 μm (histomorphometry).
Masson's trichrome
For analysis of cellular and tissue infiltration of the nerve conduits, including collagen formation, nerve sections from the negative control and experimental animals were stained for Masson's Trichrome. Sectioned nerves were first deparaffinized with Xylene and rehydrated with decreasing percent alcohol solutions (100% followed by 90% and then DI water). Solutions from a Masson's trichrome kit were then used according to the protocol published by Di Scipio et al.15
Immunohistochemistry
For fluorescent observation of Schwann cells, IHC was performed on explanted nerve samples. Paraffin-embedded specimens fixed in osmium tetroxide were first deparaffinized as described above and then etched with H2O2 for 10 min as described in ref.15 The samples were then blocked with 5% fetal bovine serum (FBS) with 0.02% triton-X in PBS for 1 h at room temperature. Antibodies against S-100 protein were then added overnight at 4°C (1:400 in 2.5% FBS and 0.02% triton-X in PBS). The samples were then washed three times with PBS and the secondary antibody was added for 1 h at room temperature (1:1000 in 2.5% FBS and 0.02% triton-X in PBS). The samples were then washed thrice again and the nuclei were detected using 4′,6-diamidino-2-phenylindole (0.6 μg/mL). The slides were then mounted with a fluorescent mounting media.
Histomorphometry
Nerve guides were fixed using 2.5% glutaraldehyde, embedded in epon, and cut into 0.5-μm cross sections using an ultramicrotome (Reichert Ultracut). Sections were then mounted onto glass slides and stained with 1% toluidine blue dye for imaging. A Hitachi (model KP-M1AN) digitizing camera was mounted on a Zeiss Primo Star microscope for image acquisition. A 100× oil immersion objective lens was used to produce digital images at a final magnification of 1000×, with a pixel size of 0.125 μm as calibrated with a stage micrometer.
The Leco IA32 Image Analysis System (Leco) with custom calculation routines (macros) was used as developed by Hunter et al.16 8-bit monochrome images were acquired and thresholded for determining myelin composition. As described by Hunter et al., manual adjustments were made to photomicrographs displayed on an attached monitor such that debris and nonviable nerve fibers were removed. Viable axons were defined as dark myelin rings enclosing clear fiber areas devoid of debris or cell nuclei. Myelin width, axon width, and fiber diameter were then automatically calculated through software analysis of red and green bitplane identifiers. From these primary measurements, g-ratio (the ratio of the axonal diameter divided by the diameter of the axon and its myelin sheath) and nerve fiber density (fiber number/mm2) were calculated.
Statistical analysis
A minimum repetition value of four was used when measuring GDNF release from nerve guides. Results are expressed as the mean ± standard deviation. Analysis of variance was used to determine statistical significance between experimental groups. The least significant difference method was used for multiple comparisons with p < 0.05.
Results
GDNF release from nerve guides with double-walled microspheres
Long-term release studies of GDNF from PCL nerve guides with double-walled microspheres were performed to approximate the release profile of the growth factor in vivo. Nerve guides embedded with double-walled microspheres encapsulating GDNF were incubated in PBS and released growth factor was quantified using an ELISA system. As shown in Figure 4, the release of GDNF from the PCL nerve guides did not exhibit the typical burst release profile seen in single-walled microsphere studies. At day 3, only ∼2.9% of the total released protein is liberated into solution. GDNF release is nearly linear until day 56, at which point ∼89.0% of the total growth factor is released. After this point, GDNF release is consistent to day 112, when the 16 week in vivo studies were complete. Assuming that the majority of microspheres weighed for nerve guide preparation were successfully embedded and secured into the nerve guide walls, the encapsulation efficiency for GDNF nerve guide production was ∼1%.
FIG. 4.
Cumulative release of GDNF (pg) from double-walled microspheres embedded in PCL nerve guides (1.7 cm in length). Values expressed as mean ± standard deviation (n = 4). GDNF, glial cell line-derived neurotrophic factor; PCL, poly(caprolactone).
Implantation of PCL nerve guides
Upon exposure of the injured sciatic nerve at sacrifice, remaining PCL conduits were soft and pliable and both sutures intact. Nerve guides were well vascularized with a soft fibrous coating (Fig. 5A). The proximal and distal ends of the nerve guides were completely sealed with a fibrous capsule and small neuromas were apparent at both guide ends. Before separating the nerve guide from the regenerated nerve, a pinch test was administered proximal to any anastamosis to determine if a muscle reflex could be observed. In 75% of the animals treated with an empty PCL conduit (e.g., 12/16 rats), a lower limb reflex was observed. However, often the regenerated nerve through the negative control conduits was delicate, and attempts to remove the conduit for placement of a nerve cuff resulted in disruption in nerve continuity. It was also noted that the regenerating nerve grew adjacent or into the porous walls of the PCL conduit.
FIG. 5.
(A) Photo of exposed empty PCL nerve guide 16 weeks after implantation (white arrow). The proximal and distal ends of the guide are indicated with the characters P and D. (B) Photo of GDNF releasing PCL nerve guide (white arrow) that has been longitudinally sectioned revealing regenerated nerve (white arrow head). Color images available online at www.liebertonline.com/tea
Nerve guides implanted with GDNF releasing microspheres were also observed as well vascularized and sheathed in a soft fibrous coating. Results from a preliminary pinch test indicated that lower limb reinnervation was seen in 100% of the experimental animals. Furthermore, the nerves appeared to grow through the open lumen of the nerve guides and could be easily separated from the conduits for electrophysiology studies (Fig. 5B).
Functional assessment of nerve regeneration: Video gait kinematics
Gait kinematics from animals receiving a PCL nerve guide without growth factor (n = 3) and animals which received GDNF releasing guides (n = 16) after sciatic nerve injury are shown in Figure 6. At the week 1 time point, all animals show a large reduction in the RoM at the ankle during the stance and swing phases; the stance-phase RoM for the knee was also reduced. Injured animals are unable to generate muscle force to extend the knee, reaching only ∼90° of extension during stance (Fig. 6C) compared to 130° in the healthy animal. The ankle, which normally flexes ∼70° to lift the foot during the swing phase, extends passively during swing after injury (Fig. 6E). To compensate for reduced motion at the ankle and knee, the hip joint shows a large increase in RoM as the hip is hyperflexed to provide sufficient ground clearance, compensating for the reduced motion at the ankle and knee. By week 15, the stance phase RoM for the ankle and knee return to baseline levels, and the RoM between experimental and negative control animals was not significantly different between groups at any time point.
FIG. 6.
Hip range of motion (degrees) at baseline (week 0) and sequential timepoints after injury for swing phase (A) and stance phase (B) of the gait cycle. Knee range of motion during swing (C) and stance (D). Ankle range of motion (degrees) in swing (E) and stance (F). For all graphs, GDNF animals are represented with (♦), control PCL guides are ( ) and isografts at week 15 are (▴).
) and isografts at week 15 are (▴).
Functional assessment of nerve regeneration: Gastrocnemius twitch force
The mean measured gastroc twitch force was 0.59 ±0.28 N, 0.44 ± 0.22 N, and 0.07 ± 0.07 N for animals treated with an isograft (n = 11), a nerve conduit with GDNF (n = 6) and animals treated with a PCL conduit and no growth factors (n = 7), respectively. The average twitch force between isograft animals and those within the experimental GDNF group was not significantly different (p = 0.1754), whereas both of these groups showed significantly improved twitch force above negative control animals (Fig. 7).
FIG. 7.
Average gastrocnemius contraction force (N) with error bars representing standard deviation. * = p < 0.05.
Functional assessment of nerve regeneration: Gastrocnemius muscle wet weight
The wet weight of recovered gastroc muscles from injured legs as normalized to contralateral uninjured controls are recorded in Table 1. The normalized values of muscles from animals treated with isografts as a positive control for nerve regeneration were statistically higher than both the experimental GDNF group as well as the PCL guides without growth factor delivery (p < 0.01).
Table 1.
Recorded Wet Weights of Injured Gastrocnemius Muscle as Normalized to Contralateral Control
| Treatment description | Gastrocnemius wet weight (%) |
|---|---|
| Isograft (n = 11) | 46.6 ± 12.4a |
| PCL guide + GDNF (n = 7) | 24.8 ± 5.7 |
| Empty PCL guide (n = 6) | 21.7 ± 4.4 |
Indicates statistical significance from experimental groups, p < 0.01.
PCL, poly(caprolactone); GDNF, glial cell line-derived neurotrophic factor.
Histological analysis: Masson's trichrome
The regeneration of nerve across a 1.5-cm defect was evaluated at 16 weeks. Low magnification transverse sections of the proximal portion of negative control conduits reveal a high degree of collagen content within the lumen of the guide (blue) with tissue integration throughout the entirety of the guide lumen (Fig. 8A). Regenerated nerve is evident near the PCL nerve guide wall (black box) and, as evident at higher magnification, is disorganized and sparse (Fig. 8B). Nerve tissue is also evident within the lumen of nerve guides releasing GDNF; however, fibers are located in the center of the lumen surrounded by newly formed tissue (black circle, Fig. 8C). High-magnification light micrographs reveal a large number of small nerve fibers that are well organized and thinly myelinated (Fig. 8D).
FIG. 8.
Transverse sections of the proximal segment of guides explanted after 16 weeks as observed with Masson's trichrome stain. (A) Low-magnification brightfield micrographs taken of negative control guides with regenerated nerve tissue indicated within black box. (B) High magnification micrographs showing detailed nerve tissue organization. (C) Low magnification transverse section from guides releasing GDNF with centrally located nerve tissue circled in black. (D) High-magnification brightfield image of nerve fiber organization after GDNF treatment. Scale bars are 100 μm. NG = nerve guide. Color images available online at www.liebertonline.com/tea
Low-magnification brightfield images of transverse sections from control PCL guides reveal that regenerated nerve tissue was not evident within mid (Fig. 9A) or distal segments (Fig. 9B). Tissue integration appears incomplete and few blood vessels are observed. However, in both the mid and distal regions of conduits releasing GDNF, there was regenerated nerve tissue within the lumen of the guides. Low magnification micrographs of the midline of explanted conduits (Fig. 9C) show collagen formation and tissue integration supporting the regenerated nerve fibers seen at higher magnification within Figure 9D. Additionally, nerve tissue, including collagen and axons, is also evident within distal regions of the GDNF releasing conduits within both low (Fig. 9E) and high magnification (Fig. 9F) micrographs.
FIG. 9.
Transverse sections from guides explanted 16 weeks post injury observed with Masson's trichrome stain. Low-magnification brightfield micrographs taken of negative control guides from the mid (A) and distal (B) sections of the explanted guide. (C) Low-magnification mid-transverse section from guides releasing. (D) High-magnification brightfield image of nerve fiber organization after GDNF treatment. (E) Low-magnification distal transverse section from guides releasing. (F) High-magnification brightfield image showing detail nerve fiber organization from within GDNF guides. Scale bars are 100 μm. Color images available online at www.liebertonline.com/tea
Histological analysis: IHC
Nerve tissue was evident in the proximal region of explanted conduits 16 weeks after sciatic nerve transection and conduit implantation. Fluorescent images reveal the presence of both nerve fibers (neurofilament proteins: green) and Schwann cells (S-100: red) in both negative control PCL guides and guides releasing GDNF. However, nerve tissue within control PCL guides (Fig. 10A) was localized to the internal border of the nerve guide wall and appears disorganized, whereas nerve tissue was centrally located and well organized within GDNF releasing guides (Fig. 10B). Schwann cells and nerve fibers were not detectable within the middle (Fig. 10C) or distal segments (Fig. 10E) of control nerve guides, whereas robust Schwann cell populations were observed across the entire length of GDNF releasing guides (Fig. 10D, F).
FIG. 10.
Fluorescent micrographs of proximal transverse sections of negative control guides (A) and guides releasing GDNF (B). Fluorescent micrograph from middle segment of control (C) and GDNF (D) nerve guides. Distal transverse section from negative control (E) and GDNF releasing nerve guides (F). Red indicates S100 labeling of Schwann cells. Neurofilament proteins within nerve fibers are green. 4′,6-Diamidino-2-phenylindole is blue. Scale bars are 100 μm. Color images available online at www.liebertonline.com/tea
Histological analysis: G-ratio and nerve fiber density
Monochrome images of the proximal nerve stump, proximal isograft or nerve guide, distal isograft or nerve guide, and distal nerve stump were acquired and thresholded to identify viable axons. Using semi-automated software, myelin width, axon width, and fiber diameter were calculated. From these primary measurements, g-ratio and nerve fiber density (fiber number/mm2) were determined. Within the isograft-positive control nerve samples, the g-ratio of axons was consistent within each section from the proximal nerve stump to the distal nerve stump with a measured range of 0.40 to 0.42 (Fig. 11A). The g-ratio of nerve fibers within GDNF releasing guides were higher for all measured transverse sections, with average values of 0.55, 0.59, 0.56, and 0.53 for the proximal nerve stump, proximal graft or guide, middle graft or guide, and distal nerve stump sections, respectively, which are not statistically significantly different from within measured groups. These measured values from GDNF guides approach an uninjured g-ratio range of 0.6–0.7 (gray bar). Fibers from control PCL guides were observed to have a lower g-ratio value within both the proximal nerve stump (0.47) and the proximal segment (0.45) of the explanted nerve guide. No fibers were evident in the mid or distal regions of negative control PCL guides for measurement calculations.
FIG. 11.
(A) Calculated g-ratio of fibers within transverse segments of the PNS, PG, DG, and DNS. (B) Calculated nerve fiber density (fibers per mm2). Treatment groups are isograft (▪), GDNF nerve guide ( ) and control nerve guide (
) and control nerve guide ( ). Values are expressed as mean ± standard deviation. Gray horizontal bar indicates normal, uninjured g-ratio values. PNS, proximal nerve stump; PG, proximal graft or guide; DG, distal graft or guide; DNS, distal nerve stump. * = p < 0.05.
). Values are expressed as mean ± standard deviation. Gray horizontal bar indicates normal, uninjured g-ratio values. PNS, proximal nerve stump; PG, proximal graft or guide; DG, distal graft or guide; DNS, distal nerve stump. * = p < 0.05.
Evaluation of nerve fiber density throughout the length of explanted nerve samples reveals an increased density of fibers per mm2 for both the GDNF and control nerve guides as compared to isograft positive controls, an indication of nerve regeneration (Fig. 11B). As seen in fiber g-ratio results, isograft sections had a small range of fiber densities (∼11,300–12,200) progressing from the proximal to the distal nerve stumps, indicating limited axonal sprouting occurred within the graft. The density of fibers within GDNF releasing guides was higher than negative control guides for all measured segments and there were no viable nerve fibers in negative control nerve guides beyond the mid region of the explanted conduits.
Discussion
The efficacy of GDNF toward improving peripheral nerve regeneration through nerve guidance conduits has been evaluated in vivo. Patel et al. reported chitosan scaffolds blended with laminin-1 and 5 μg GDNF were implanted across a 10-mm gap in the Lewis rat sciatic nerve.9 After 12 weeks, the chitosan/GDNF guides resulted in decreased gastrocnemius muscle atrophy and restoration of functional strength that was comparable to autograft controls. Behavioral testing indicated that GDNF treatment groups regained sensation and improved gait kinematics. In addition, silicone conduits filled with GDNF gene-modified Schwann cells have resulted in significantly improved nerve conduction velocity, number and density of regenerated nerves, and the thickness of the myelin sheath of regenerated nerves than that seen in controls across a 10-mm defect in the Wistar rat.10 Finally, nerve guides with a GDNF releasing rod increased the number of myelinated axons and the overall number of regenerated axons was four-fold higher than nerve guides with nerve growth factor.7
Our previous work examining GDNF release from nerve guides for 6 weeks suggested that bioactive GDNF was delivered to endogenous cell populations and markedly improved cellular integration and tissue formation across the nerve injury.11 Although nerve gaps treated with empty microsphere conduits resulted in incomplete fibrotic tissue formation in the center of proximal segment of excised nerve guides, nerve guides releasing GDNF had an increase in cellular infiltration and tissue integration within the lumen of the conduits. Additionally, we observed an increase in cellular infiltration and collagen content after GDNF treatment, and we hypothesize that this lead to an improved scaffold for Schwann cell migration and supported axonal outgrowth within this longer 16-week in vivo study.
Within this study, we have shown that GDNF is released from nerve guides in vitro for over 100 days. When implanted into rat sciatic nerve defects, a higher density of nerve tissue regenerated through the center of conduits as opposed to negative control guides (e.g., empty microsphere PCL conduits), which produced nerve tissue bolstered by the wall of the nerve guide. Regenerated nerve tissue from within GDNF releasing guides appeared to have an increased level of collagen and intercellular tissue as observed with MTC. Quantification of high magnification light micrographs through software analysis supported visual impressions of improved nerve repair with GDNF treatment. Animals receiving GDNF had a g-ratio that approached native, uninjured levels, and were improved above both isografts and negative controls. Additionally, the mean nerve fiber density of regenerated nerves was highest within conduits releasing GDNF, and was statistically higher than isograft controls at the proximal end of conduits (p < 0.05). Finally, immunofluorescent micrographs identifying Schwann cells show a large population of Schwann cells throughout the length of conduits releasing GDNF, whereas very few Schwann cells are detectable in the middle and distal segments of negative control guides. The presence of Schwann cells within the lumen of the guides is a positive indication for nerve repair, as several studies have shown that higher numbers of Schwann cells result in more robust nerve regeneration.17–19
Analysis of muscle reinnervation through quantification techniques of rat gait proved challenging using the techniques described herein. While mass loss was statistically equivalent when comparing wet muscle weight in GDNF treated and empty nerve guides, muscle function through twitch force may be more indicative of nerve regrowth and reinnervation of target end organs. This study suggests that measuring muscle twitch force is an improved method of observing nerve regeneration to target muscles over the described timepoint of 15 weeks. It is very possible that at a longer timepoint, the muscle wet weights between the control and experimental groups would have been different due to the continued lack of nerve innervation in the gastrocnemius muscle of rats treated with empty nerve guides. No significant difference in joint angle RoM was measured between the GDNF experimental group and the negative control. The development of a technique for analyzing gait kinematics through video recording was undertaken as a method of circumventing those challenges seen with sciatic functional index measurement.20 However, unique obstacles were presented within this method of gait analysis. First, animal behavior is varied, and often animals were observed as walking with a unique head position, speed, or step pattern. In addition, animals would traverse the walkway only a few times before losing interest in the activity and could not be trained to proceed with a smooth gait pattern despite incentives provided by the operator. Because of this, step cycles were used for measuring joint angles from only those steps that were consecutive and were determined to be of a walking nature (at least one foot always on the ground). The use of a treadmill would greatly improve the consistency seen in joint angle measurements and may allow this technique to be a more robust method for detecting early improvements in gait kinematics after lower limb nerve injury.
Conclusions
Within this study, we have shown that GDNF can be successfully encapsulated in double-walled microspheres and released in a controlled manner from PCL nerve guides for over 100 days in vitro and improves nerve regeneration in vivo. Upon the initial exposure of the site of nerve injury, nerve tissue was observed exiting the distal end of implanted conduits in only 70% of the control PCL guides, whereas 100% of the animals implanted with GDNF releasing guides had nerve trunks throughout the length of the conduits and integrating into the distal target muscles. In addition, the measured gastrocnemius twitch force, an indication of muscle atrophy and reinnervation, was significantly improved by a difference of sixfold in animals treated with GDNF releasing conduits as opposed to those that received empty PCL conduits. Markedly, the twitch force between animals treated with isografts, considered to be the gold standard for nerve repair, was not significantly different from animals receiving GDNF releasing conduits. Although this study evaluated nerve repair after treatment with a single growth factor, the nerve guide design is modular; therefore, these PCL conduits described herein lend themselves easily toward investigation of a variety of different therapeutics for improved nerve regeneration.
Acknowledgments
This work was primarily supported by the National Science Foundation, DMR-0705948 (K.G.M.), and Department of Defense Armed Forces Institute for Regenerative Medicine W81XWH-08-2-0032 (K.G.M.). Additional support was provided by the Telemedicine and Advanced Technology Research Center of U.S. Army Medical Research and Material Command Agreement W81XWH-07-1-0716 (D.J.W.), National Science Foundation IGERT DGE-0549352 (D.J.B.), and NIH grants from the NIBIB (1R01EB007749) and NINDS (1R21NS056136) (D.J.W.). The authors would also like to thank the Center for Biologic Imaging for image analysis, Amir Mahan Ghaznavi for surgical assistance, Sami Tuffaha and Mostafa Ramadan for assistance with nerve conductance studies, and Laura McKee for assistance with axon counting.
Disclosure Statement
No competing financial interests exist.
References
- 1.Schlosshauer B. Dreesmann L. Schaller H.-E. Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey [review] Neurosurgery. 2006;59:740. doi: 10.1227/01.NEU.0000235197.36789.42. [DOI] [PubMed] [Google Scholar]
- 2.Valero-Cabré A. Tsironis K. Skouras E. Perego G. Navarro X. Neiss W. Superior muscle reinnervation after autologous nerve graft or poly-L-lactide-epsilon-caprolactone (PLC) tube implantation in comparison to silicone tube repair. J Neurosci Res. 2001;63:214. doi: 10.1002/1097-4547(20010115)63:2<214::AID-JNR1014>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Pfister L.A. Papaloïzos M. Merkle H.P. Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 4.Iwase T. Jung C. Bae H. Zhang M. Solivan B. Glial Cell Line Derived Neurotrophic Factor-Induced Signaling in Schwann Cells. J Neurochem. 2005;94:1488. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- 5.Li L. Wu W. Lin L. Lei M. Oppenheim R. Houenou L. Rescue of Adult Mouse Motorneurons from Injury-Induced Cell Death by Glial Cell Line-Derived Neurotrophic Factor. Proc Natl Acad Sci. 1995;92:9771. doi: 10.1073/pnas.92.21.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannotti C. Li H. Yan P. Lu X. Wirthlin L. Xu X. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol. 2003;183:379. doi: 10.1016/s0014-4886(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 7.Fine E.G. Decosterd I. Papaloizos M. Zurn A.D. Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 8.Barras F. Pasche P. Bouche N. Aebischer P. Zurn A.D. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J Neurosci Res. 2002;70:746. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- 9.Patel M. Mao L. Wu B. VandeVord P.J. GDNF-chitosan blended nerve guides: a functional study. J Tissue Eng Regen Med. 2007;1:360. doi: 10.1002/term.44. [DOI] [PubMed] [Google Scholar]
- 10.Li Q. Ping P. Jiang H. Liu K. Nerve conduit filled with GDNF gene-modified schwann cells enhances regeneration of the peripheral nerve. Microsurgery. 2006;26:116. doi: 10.1002/micr.20192. [DOI] [PubMed] [Google Scholar]
- 11.Kokai L.E. Ghaznavi A.M. Marra K.G. Incorporation of double-walled microspheres into polymer nerve guides for the sustained delivery of glial cell line-derived neurotrophic factor. Biomaterials. 2010;31:2313. doi: 10.1016/j.biomaterials.2009.11.075. [DOI] [PubMed] [Google Scholar]
- 12.Kokai L.E. Tan H. Jhunjhunwala S. Little S.R. Frank J.W. Marra K.G. Protein bioactivity and polymer orientation is affected by stabilizer incorporation for double-walled microspheres. J Controlled Release. 2010;25:168. doi: 10.1016/j.jconrel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Jiang C. Moore M. Zhang X. Klassen H. Langer R. Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007;13:1783. [PubMed] [Google Scholar]
- 14.Kokai L.E. Lin Y.-C. Oyster N.M. Marra K.G. Diffusion of soluble factors through degradable polymer nerve guides: Controlling manufacturing parameters. Acta Biomaterialia. 2009;5:2540. doi: 10.1016/j.actbio.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Di Scipio F. Raimondo S. Tos P. Geuna S. A simple protocol for paraffin-embedded myelin sheath staining with osmium tetroxide for light microscope observation. Microsc Res Tech. 2008;71:497. doi: 10.1002/jemt.20577. [DOI] [PubMed] [Google Scholar]
- 16.Hunter D.A. Moradzadeh A. Whitlock E.L. Brenner M.J. Myckatyn T.M. Wei C.H. Tung T.H.H. Mackinnon S.E. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkowski G.E. Miller C.A. Jeftinija S. Mallapragada S.K. Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration. J Neural Eng. 2004;1:151. doi: 10.1088/1741-2560/1/3/004. [DOI] [PubMed] [Google Scholar]
- 18.Kim S.-M. Lee S.-K. Lee J.-H. Peripheral nerve regeneration using a three dimensionally cultured Schwann cell conduit. J Craniofac Surg. 2007;18:475. doi: 10.1097/01.scs.0000249362.41170.f3. [DOI] [PubMed] [Google Scholar]
- 19.Guenard V. Kleitman N. Morrissey T.K. Bunge R.P. Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12:3310. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown C.J. Mackinnon S.E. Evans P.J. Bain J.R. Makino A.P. Hunter D.A. Hare GMT. Self-evaluation of walking-track measurement using a sciatic function index. Microsurgery. 1989;10:226. doi: 10.1002/micr.1920100317. [DOI] [PubMed] [Google Scholar]