Abstract
This study describes the use of oligo [(polyethylene glycol) fumarate] (OPF) hydrogel scaffolds as vehicles for sustained delivery of dibutyryl cyclic adenosine monophosphate (dbcAMP) to the transected spinal cord. dbcAMP was encapsulated in poly(lactic-co-glycolic acid) (PLGA) microspheres, which were embedded within the scaffolds architecture. Functionality of the released dbcAMP was assessed using neurite outgrowth assays in PC12 cells and by delivery to the transected spinal cord within OPF seven channel scaffolds, which had been loaded with Schwann cells or mesenchymal stem cells (MSCs). Our results showed that encapsulation of dbcAMP in microspheres lead to prolonged release and continued functionality in vitro. These microspheres were then successfully incorporated into OPF scaffolds and implanted in the transected thoracic spinal cord. Sustained delivery of dbcAMP inhibited axonal regeneration in the presence of Schwann cells but rescued MSC-induced inhibition of axonal regeneration. dbcAMP was also shown to reduce capillary formation in the presence of MSCs, which was coupled with significant functional improvements. Our findings demonstrate the feasibility of incorporating PLGA microsphere technology for spinal cord transection studies. It represents a novel sustained delivery mechanism within the transected spinal cord and provides a platform for potential delivery of other therapeutic agents.
Introduction
Of the 259,000 persons currently living in the United States with spinal cord injury (SCI), 25.6% and 20.4% have complete paraplegia and tetraplegia, respectively (National Spinal Cord Injury Database). Pathological and imaging studies demonstrate tissue destruction with cysts and gliosis in the area of injury and atrophy in adjacent segments of the spinal cord.1,2 Re-establishing function below the block requires rebuilding functional tissue within the necrotic lesioned area.3 Tissue bridges may carry regenerating axons from above the lesion to roots or muscles below3 and could potentially replace the cystic/collagenous areas with functional tissue. Biodegradable polymer implants have been shown to stimulate axon regeneration in the peripheral nervous system by providing structural stability, prolonged release of growth-promoting agents,4–10 and a reservoir for sustained therapeutic drug delivery.11 This approach also provides a platform for evaluating the effectiveness of specific interventions in the spinal cord.12–18 Insertion of biodegradable polymers into the transected spinal cord could potentially act to guide nerve fibers through the injury, and also allow for the quantification of nerve regeneration.6,19,20
Oligo[(polyethylene glycol) fumarate] (OPF) is a water-soluble material21 that can be cross-linked using ultraviolet light to form biodegradable synthetic hydrogels.22,23 The molecular weight of the poly(ethylene glycol) used in macromer formation controls the mechanical and degradation properties of the OPF hydrogels.23,24 Potential applications of these hydrogels include bone tissue engineering,24–27 scaffold material for marrow stromal cell attachment and differentiation,28–31 cell encapsulation,30,31 cartilage tissue engineering,32 and the controlled release of various compounds.33–35 Recent studies suggest applications in the nervous system using positively charged hydrogels.36 These researchers demonstrate the capability of OPF hydrogels for sustaining growth of both primary nerve cells and neural support cells that are critical for regeneration.
A common method for the controlled release of potential therapeutic agents is by encapsulation in poly(lactic-co-glycolic acid) (PLGA) microspheres. The biocompatibility,37 degradation kinetics,11 and nontoxicity38 of PLGA make it an attractive delivery vehicle. Formulation of microspheres commonly involves the water-in-oil-in-water emulsion and solvent evaporation technique, which is suitable for incorporation of hydrophilic compounds.39,40 PLGA microspheres may be used for the controlled release and in vivo localization of proteins39,41–43 and drugs.40,44
Novel approaches to PLGA microsphere incorporation into three-dimensional biodegradable scaffolds include fusion of protein loaded spheres into scaffolds using dichloromethane vapor.45
While incorporation of PLGA microspheres into three-dimensional OPF scaffolds has not yet been demonstrated, release of DNA33,34,46 and growth factors35 from gelatin microspheres within OPF hydrogels has been shown. To assess the feasibility of incorporating OPF hydrogel and PLGA microsphere technology, we sought to release dibutyryl cyclic adenosine monophosphate (dbcAMP) to the transected spinal cord. dbcAMP is a cAMP analog that is membrane permeable and preferentially activates protein kinase A.47 cAMP mediates diverse functions within the nervous system through its binding to protein kinase A. These functions include the following:
Cell survival: in cultures of rat sympathetic and sensory neurons47 and retinal ganglion cells.48
Axon guidance: in the presence of other factors (i.e., Brain-derived neurotrophic factor (BDNF) or acetylcholine), cAMP can modulate behaviors of growth cones during pathfinding.49
Neurite outgrowth: in cultures of rat sympathetic and sensory neurons47 and in xenopus laevis embryonic neuronal culture50 (mediated in part by the phosphorylation of synapsins in nerve terminals50).
Axonal regeneration: by promoting extension of axons into the peripheral nervous system, contributing to functional recovery in SCI51–54 and in the event of ventral motor neuron ablation.55
The main objective of this study was to establish a novel method of delivery of dbcAMP to the transected spinal cord via PLGA microsphere-loaded OPF scaffolds.
Materials and Methods
Fabrication of dbcAMP-encapsulating PLGA microspheres
Microspheres were fabricated using a water-in-oil-in-water double emulsion, solvent evaporation technique.56 Briefly, 500 mg 50:50 PLGA (Lakeshore Biomaterials) was dissolved in 1.25 mL methylene chloride (Sigma Aldrich) in a glass test tube for 2–3 h. While continually vortexing, 10 μM dbcAMP (Calbiochem) and 2 mL of 2% polyvinyl alcohol (PVA) (Sigma Aldrich) was added to the dissolved PLGA. For the empty microspheres (used as a control), sterile water was added instead of dbcAMP. This PLGA/PVA solution was then poured into a beaker containing 100 mL 0.3% PVA. About 100 mL 2% isopropyl alcohol (Sigma Aldrich) was added to this mix, which was then stirred for at least 1 h to evaporate the methylene chloride. Microspheres were washed with distilled H2O, frozen at −20°C, and placed in a high vacuum pump to dry (Virtis freeze mobile; Virtis Company). Microspheres were stored in a desiccator at 4°C until use.
Fabrication of OPF hydrogel discs
Hydrogel discs were made by dissolving OPF with initiator and distilled H2O at 37°C for 10 min according to a previously described method.27,36 After centrifugation, N-vinyl pyrrolidinone (NVP) crosslinker was added. Ninety milligrams of microspheres was added to 1 mL of OPF solution, which was then poured into glass molds and crosslinked using UV light. As a control, dbcAMP was added directly into charged or uncharged OPF discs.
Molds were cut into discs measuring 15 mm in diameter and 1 mm thickness, and placed in a high-vacuum pump to dry. OPF discs were stored in a desiccator at 4°C until use. Before use, OPF discs were soaked in sterile water, followed by washes in 80% ethanol, 40% ethanol, and sterile water (x2). To assess the release of dbcAMP from these OPF discs, Dulbecco's modified Eagle's medium (DMEM) (1 mL per disc) was added to each well containing the microsphere–hydrogel discs (n = 3), the charged hydrogel discs with dbcAMP (n = 3), and to the uncharged hydrogel discs with dbcAMP (n = 3), which were then placed in an incubator at 37°C, 5% CO2. The medium was removed every 3 days and stored at −20°C until enzyme linked immunosorbent assay (ELISA) analysis. Fresh DMEM was placed on the discs every 3 days. dbcAMP was analyzed using a cyclic AMP assay ELISA (R&D Systems), which could also be used to detect dbcAMP. Manufacturer's instructions were followed.
Neurite outgrowth functional assay
PC12 cells were seeded at 3000 cells/cm2 in poly-l-lysine-coated (Sigma Aldrich) 12-well plastic tissue culture dishes and were fed with the PC12 cell culture medium (DMEM high glucose with l-glutamine, 10% horse serum, 5% fetal bovine serum [both sera were heat inactivated at 57°C for 1 h], and 0.5% penicillin–streptomycin [Sigma Aldrich]). Cells were allowed to adhere overnight.
Before use, OPF discs were soaked in sterile water, followed by washes in 80% ethanol, 40% ethanol, and sterile water (two times). OPF discs containing empty microspheres versus dbcAMP-containing microspheres were inserted in transwells and incubated with the PC12 cells. Light microscopic images were acquired after 48 h. Random fields of view were taken from all of the samples (n = 5) and neurite outgrowth was assessed. Total neurite length was measured using ImageJ software.
dbcAMP concentration assay
A dbcAMP concentration assay was performed to assess if a minimal concentration of dbcAMP was required to promote neurite outgrowth. PC12 cells were seeded as described above and were left to adhere for 24 h. dbcAMP was added at concentrations of 0, 0.1, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, and 256 nmol/ml. Light microscopic images were acquired after 24 h. Random fields of view were taken from all of the samples (n = 9) and neurite outgrowth was assessed. Total neurite length was measured using ImageJ software.
Fabrication of OPF hydrogel 7 channel scaffolds
Twenty-five milligrams of PLGA microspheres was added to 250 μL of OPF, which was then vortexed. This OPF–microsphere solution was injected into a cylindrical glass mold and seven wires were inserted to form the channels. After crosslinking with UV light, scaffolds were removed from the molds, hydrated in phosphate-buffered saline (PBS) for 30 min, cut to 2 mm lengths, placed in a high-vacuum pump to dry, and stored in a desiccator at 4°C until use. These cylindrical scaffolds were 2.6 mm in diameter and 2 mm length, with internal channels measuring 450 μm in diameter.
To assess the release of dbcAMP from these OPF scaffolds in vitro, we subjected the scaffolds to the same sterilization process that would be used when the scaffolds would be put into the transected spinal cord. Scaffolds were disinfected by immersion in 80% ethanol overnight, then thoroughly rinsed in several changes of PBS, and left in PBS for at least 24 h at 4°C.
DMEM (300 μL per scaffold) was then added to each scaffold (n = 5), which were then placed in an incubator at 37°C, 5% CO2. The medium was removed every 3 days and stored at −20°C until ELISA analysis. Fresh DMEM was placed on the discs every 3 days. Analysis of dbcAMP was performed as described above using a cyclic AMP assay ELISA.
Isolation and characterization of Schwann cells and mesenchymal stem cells
Mesenchymal stem cells (MSCs) were isolated from the bone marrow of 8- to 12-week-old male or female Sprague Dawley rats (transgenic rats expressing green fluorescent protein [GFP]—green rats CZ-004 [SD TgN(act-EGFP)OsbCZ-004] [Osaka University, Japan]), and extensively characterized as we have previously published.37,57 Schwann cells (SCs) were isolated from the sciatic nerve of 2- to 5-day-old newborn rats, and characterized by staining for the marker S-100 as we have previously published.58
Preparation of OPF scaffolds for surgeries
Forty-eight hours before surgery, scaffolds were sterilized by immersion in 80% ethanol overnight, and then thoroughly rinsed in several changes of PBS. These were then stored in PBS at 4°C until loading. Twenty-four hours before surgery, scaffolds were placed in the appropriate cell culture medium. Cells (SCs or MSCs) were resuspended in undiluted Matrigel (BD Biosciences) at a density of 50,000 cells/μL and seeded into scaffolds using a pipette with gel-loading pipette tips. About 8 μL cell suspension was required to fill each scaffold, giving a total of 400,000 cells per scaffold. Optimal cell number was based on previous SC transplantation studies.36,58–60 The same cell number was chosen for MSC delivery to make the biological comparison of MSCs to SCs more clear and significant. The scaffolds for the control animals were also prewet with cell culture medium and were loaded with Matrigel alone without any cells.
Spinal cord transection surgery and postoperative care
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic (IACUC number A12908). Adult Sprague-Dawley rats (250–300 g) were anesthetized with 60 mg/kg ketamine (Fort Dodge Animal Health) and 2.5 mg/kg xylazine (Ben Venue Laboratories) by intraperitoneal injection. Operations were performed using sterile technique and with the aid of a Zeiss Superlux 40 surgical microscope. A 2-cm midline incision was made along the T7 to T10 spinous processes. The thoracolumbar fascia and paraspinal musculature were incised along the spinous processes and retracted. A T8-9 laminectomy was performed, and the cord was transected using a no. 11 blade, which was confirmed by observation. The site was irrigated with normal saline, and bleeding was controlled using gauze and cotton-tipped applicators. The implant was placed in the transection gap and aligned to assure good contact and no blood clots at the rostral and caudal cord–scaffold interfaces. Since the scaffold was 2 mm in length, all transections resulted in a 2-mm gap between spinal stumps.
Surgeries were performed on 70 rats with an n = 10 per group. A mortality rate of ∼10% was observed. Groups are outlined in Table 1.
Table 1.
Animal Groups
| Group | Animal number | Microspheres (MS) | Cell type |
|---|---|---|---|
| 1: Control | 10 | / | / |
| 2: Empty MS | 10 | Empty | / |
| 3: dbcAMP MS | 10 | dbcAMP | / |
| 4: SCs | 10 | / | Schwann cells |
| 5: SCs + dbcAMP MS | 10 | dbcAMP | Schwann cells |
| 6: MSCs | 10 | / | Mesenchymal stem cells |
| 7: MSCs + dbcAMP MS | 10 | dbcAMP | Mesenchymal stem cells |
dbcAMP, dibutyryl cyclic adenosine monophosphate.
Postoperatively, animals were given buprenorphine 0.05 mg/kg subcutaneously for pain, saline, and Baytril intramuscularly for the first 5 days. For the duration of the experiment, bladder voiding and observation of the animals were performed twice daily. Saline and Baytril were used to treat urinary tract infections, and aluminium spray was applied to limit autophagia.
Functional analysis
Functional recovery of experimental and control animals was assessed by performing open field testing using the Basso, Beattie and Bresnahan (BBB) locomotor rating scale 2 weeks and 4 weeks after surgery. Each rat was observed in an open plastic box for 5 min, and three independent, blinded observers recorded hind limb joint movements, weight support, toe clearance, tail position, coordination of gait, and paw position.
Tissue processing
One month after surgery, animals were euthanized with pentobarbital (Fort Dodge Animal Health) and fixed by transcardial perfusion with 4% paraformaldehyde. The spinal column was removed en bloc and postfixed in 4% paraformaldehyde for 48 h. The spinal cord was then removed and postfixed in 4% paraformaldehyde for an additional 24 to 48 h. A 1-cm region of the spinal cord containing the scaffold was embedded in paraffin and 8-μm-thick serial transverse sections cut with a Reichert Jung tabletop microtome (Leica).
Axonal profile counting
Neurofilament staining was used to identify axons within the channels of the scaffold. One slide was randomly selected at three levels through the scaffold in each animal. Slides were warmed in an oven, deparaffinized in 5 changes of xylol, followed by rehydration in descending alcohol concentrations and distilled H2O. Sections were blocked for endogenous peroxidases using 3% hydrogen peroxide (Sigma Aldrich) and antigen retrieval was carried out by steaming in pH 8.0 target retrieval solution (Innogenex). Nonspecific proteins were blocked by incubation in 20% goat serum (Sigma Aldrich) in PBS + 0.4% triton X (Sigma Aldrich) to permeabilize the membrane. The neurofilament primary antibody (monoclonal IgG, 1:100; Dako) was incubated overnight at room temperature. The secondary biotinylated antibody (rat absorbed, rabbit anti-mouse IgG, 1:100; Dako) was incubated for 90 min at room temp, followed by application of a peroxidase-streptavidin (Dako) tertiary link. Staining was detected using diaminobenzidine (Sigma Aldrich), followed by a rinse in distilled H2O and dehydration in ascending alcohol concentrations and xylol. Slides were cover slipped with a synthetic xylol-based mounting medium.
For each animal (n = 8 per group), the total axon number within the scaffold channels was counted by a blinded observer. Images were acquired using a Zeiss AxioCam loaded on a Zeiss Axio Imager Z1 microscope.
Capillary counting
von Willibrand factor staining, a blood glycoprotein produced by the endothelium, was used to identify capillaries within the channels of the scaffold. Slides were selected, deparaffinized, and rehydrated as described above. Antigen retrieval was carried out by steaming in pH 8.0 target retrieval solution (Innogenex) and nonspecific proteins were blocked with 20% goat serum in PBS + 0.4% triton X (Sigma Aldrich). The von Willibrand factor primary antibody (rabbit polyclonal; 1:800; Abcam) was applied for 1 h at room temperature, followed by detection with a Cy3 secondary antibody (goat anti rabbit, 1:50; Dako) for 1 h at room temperature. Slides were cover slipped with Vectashield hard set mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector labs).
For each animal (n = 4 per group), the total capillary number within the scaffold channels was counted by a blinded observer. Images were acquired using a Zeiss AxioCam loaded on a Zeiss Axio Imager.Z1 microscope.
Analysis of scar and cyst formation
The interface (i.e., necrotic tissue between the scaffold and normal spinal cord) rostral and caudal to the implanted scaffold was measured. Slides were selected at 200-μm intervals from the scaffold within this interface region. Slides were selected, deparaffinized, and rehydrated as described above. Sections were stained with hematoxylin (Thermo Fisher Scientific) followed by Gomori's trichrome stain solution (Sigma Aldrich). After rinsing in 1% acetic acid and distilled H2O, sections were dehydrated in ascending alcohol concentrations and five changes of xylol. Slides were cover slipped with a synthetic xylol-based mounting medium.
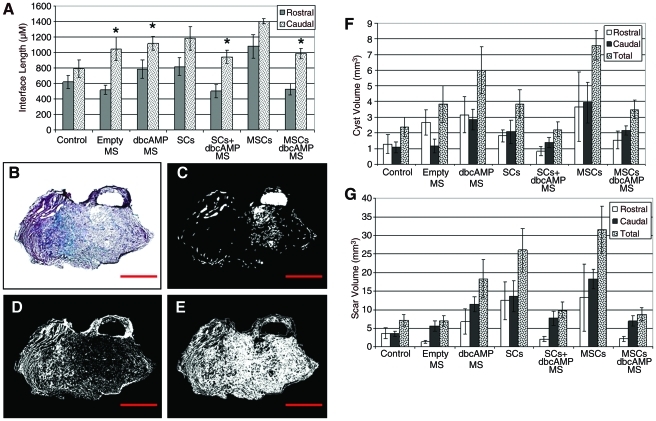
Images were acquired using a Zeiss AxioCam loaded on a Zeiss Axio Imager Z1 microscope. Zeiss KS400 software was used to determine the volume of normal cord, scar formation, and cyst formation. The same parameters for making these measurements were applied to all tissue analyzed. All analyses were carried out by a blinded observer (n = 8 per group). Cysts were identified as fluid-filled cavities (Fig. 8C), whereas scar was identified as spinal cord tissue that was infiltrated with collagen (Fig. 8D), since collagen is not normally found in the spinal cord. The same parameters were used for assessing all images from all groups.
FIG. 8.
Sustained delivery of dbcAMP reduced cyst and scar formation in the presence of SCs or MSCs. Graphic representation of the interface length (i.e., necrotic tissue between the implanted scaffold and normal spinal cord tissue) rostral and caudal to the implanted scaffold (A). Error bars represent standard error of the mean. *p < 0.05 compared to the rostral length. Trichrome staining of collagen fibers (blue) in the transversely sectioned spinal cord (B). (C–E) represent computer-generated images of what was deemed to be cyst (C), scar (D), and normal tissue (E). Graphic representation comparing the volume of cyst (F) and scar (G) formation between treatment groups. This was assessed in the rostral interface, caudal interface, and a combination of both (total). Error bars represent standard error of the mean (n = 8). Color images available online at www.liebertonline.com/tea
Statistical analysis
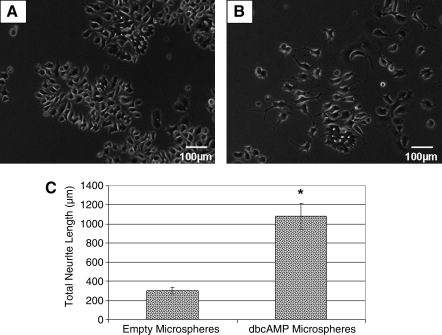
Student's unpaired t-test was used to analyze the neurite outgrowth data after exposure of PC12 cells to encapsulated dbcAMP (Fig. 2).
FIG. 2.
Functionality of dbcAMP was unaffected by the encapsulation process. Light microscopy images of PC12 cells cultured with empty PLGA microspheres (A) or dbcAMP-containing PLGA microspheres (B). Magnification: 100× (A, B). Graphic representation of neurite outgrowth from PC12 cells as measured by ImageJ software (C). Error bars represent standard error of the mean (n = 5). *p = 0.0007.
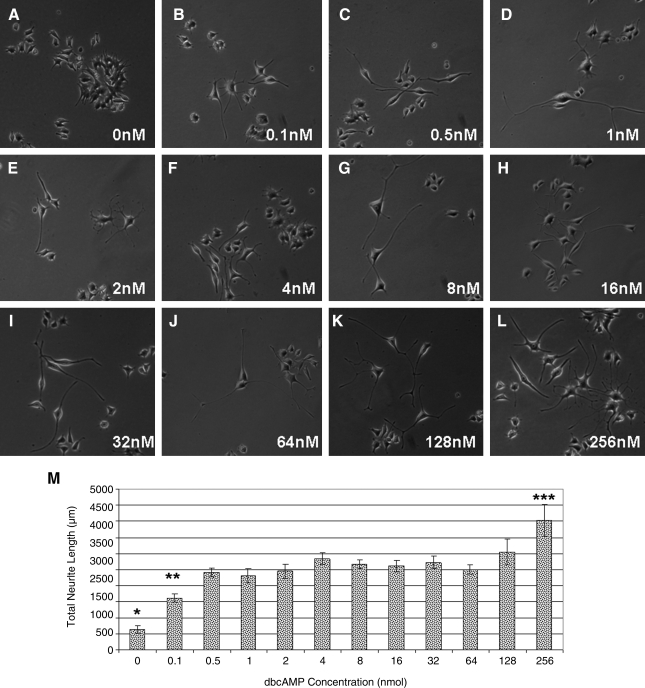
One-way analysis of the variance was used to determine if there was a significant difference in neurite length after exposure to different concentrations of dbcAMP, with post hoc analysis performed using the Bonferroni test (Fig. 3).
FIG. 3.
Low concentrations of dbcAMP were required to promote neurite outgrowth. Light microscopy images of PC12 cells exposed to 0, 0.1, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, and 256 nmol dbcAMP (A–L). Magnification: 100 × (A–L). Graphic representation of neurite outgrowth from PC12 cells as measured by ImageJ software (M). Error bars represent standard error of the mean (n = 9). *p < 0.001 compared to all other samples. **p < 0.05 compared to 4, 128, and 256 nmol samples. ***p < 0.05 compared to all other samples except 128 nmol sample.
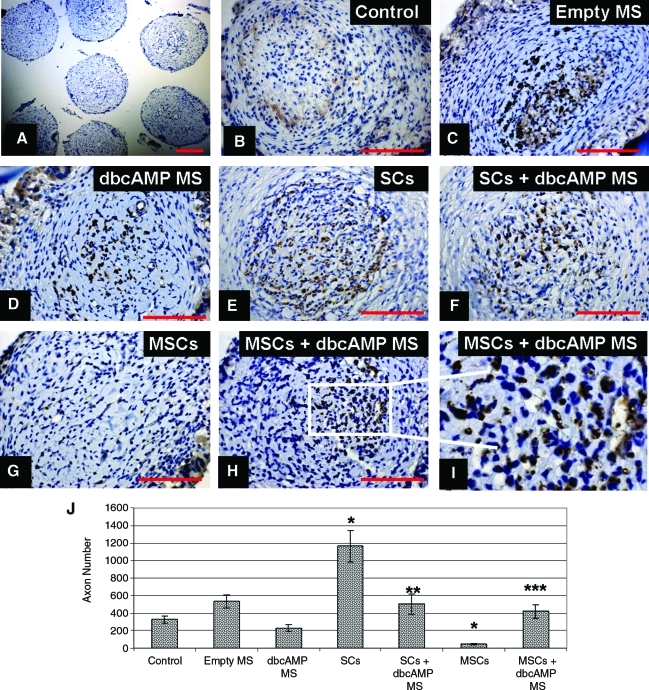
Nonparametric analysis of the variance (Kruskal–Wallis test) was used to determine if there was a significant difference in median neurofilament axonal counts, capillary counts, scar and cyst formation, and BBB scores between the groups (Figs. 6–9).
FIG. 6.
Sustained delivery of dbcAMP alone and in the presence of SCs inhibited axonal regeneration but rescued MSC-induced inhibition of axonal regeneration. Light microscopy analysis of neurofilament-labeled axons (brown) and hematoxylin-labeled nuclei (blue) in the transversely sectioned scaffold (A) at a low magnification (100×) and at higher magnification (400×) in the control (B), empty MS (C), dbcAMP MS (D), SCs (E), SCs + dbcAMP (F), MSCs (G), and MSCs + dbcAMP (H, I) groups. Scale bar: 200 μm (A) and 100 μm (B–H). Graphic representation of axonal counts between the different treatment groups (J). Error bars represent standard error of the mean (n = 8). *p < 0.05 compared to the control group. **p < 0.05 compared to the SC group. ***p < 0.05 compared to the MSC group. SC, Schwann cell; MSC, mesenchymal stem cell. Color images available online at www.liebertonline.com/tea
FIG. 9.
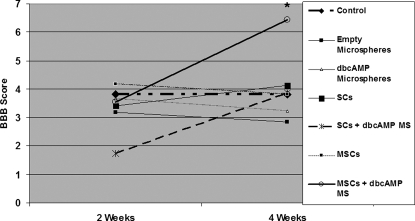
Sustained delivery of dbcAMP in the presence of MSCs significantly improved functional recovery 4 weeks post-transection. Graphic representation of Basso, Beattie and Bresnahan (BBB) scores in the following groups: control (receiving matrigel-filled OPF scaffolds), empty microspheres (receiving matrigel-filled OPF scaffolds with embedded empty microspheres), dbcAMP microspheres (receiving matrigel-filled OPF scaffolds with embedded dbcAMP-containing microspheres), SCs (receiving SC-filled OPF scaffolds), SCs + dbcAMP (receiving SC-filled OPF scaffolds with embedded dbcAMP-containing microspheres), MSCs (receiving MSC-filled OPF scaffolds), and MSCs + dbcAMP (receiving MSC-filled OPF scaffolds with embedded dbcAMP-containing microspheres). Error bars represent standard error of the mean (n = 8). *p < 0.05 when compared to the empty microsphere and dbcAMP microsphere groups.
Values of p ≤ 0.05 were considered statistically significant. Graph Pad Prism 4 software was used for all analyses.
Results
Encapsulation of dbcAMP in PLGA microspheres led to prolonged release in vitro
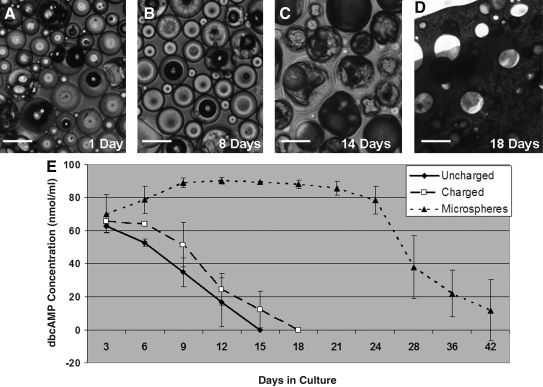
dbcAMP was encapsulated within 50:50 PLGA microspheres using a water-in-oil-in-water double emulsion, solvent evaporation technique. Light microscopy analysis revealed degradation of the microspheres over time when cultured at 37°C in tissue culture media (Fig. 1A–D).
FIG. 1.
Encapsulation of dbcAMP in PLGA microspheres lead to prolonged release in vitro. Light microscopy images of 50:50 PLGA microspheres degrading over time (A–D). Magnification: 100×(A–D). Scale bar: 100 μm. Graphic representation of dbcAMP release from PLGA microspheres embedded in OPF discs, from uncharged OPF discs without microspheres and from positively charged OPF discs without microspheres (E). Error bars represent standard deviation (n = 3). OPF, oligo [(polyethylene glycol) fumarate]; dbcAMP, dibutyryl cyclic adenosine monophosphate; PLGA, poly(lactic-co-glycolic acid).
Incorporation of these microspheres into OPF discs and subsequent culture at 37°C in tissue culture media revealed a stable release of dbcAMP over a 42-day period (Fig. 1E). The highest concentration of dbcAMP to be released was 90.25 ± 2.14 nmol/ml at the 12-day time-point. Approximately the same concentration of dbcAMP was released at each time-point from 12 to 24 days, at which time dbcAMP concentration progressively decreased to 11.89 ± 18.28 nmol/ml at 42 days.
As a control, dbcAMP was directly loaded into either charged or uncharged OPF (without microspheres), which revealed a faster rate of release over an 18-day period (Fig. 1E). The highest concentration released here was 65.73 ± 1.75 nmol/ml at the 3-day time-point, which instantly decreased over a 15–18-day period for uncharged and charged OPF, respectively.
Functionality of dbcAMP was unaffected by the encapsulation process
Either dbcAMP or empty microspheres were loaded into OPF discs, which were cultured with PC12 cells. Light microscopic images were acquired randomly from all of the samples (n = 5) and neurite outgrowth was measured using ImageJ software.
OPF discs loaded with dbcAMP microspheres revealed significantly greater neurite outgrowth (1079 ± 135.59 μm) (Fig. 2B, C) compared with those loaded with empty microspheres (302.63 ± 33.37 μm) (Fig. 2A, C) (p = 0.0007).
Low concentrations of dbcAMP were required to promote neurite outgrowth
To assess the minimum concentration and the dose–response characteristics of dbcAMP on neurite outgrowth, dbcAMP was added to PC12 cells at concentrations of 0, 0.1, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, and 256 nmol. Light microscopic images were acquired randomly from all of the samples (n = 9) and neurite outgrowth was measured using ImageJ software. Significantly greater neurite outgrowth was observed in all treated samples compared with the untreated control samples (Fig. 3A–M) (p < 0.001). There was no significant difference in neurite outgrowth observed between those samples receiving 0.5 to 128 nmol dbcAMP (Fig. 3C–K, M). Neurite outgrowth ranged from 2410.95 ± 138.16 to 3036.88 ± 398.95 μm from 0.5 to 128 nmol, respectively. The most extensive neurite outgrowth (4029.87 ± 495.67) was observed in those samples treated with 256 nmol dbcAMP, which was significantly greater than all other samples except those treated with 128 nmol dbcAMP (p < 0.05).
Microspheres could be successfully loaded into hydrogel scaffolds and delivered to the transected spinal cord
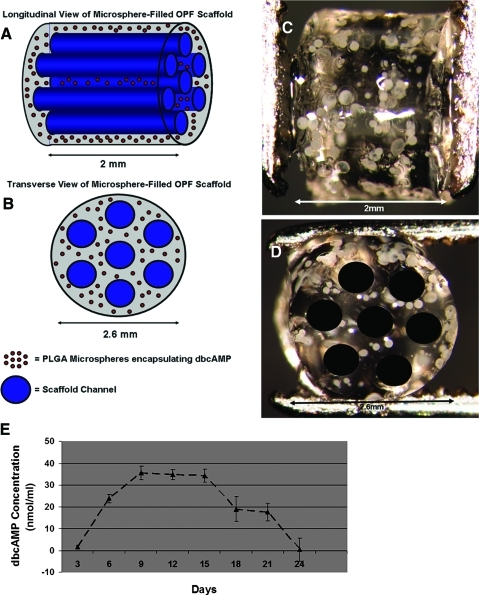
The OPF–microsphere solution was injected into a glass mold with seven wires, resulting in cylindrical scaffolds with a length and diameter of 2 and 2.6 mm, respectively (Fig. 4A–D). The release profile of dbcAMP from these 2-mm scaffolds is shown in Figure 4E. At 3 days, the scaffold releases 2.5 ng/mL, which increases to 35 ng/mL at 9 days and steadily decreases over a 24-day period (Fig. 4E).
FIG. 4.
Microspheres could be successfully loaded into hydrogel scaffolds and delivered to the transected spinal cord. Schematic (A, B) and light microscopy (C, D) images of longitudinal (A, C) and transverse (B, D) views of the 7 channel OPF scaffold with embedded microspheres. Magnification: 25× (C, D). Graphic representation of dbcAMP secreted from 2 mm cut OPF scaffold (E). Error bars represent standard deviation (n = 3). Color images available online at www.liebertonline.com/tea
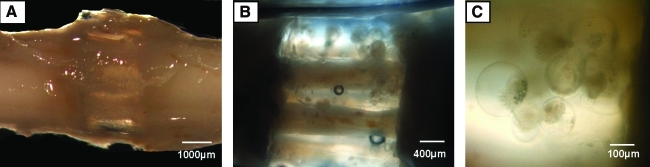
These scaffolds were then successfully delivered to the transected spinal cord, with tissue observed within the scaffold channels 1 month postimplantation (Fig. 5A, B). At this time, microspheres were shown to degrade within the pores they initially filled in the OPF scaffold before implantation (Fig. 5C).
FIG. 5.
Microspheres degraded within the pores they formed in the OPF scaffold postimplantation into the transected spinal cord. Light microscopy images of OPF scaffolds filled with microspheres, 1 month postimplantation into the transected rat spinal cord (A–C). Magnification: 8× (A), 25× (B), and 100× (C). Color images available online at www.liebertonline.com/tea
Sustained delivery of dbcAMP alone and in the presence of SCs inhibited axonal regeneration
Neurofilament staining was used to identify axon profiles within the scaffold channels, with hematoxylin used to counterstain the nuclei (Fig. 6A–I). Axons were counted by a blinded observer under a 40× oil objective (Fig. 6B–H). Quantification of the diaminobenzidine-stained axons revealed that significantly more regeneration occurred in the group that received SCs (1165 ± 178 axons) (Fig. 6E, J) compared with the control (327 ± 44 axons) (Fig. 6B, J) (p < 0.05).
However, when a sustained release of dbcAMP was combined with SC delivery, this regeneration was significantly reduced (502 ± 118 axons) (Fig. 6F, J) (p < 0.05). This was consistent with the finding that sustained delivery of dbcAMP alone revealed a trend toward decreased regeneration (229 ± 37 axons) when compared with the control group (327 ± 44 axons) (Fig. 6D, J). We demonstrate that this decrease in regeneration was not due to the presence of the microspheres themselves, since delivery of empty microspheres did not negatively effect regeneration (535 ± 76 axons) (Fig. 6C, J).
Sustained delivery of dbcAMP rescued MSC-induced inhibition of axonal regeneration
When sustained release of dbcAMP was combined with MSC transplantation, the inhibitory effect of MSCs on regeneration was negated (Fig. 6G–J). Delivery of MSCs alone was shown to significantly decrease regeneration (45 ± 12 axons) compared with the control (327 ± 44 axons), whereas in combination with dbcAMP, significantly more regeneration (420 ± 80 axons) was seen compared with the group receiving MSCs alone (Fig. 6J) (p < 0.05). There was also a trend toward increased regeneration in the MSCs + dbcAMP MS group compared with the dbcAMP MS group (Fig. 6J).
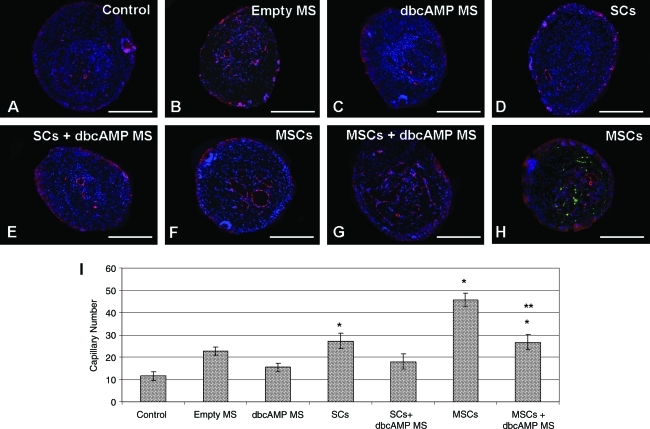
Sustained delivery of dbcAMP reduced capillary formation in the presence of SCs and MSCs
von Willebrand factor staining was used to identify capillaries within the scaffold channels, with DAPI used to stain the nuclei (Fig. 7A–H). Capillaries were counted by a blinded observer under a 20× objective (Fig. 7I). To assess the localization of GFP-MSCs with von Willebrand factor–labeled endothelial cells, images from the fluorescein isothiocyanate (FITC), tetramethyl rhodamine isothiocyanate (TRITC), and DAPI channels were taken and merged (Fig. 7H). Although MSCs were observed in the vicinity of the endothelial cells, they did not co-localize with them.
FIG. 7.
Sustained delivery of dbcAMP reduced capillary formation in the presence of SCs and MSCs. Fluorescent microscopy analysis of von Willebrand factor–labeled capillaries (red) and 4′,6-diamidino-2-phenylindole (DAPI)-labeled nuclei (A–G) in the transversely sectioned scaffold in the control (A), empty MS (B), dbcAMP MS (C), SCs (D), SCs + dbcAMP (E), MSCs (F), and MSCs+dbcAMP (G) groups. Green fluorescent protein expression was also observed in the groups that received green fluorescent protein MSCs (H). Scale bar: 100 μm (A–H). Magnification: 400× (A–H). Graphic representation of capillary counts between the different treatment groups (I). Error bars represent standard error of the mean (n = 4). *p < 0.05 compared to the control group. **p < 0.05 compared to the MSC group. Color images available online at www.liebertonline.com/tea
Quantification of von Willebrand factor–stained capillaries revealed that significantly more capillaries were observed in those animals that received SCs (27.25 ± 3.45) (p < 0.05), MSCs (45.67 ± 3.05) (p < 0.001), or MSCs + dbcAMP MS (26.67 ± 3.48) (p < 0.05) compared with the control (11.58 ± 1.94) (Fig. 7I). The presence of dbcAMP significantly reduced capillary formation in the group that received MSCs + dbcAMP (26.67 ± 3.48) compared with the group that received MSCs alone (45.67 ± 3.05) (p < 0.05) (Fig. 7I). There was also a trend toward reduced capillary formation in the group that received SCs + dbcAMP (18 ± 3.43) compared with the group that received SCs alone (27.25 ± 3.45) (Fig. 7I).
When axon counts were plotted against capillary counts in each analyzed section, no correlation was evident (R2 = 0.0008).
Sustained delivery of dbcAMP reduced cyst formation in the presence of SCs or MSCs
Interface measurements were made rostral and caudal to the implanted scaffold (Fig. 8A). The interface was defined as necrotic tissue between the scaffold and normal spinal cord. While no significant differences were seen in the interface lengths between groups, the caudal interface was greater than the rostral interface in all groups (Fig. 8A). This difference was significant in the groups that received empty MS, dbcAMP, SCs + dbcAMP, and MSCs + dbcAMP (p < 0.05).
Gomori's trichrome stain solution was used to identify collagen fibers infiltrating the spinal cord (Fig. 8B). These were blue/green in color. Zeiss KS400 software was used to determine the volume of cyst and scar formation. The same parameters were used to identify cyst (Fig. 8C), scar (Fig. 8D), and normal spinal cord (Fig. 8E) in all groups. No significant differences were noted between rostral and caudal cyst measurements within the groups analyzed (Fig. 8F). When the total volume occupied by cyst was calculated, the greatest cyst formation was observed in the groups that received dbcAMP (5.99 ± 1.5 mm3) or MSCs (7.59 ± 0.92 mm3) compared with the control (2.37 ± 0.62 mm3), empty MS (3.82 ± 1.15 mm3), SCs (3.84 ± 0.9 mm3), SCs + dbcAMP (2.21 ± 0.48 mm3), and MSCs + dbcAMP (3.49 ± 0.57 mm3) groups. Interestingly, the presence of dbcAMP with SCs (2.21 ± 0.48 mm3) or MSCs (3.49 ± 0.57 mm3) reduced cyst formation compared with those groups that received SCs (3.84 ± 0.9 mm3) or MSCs (7.59 ± 0.92 mm3) alone.
Sustained delivery of dbcAMP reduced scar formation in the presence of SCs or MSCs
As with analysis of cyst volume, no significant differences were noted between rostral and caudal scar measurements within the groups analyzed (Fig. 8G). When the total volume occupied by scar was calculated, the greatest scar formation was observed in the groups that received dbcAMP (18.34 ± 5.13 mm3), SCs (26.08 ± 5.91 mm3), or MSCs (31.57 ±6.41 mm3) compared with the control (7.24 ± 1.52 mm3), empty MS (7.03 ± 1.36 mm3), SCs + dbcAMP (9.89 ± 2.12 mm3), and MSCs + dbcAMP (8.81 ± 1.8 mm3) groups. As with our cyst volume observation, the presence of dbcAMP with SCs (9.89 ± 2.12 mm3) or MSCs (8.81 ± 1.8 mm3) reduced scar formation compared with those groups that received SCs (26.08 ± 5.91 mm3) or MSCs (31.57 ± 6.41 mm3) alone.
Sustained delivery of dbcAMP in the presence of MSCs significantly improved functional recovery 4 weeks post-transection
The BBB locomotor rating scale was used to assess functional recovery of the animal's motor skills 2 and 4 weeks post-transection injury (Fig. 9). No significant differences were observed between the animals at the 2-week time-point, with recorded scores of 3.81 ± 0.82, 3.19 ± 0.71, 3.67 ± 0.57, 3.41 ± 0.94, 1.74 ± 0.25, 4.19 ± 0.70, and 3.56 ± 0.63 for the control, empty MS, dbcAMP MS, SCs, SCs + dbcAMP, MSCs, and MSCs + dbcAMP groups, respectively.
At 4 weeks, the group receiving MSCs + dbcAMP showed a significant improvement in BBB score (6.44 ± 0.26) when compared with the groups receiving empty MS (2.85 ± 0.54) or dbcAMP MS (3.22 ± 0.69) (p < 0.05). The control, SCs, SCs + dbcAMP, and MSCs group had scores of 3.81 ± 0.77, 4.13 ± 1.07, 3.85 ± 0.39, and 3.81 ± 0.77, respectively.
Discussion
The objective of this study was to design a novel method of sustained delivery of potentially therapeutic agents to the transected spinal cord. This was accomplished by incorporating dbcAMP-encapsulating PLGA microspheres in an OPF hydrogel, which was then molded into the cylindrical shape of the spinal cord. Subsequent transplantation of this microsphere-embedded scaffold facilitated study of the biological effects of dbcAMP in the presence of no implanted cells, SCs, or MSCs within the injured spinal cord. We report biological effects as a result of this dbcAMP delivery in vitro and in vivo and propose that this delivery platform may be suitable for testing the effects of other potentially therapeutic agents on SCI.
This method of embedding microspheres within the scaffold architecture represents an attractive approach to microsphere delivery within the transected spinal cord. An alternative approach would have been to deliver microspheres within the channels.56 However, this approach introduces a number of complications such as (1) potential obstruction of regeneration and (2) inability to utilize the channels for cell delivery. Another limiting factor is channel size. Smaller diameter channels have been deemed optimal for spinal regeneration,59 which limits the maximum size of microspheres that could potentially be loaded within the channel itself. Our delivery method enables release of the therapeutic agent from the microsphere-embedded scaffold, without occupying the channel spaces designed for regenerating axons and cell transplantation. Further, we demonstrate that microspheres embedded within the OPF hydrogel degrade within the pores they originally occupied, confirming that they do not degrade or swell into the channels themselves.
The in vitro studies served as a platform to establish release and biological activity of dbcAMP. We confirm that the ability to control the release of compounds using microsphere technology remains unaffected by incorporation into a hydrogel substrate. Encapsulation of dbcAMP within 50:50 PLGA microspheres lead to a more prolonged release of the compound compared with direct injection into OPF in vitro. Increases in intracellular cAMP concentrations have been shown to enhance neurite outgrowth in PC12 cells.61–64 We demonstrate that exposure of PC12 cells to the dbcAMP-encapsulating microspheres resulted in neurite outgrowth, thereby confirming that functionality was unaffected by the encapsulation process.
Generation of dbcAMP-releasing scaffolds was achieved by injecting the PLGA microsphere–OPF hydrogel solution into the glass cylindrical mold and inserting seven parallel wires. We were initially concerned that the size of the scaffold needed for implantation into the spinal cord would limit the volume of dbcAMP microspheres, which could be incorporated, thereby reducing any potential effects. However, we showed that low concentrations of dbcAMP (0.5 nmol) could promote the same extent of neurite outgrowth as higher concentrations (128 nmol). This data coupled with the scaffold release data instilled confidence that our delivery system would provide enough dbcAMP to produce biological effects in an in vivo setting.
Re-establishing motor functionality below the site of an SCI requires that axons regenerate through the site of injury and form functional synapses with their targets. Assessment of this regeneration through the implanted scaffold was performed by staining for the neuron-specific neurofilament. Transversely sectioned axon profiles could then be counted using previously validated methods.58,59 The rationale for elevating cAMP at the site of injury stemmed from previous research demonstrating its effectiveness in promoting extension of axons to their peripheral targets.51–55,65 Despite these previous findings, our results pointed toward a dbcAMP-induced inhibition of axonal regeneration. This was significant when one compared the regenerative potential of SCs versus SCs + dbcAMP. SCs have consistently been shown to enhance axonal regeneration within the peripheral and central nervous system.14,66,67 However, when coupled with sustained delivery of dbcAMP, this regenerative potential was lost. Axonal counts for animals receiving SCs + dbcAMP were comparable with those observed in the control group (which showed a base-line level of endogenous axonal regeneration) and were significantly lower than those animals receiving SCs alone.
Contrary to these observations, in the presence of MSCs, dbcAMP was shown to rescue MSC-induced inhibition of axonal regeneration. The reason for these conflicting results is unclear. dbcAMP may be exerting varying effects on the SCs and MSCs, thereby altering their regenerative potential. Previous studies indicated that exposure of SCs to cAMP elevators induced expression of galactocerebroside and other molecules characteristic of mature SCs68–72 and resulted in differential expression of extracellular matrix genes.73 We speculate that the regenerative potential of SCs in the injured CNS requires maintenance of the cells in a dedifferentiated state. In the case of MSCs, cAMP elevators have been shown to stimulate neuron-like morphology.74,75 However, these morphological changes were not consistent with a real process of transdifferentiation into mature functional neurons.76,77 Indeed, specific application of dbcAMP to MSCs has been shown to have cytotoxic effects.76 These effects could potentially be involved in negating inhibition of axonal regeneration in the presence of dbcAMP.
To establish if differing effects of dbcAMP in the presence of SCs versus MSCs were due to histopathological variations, we assessed the volume of scar and cyst formation in the interface rostral and caudal to the implanted scaffold. Interestingly, we found that in the presence of SCs and MSCs, dbcAMP showed a trend toward reduced cyst and scar formation (when compared with those groups receiving SCs or MSCs alone). This is contradictory to the findings of Fouad et al., who reported deleterious effects of continuous cAMP delivery in the injured CNS.78 These researchers delivered cAMP analogs via osmotic minipumps for up to 4 weeks in two models of incomplete SCI. They reported an absence of progressive locomotor recovery, sporadic micro-hemorrhage formation and cavitation, enhanced macrophage infiltration, and tissue damage at regions beyond the immediate application site. These conflicting findings may be attributable to application of lower levels of dbcAMP, or to the use of a transection SCI model compared with an incomplete SCI model.
Disruption of the vasculature can exacerbate damage following SCI, thereby limiting restoration of function.79–81 Restoring oxygen and glucose supply to cells in the vicinity of the injury requires neovascularization of the lesioned area. To assess if increased axonal regeneration is associated with increased capillary formation and with differences in the effects of dbcAMP in the presence of SCs versus MSCs, von Willebrand factor staining of endothelial cells was performed on the transversely sectioned scaffold. SCs and MSCs were shown to significantly increase the number of capillaries formed compared with the control group. The greatest level of capillary formation occurred in the group receiving MSCs. This is not surprising given previous findings demonstrating the angiogenic differentiation potential of MSCs in vitro and in other injury models.82–85 In this study, we suggest that the lack of co-localization of the transplanted GFP-MSCs with the von Willebrand factor–stained endothelial cells points toward a promotion of angiogenesis by MSCs rather than the differentiation of MSCs themselves into endothelial cells. Indeed, we have previously demonstrated that MSCs remain in an undifferentiated state following delivery to the injured spinal cord.57 It is also worth noting that a perivascular origin for MSCs has been hypothesized.86 The rationale for this hypothesis comes from a very elegant study by Crisan et al. Cell sorting for pericytes and subsequent expansion showed that these cells were multipotent for osteogenic, chondrogenic, adipogenic, and myogenic lineages, all hallmarks of MSCs.87 In our study, the presence of dbcAMP significantly decreased capillary number in the group receiving MSCs, suggesting an alteration of MSC angiogenic potential resulting from sustained dbcAMP delivery. This result was surprising to us. One would have expected to see increased angiogenesis in the animals that received both MSCs + dbcAMP, given that MSC inhibition of axonal regeneration is negated in the presence of dbcAMP. This, however, was not the case. We speculate that the presence of dbcAMP results in decreased functionality of MSCs (i.e., reduced inhibition of axonal regeneration and reduced angiogenesis).
The MSCs + dbcAMP group also showed a significant improvement in motor function when compared with all other groups at 4 weeks. However, SCs were shown to promote the greatest level of axonal regeneration, which was not coupled with such functional improvements. This difference in functionality between the groups may be attributable to (1) the generation of more functionally mature neurons; (2) successful synapsing of regenerating axons with their targets; or (3) reduced tissue destruction (as was observed in the presence of dbcAMP).
Our findings raise a number of questions with regard to the mechanisms involved in (1) dbcAMP-mediated inhibition of axonal regeneration in the presence of SCs; (2) dbcAMP-mediated rescue of MSC-induced inhibition of axonal regeneration; (3) dbcAMP-mediated reduction of capillary formation in the presence of MSCs; and (4) functional improvements brought about by MSCs + dbcAMP delivery. We aim to address these questions in future studies.
Conclusions
Our findings demonstrate the feasibility of incorporating PLGA microsphere technology for spinal cord transection studies. It represents a novel sustained delivery mechanism within the transected spinal cord and provides a platform for potential delivery of other therapeutic agents. Biological function of the encapsulated dbcAMP was demonstrated in both in vitro and in vivo environments, confirming the effectiveness of such a delivery platform.
Acknowledgments
Grant support was provided by the NIBIB (EB02390), the Morton Cure Paralysis Foundation, and the Mayo Foundation. We sincerely thank Jim Tarara for his technical assistance with quantification of scar and cyst formation. The administrative support of Ms. Jane Meyer is also greatly appreciated.
Disclosure Statement
No competing financial interests exist between any of the authors.
References
- 1.Quencer R.M. Bunge R.P. The injured spinal cord: imaging, histopathologic clinical correlates, and basic science approaches to enhancing neural function after spinal cord injury. Spine. 1996;21:2064. doi: 10.1097/00007632-199609150-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bodley R. Imaging in chronic spinal cord injury—indications and benefits. Eur J Radiol. 2002;42:135. doi: 10.1016/s0720-048x(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 3.Tadie M. Liu S. Robert R. Guiheneuc P. Pereon Y. Perrouin-Verbe B. Mathe J.F. Partial return of motor function in paralyzed legs after surgical bypass of the lesion site by nerve autografts three years after spinal cord injury. J Neurotrauma. 2002;19:909. doi: 10.1089/089771502320317069. [DOI] [PubMed] [Google Scholar]
- 4.Murphy W. Peters M. Kohn D. Mooney D. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 5.Lu L. Zhu X. Valenzuela R. Currier B. Yaszemski M. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin Orthop. 2001;391:S251. doi: 10.1097/00003086-200110001-00024. [DOI] [PubMed] [Google Scholar]
- 6.Maquet V. Martin D. Scholtes F. Franzen R. Schoenen J. Moonen G. Jerme R. Poly(D,L-lactide) foams modified by poly(ethylene oxide)-block-poly(D,L-lactide) copolymers and a-FGF: in vitro and in vivo evaluation for spinal cord regeneration. Biomaterials. 2001;22:1137. doi: 10.1016/s0142-9612(00)00357-4. [DOI] [PubMed] [Google Scholar]
- 7.Jain R. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 8.Eliaz R. Wallach D. Kost J. Delivery of soluble tumor necrosis factor receptor from in-situ forming PLGA implants: in-vivo. Pharm Res. 2000;17:1546. doi: 10.1023/a:1007621512647. [DOI] [PubMed] [Google Scholar]
- 9.Gumusderelioglu M. Deniz G. Sustained release of mitomycin-C from poly(dl-lactide)/poly(dl-lactide-co-glycolide) films. J Biomater Sci Polym Ed. 2000;11:1039. doi: 10.1163/156856200743562. [DOI] [PubMed] [Google Scholar]
- 10.Xu X. Yee W.C. Hwang P.Y. Yu H. Wan A.C. Gao S. Boon K.L. Mao H.Q. Leong K.W. Wang S. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24:2405. doi: 10.1016/s0142-9612(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 11.Friedman J. Windebank A. Moore M. Spinner R. Currier B. Yazemski M. Biodegradeable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery. 2002;51:742. [PubMed] [Google Scholar]
- 12.Bunge M. Bridging areas of injury in the spinal cord. Neuroscientist. 2001;7:325. doi: 10.1177/107385840100700409. [DOI] [PubMed] [Google Scholar]
- 13.Bunge M.B. Bridging the transected or contused adult rat spinal cord with Schwann cell and olfactory ensheathing glia transplants. Prog Brain Res. 2002;137:275. doi: 10.1016/s0079-6123(02)37021-3. [DOI] [PubMed] [Google Scholar]
- 14.Takami T. Oudega M. Bates M.L. Wood P.M. Kleitman N. Bunge M.B. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novikova L.N. Novikov L.N. Kellerth J.O. Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Curr Opin Neurol. 2003;16:711. doi: 10.1097/01.wco.0000102620.38669.3e. [DOI] [PubMed] [Google Scholar]
- 16.Novikova L.N. Pettersson J. Brohlin M. Wiberg M. Novikov L.N. Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials. 2008;29:1198. doi: 10.1016/j.biomaterials.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig E.S. McDonald J.W. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17:121. doi: 10.1097/00019052-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Xu X.M. Guenard V. Kleitman N. Bunge M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 19.Moore M.J. Friedman J.A. Lewellyn E.B. Mantila S.M. Krych A.J. Ameenuddin S. Knight A.M. Lu L. Currier B.L. Spinner R.J. Marsh R.W. Windebank A.J. Yaszemski M.J. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 20.Friedman J.A. Windebank A.J. Moore M.J. Spinner R.J. Currier B.L. Yaszemski M.J. Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery. 2002;51:742. [PubMed] [Google Scholar]
- 21.Jo S. Shin H. Mikos A.G. Modification of oligo(poly(ethylene glycol) fumarate) macromer with a GRGD peptide for the preparation of functionalized polymer networks. Biomacromolecules. 2001;2:255. doi: 10.1021/bm000107e. [DOI] [PubMed] [Google Scholar]
- 22.Shin H. Quinten Ruhe P. Mikos A.G. Jansen J.A. In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials. 2003;24:3201. doi: 10.1016/s0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 23.Temenoff J.S. Athanasiou K.A. LeBaron R.G. Mikos A.G. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59:429. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 24.Temenoff J.S. Steinbis E.S. Mikos A.G. Effect of drying history on swelling properties and cell attachment to oligo(poly(ethylene glycol) fumarate) hydrogels for guided tissue regeneration applications. J Biomater Sci Polym Ed. 2003;14:989. doi: 10.1163/156856203322381465. [DOI] [PubMed] [Google Scholar]
- 25.Shin H. Jo S. Mikos A.G. Modulation of marrow stromal osteoblast adhesion on biomimetic oligo[poly(ethylene glycol) fumarate] hydrogels modified with Arg-Gly-Asp peptides and a poly(ethyleneglycol) spacer. J Biomed Mater Res. 2002;61:169. doi: 10.1002/jbm.10193. [DOI] [PubMed] [Google Scholar]
- 26.Shin H. Zygourakis K. Farach-Carson M.C. Yaszemski M.J. Mikos A.G. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials. 2004;25:895. doi: 10.1016/s0142-9612(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 27.Dadsetan M. Hefferan T.E. Szatkowski J.P. Mishra P.K. Macura S.I. Lu L. Yaszemski M.J. Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials. 2008;29:2193. doi: 10.1016/j.biomaterials.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin H. Temenoff J.S. Bowden G.C. Zygourakis K. Farach-Carson M.C. Yaszemski M.J. Mikos A.G. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26:3645. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 29.Shin H. Zygourakis K. Farach-Carson M.C. Yaszemski M.J. Mikos A.G. Modulation of differentiation and mineralization of marrow stromal cells cultured on biomimetic hydrogels modified with Arg-Gly-Asp containing peptides. J Biomed Mater Res A. 2004;69:535. doi: 10.1002/jbm.a.30027. [DOI] [PubMed] [Google Scholar]
- 30.Temenoff J.S. Park H. Jabbari E. Conway D.E. Sheffield T.L. Ambrose C.G. Mikos A.G. Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules. 2004;5:5. doi: 10.1021/bm030067p. [DOI] [PubMed] [Google Scholar]
- 31.Temenoff J.S. Park H. Jabbari E. Sheffield T.L. LeBaron R.G. Ambrose C.G. Mikos A.G. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70:235. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 32.Dadsetan M. Szatkowski J.P. Yaszemski M.J. Lu L. Characterization of photo-cross-linked oligo[poly(ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules. 2007;8:1702. doi: 10.1021/bm070052h. [DOI] [PubMed] [Google Scholar]
- 33.Kasper F.K. Jerkins E. Tanahashi K. Barry M.A. Tabata Y. Mikos A.G. Characterization of DNA release from composites of oligo(poly(ethylene glycol) fumarate) and cationized gelatin microspheres in vitro. J Biomed Mater Res A. 2006;78:823. doi: 10.1002/jbm.a.30736. [DOI] [PubMed] [Google Scholar]
- 34.Kasper F.K. Kushibiki T. Kimura Y. Mikos A.G. Tabata Y. In vivo release of plasmid DNA from composites of oligo(poly(ethylene glycol)fumarate) and cationized gelatin microspheres. J Control Release. 2005;107:547. doi: 10.1016/j.jconrel.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Holland T.A. Tabata Y. Mikos A.G. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Dadsetan M. Knight A.M. Lu L. Windebank A.J. Yaszemski M.J. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials. 2009;30:3874. doi: 10.1016/j.biomaterials.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rooney G.E. Moran C. McMahon S.S. Ritter T. Maenz M. Flugel A. Dockery P. O'Brien T. Howard L. Windebank A.J. Barry F.P. Gene-modified mesenchymal stem cells express functionally active nerve growth factor on an engineered poly lactic glycolic acid (PLGA) substrate. Tissue Eng Part A. 2008;14:681. doi: 10.1089/tea.2007.0260. [DOI] [PubMed] [Google Scholar]
- 38.Wu X.S. Wang N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: biodegradation. J Biomater Sci Polym Ed. 2001;12:21. doi: 10.1163/156856201744425. [DOI] [PubMed] [Google Scholar]
- 39.Cohen S. Yoshioka T. Lucarelli M. Hwang L.H. Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8:713. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 40.Bilati U. Allemann E. Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Lee J. Lee K.Y. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739. doi: 10.1007/s11095-009-9884-4. [DOI] [PubMed] [Google Scholar]
- 42.Wang X. Wenk E. Zhang X. Meinel L. Vunjak-Novakovic G. Kaplan D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134:81. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaklenec A. Hinckfuss A. Bilgen B. Ciombor D.M. Aaron R. Mathiowitz E. Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials. 2008;29:1518. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Jiang W. Gupta R.K. Deshpande M.C. Schwendeman S.P. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Jaklenec A. Wan E. Murray M.E. Mathiowitz E. Novel scaffolds fabricated from protein-loaded microspheres for tissue engineering. Biomaterials. 2008;29:185. doi: 10.1016/j.biomaterials.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Kasper F.K. Seidlits S.K. Tang A. Crowther R.S. Carney D.H. Barry M.A. Mikos A.G. In vitro release of plasmid DNA from oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2005;104:521. doi: 10.1016/j.jconrel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Rydel R.E. Greene L.A. cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc Natl Acad Sci USA. 1988;85:1257. doi: 10.1073/pnas.85.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer-Franke A. Kaplan M.R. Pfrieger F.W. Barres B.A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 49.Song H.J. Ming G.L. Poo M.M. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 50.Kao H.T. Song H.J. Porton B. Ming G.L. Hoh J. Abraham M. Czernik A.J. Pieribone V.A. Poo M.M. Greengard P. A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci. 2002;5:431. doi: 10.1038/nn840. [DOI] [PubMed] [Google Scholar]
- 51.Pearse D.D. Pereira F.C. Marcillo A.E. Bates M.L. Berrocal Y.A. Filbin M.T. Bunge M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 52.Lu P. Yang H. Jones L.L. Filbin M.T. Tuszynski M.H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neumann S. Bradke F. Tessier-Lavigne M. Basbaum A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 54.Qiu J. Cai D. Dai H. McAtee M. Hoffman P.N. Bregman B.S. Filbin M.T. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 55.Deshpande D.M. Kim Y.S. Martinez T. Carmen J. Dike S. Shats I. Rubin L.L. Drummond J. Krishnan C. Hoke A. Maragakis N. Shefner J. Rothstein J.D. Kerr D.A. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006;60:32. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- 56.de Boer R. Knight A.M. Spinner R.J. Malessy M. Yaszemski M.J. Windebank A.J. In vitro and in vivo release of nerve growth factor from biodegradable poly-lactic-co-glycolic-acid microspheres. J Biomed Mater Res Part A. 2010;95:1067. doi: 10.1002/jbm.a.32900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rooney G.E. McMahon S.S. Ritter T. Garcia Y. Moran C. Madigan N.N. Fluegel A. Dockery P. O'Brien T. Howard L. Windebank A.J. Barry F. Neurotrophic factor-expressing mesenchymal stem cells survive transplantation into the contused spinal cord without differentiating into neural cells. Tissue Eng Part A. 2009;15:3049. doi: 10.1089/ten.TEA.2009.0045. [DOI] [PubMed] [Google Scholar]
- 58.Olson H.E. Rooney G.E. Gross L. Nesbitt J.J. Galvin K.E. Knight A. Chen B. Yaszemski M.J. Windebank A.J. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15:1797. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krych A.J. Rooney G.E. Chen B. Schermerhorn T.C. Ameenuddin S. Gross L. Moore M.J. Currier B.L. Spinner R.J. Friedman J.A. Yaszemski M.J. Windebank A.J. Relationship between scaffold channel diameter and number of regenerating axons in the transected rat spinal cord. Acta Biomater. 2009;5:2551. doi: 10.1016/j.actbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen B.K. Knight A.M. de Ruiter G.C. Spinner R.J. Yaszemski M.J. Currier B.L. Windebank A.J. Axon regeneration through scaffold into distal spinal cord after transection. J Neurotrauma. 2009;26:1759. doi: 10.1089/neu.2008-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gysbers J.W. Rathbone M.P. Neurite outgrowth in PC12 cells is enhanced by guanosine through both cAMP-dependent and -independent mechanisms. Neurosci Lett. 1996;220:175. doi: 10.1016/s0304-3940(96)13253-5. [DOI] [PubMed] [Google Scholar]
- 62.Shimomura A. Okamoto Y. Hirata Y. Kobayashi M. Kawakami K. Kiuchi K. Wakabayashi T. Hagiwara M. Dominant negative ATF1 blocks cyclic AMP-induced neurite outgrowth in PC12D cells. J Neurochem. 1998;70:1029. doi: 10.1046/j.1471-4159.1998.70031029.x. [DOI] [PubMed] [Google Scholar]
- 63.Kano Y. Nohno T. Takahashi R. Hasegawa T. Hiragami F. Kawamura K. Motoda H. Sugiyama T. cAMP and calcium ionophore induce outgrowth of neuronal processes in PC12 mutant cells in which nerve growth factor-induced outgrowth of neuronal processes is impaired. Neurosci Lett. 2001;303:21. doi: 10.1016/s0304-3940(01)01676-7. [DOI] [PubMed] [Google Scholar]
- 64.Piiper A. Dikic I. Lutz M.P. Leser J. Kronenberger B. Elez R. Cramer H. Muller-Esterl W. Zeuzem S. Cyclic AMP induces transactivation of the receptors for epidermal growth factor and nerve growth factor, thereby modulating activation of MAP kinase, Akt, and neurite outgrowth in PC12 cells. J Biol Chem. 2002;277:43623. doi: 10.1074/jbc.M203926200. [DOI] [PubMed] [Google Scholar]
- 65.Harper J.M. Krishnan C. Darman J.S. Deshpande D.M. Peck S. Shats I. Backovic S. Rothstein J.D. Kerr D.A. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci USA. 2004;101:7123. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X. Guenard V. Kleitman N. Bunge M. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 67.Oudega M. Gautier S.E. Chapon P. Fragoso M. Bates M.L. Parel J.M. Bunge M.B. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22:1125. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 68.Unsicker K. Chamley J.H. Effects of dbcAMP and theophylline on rat adrenal medulla grown in tissue culture. Histochemistry. 1976;46:197. doi: 10.1007/BF02462783. [DOI] [PubMed] [Google Scholar]
- 69.Sobue G. Shuman S. Pleasure D. Schwann cell responses to cyclic AMP: proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986;362:23. doi: 10.1016/0006-8993(86)91394-6. [DOI] [PubMed] [Google Scholar]
- 70.Sobue G. Yasuda T. Mitsuma T. Pleasure D. Schwann cell galactocerebroside of unmyelinated fibers is inducible by derivatives of adenosine 3',5'-monophosphate. Neurosci Lett. 1986;72:253. doi: 10.1016/0304-3940(86)90522-7. [DOI] [PubMed] [Google Scholar]
- 71.Morgan L. Jessen K.R. Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112:457. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monje P.V. Rendon S. Athauda G. Bates M. Wood P.M. Bunge M.B. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia. 2009;57:947. doi: 10.1002/glia.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto M. Sobue G. Li M. Mitsuma T. Kimata K. Yamada Y. cAMP-dependent differential regulation of extracellular matrix (ECM) gene expression in cultured rat Schwann cells. Brain Res. 1994;653:335. doi: 10.1016/0006-8993(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 74.Suon S. Jin H. Donaldson A.E. Caterson E.J. Tuan R.S. Deschennes G. Marshall C. Iacovitti L. Transient differentiation of adult human bone marrow cells into neuron-like cells in culture: development of morphological and biochemical traits is mediated by different molecular mechanisms. Stem Cells Dev. 2004;13:625. doi: 10.1089/scd.2004.13.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chu M.S. Chang C.F. Yang C.C. Bau Y.C. Ho L.L. Hung S.C. Signalling pathway in the induction of neurite outgrowth in human mesenchymal stem cells. Cell Signal. 2006;18:519. doi: 10.1016/j.cellsig.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 76.Zwart I. Hill A.J. Girdlestone J. Manca M.F. Navarrete R. Navarrete C. Jen L.S. Analysis of neural potential of human umbilical cord blood-derived multipotent mesenchymal stem cells in response to a range of neurogenic stimuli. J Neurosci Res. 2008;86:1902. doi: 10.1002/jnr.21649. [DOI] [PubMed] [Google Scholar]
- 77.Rooney G.E. Howard L. O'Brien T. Windebank A.J. Barry F.P. Elevation of cAMP in mesenchymal stem cells transiently upregulates neural markers rather than inducing neural differentiation. Stem Cells Dev. 2009;18:387. doi: 10.1089/scd.2008.0080. [DOI] [PubMed] [Google Scholar]
- 78.Fouad K. Ghosh M. Vavrek R. Tse A.D. Pearse D.D. Dose and chemical modification considerations for continuous cyclic AMP analog delivery to the injured CNS. J Neurotrauma. 2009;26:733. doi: 10.1089/neu.2008.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rooney G.E. Endo T. Ameenuddin S. Chen B. Vaishya S. Gross L. Schiefer T.K. Currier B.L. Spinner R.J. Yaszemski M.J. Windebank A.J. Importance of the vasculature in cyst formation after spinal cord injury. J Neurosurg Spine. 2009;11:432. doi: 10.3171/2009.4.SPINE08784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Norenberg M.D. Smith J. Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 81.Dumont R.J. Okonkwo D.O. Verma S. Hurlbert R.J. Boulos P.T. Ellegala D.B. Dumont A.S. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 82.Wu Y. Chen L. Scott P.G. Tredget E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem cells. 2007;25:2648. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 83.Alviano F. Fossati V. Marchionni C. Arpinati M. Bonsi L. Franchina M. Lanzoni G. Cantoni S. Cavallini C. Bianchi F. Tazzari P.L. Pasquinelli G. Foroni L. Ventura C. Grossi A. Bagnara G.P. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duffy G.P. Ahsan T. O'Brien T. Barry F. Nerem R.M. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng Part A. 2009;15:2459. doi: 10.1089/ten.TEA.2008.0341. [DOI] [PubMed] [Google Scholar]
- 85.Huang N.F. Lam A. Fang Q. Sievers R.E. Li S. Lee R.J. Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regen Med. 2009;4:527. doi: 10.2217/rme.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3:229. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 87.Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.N. Traas J. Schugar R. Deasy B.M. Badylak S. Buhring H.J. Giacobino J.P. Lazzari L. Huard J. Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]