Abstract
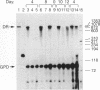
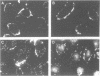
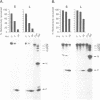
Augmented biological activity in vitro has been demonstrated in oligonucleotides (oligos) modified to provide nuclease resistance, to enhance cellular uptake or to increase target affinity. How chemical modification affects the duration of effect of an oligo with potent activity has not been investigated directly. We postulated that modification with internucleotide phosphorothioates and 3' alkylamine provided additional nuclease protection which could significantly extend the biological activity of a 26 mer, (T2). We showed this analog, sT2a, could maximally inhibit interferon gamma-induced HLA-DR mRNA synthesis and surface expression in both HeLa and retinal pigmented epithelial cells and could continue to be effective, in the absence of oligo, 15 days following initial oligo treatment; an effect not observed with its 3'amine counterpart, T2a. In vitro stability studies confirmed that sT2a conferred the greatest stability to nucleases and that cellular accumulation of 32P-sT2a in both cell types was also greater than other T2 oligos. Using confocal microscopy, we revealed that the intracellular distribution of sT2a favored greater nuclear accumulation and release of oligo from cytoplasmic vesicles; a pattern not observed with T2a. These results suggest that phosphorothioate-3'amine modification could increase the duration of effect of T2 oligo by altering nuclease resistance as well as intracellular accumulation and distribution; factors known to affect biological availability.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar S., Kole R., Juliano R. L. Stability of antisense DNA oligodeoxynucleotide analogs in cellular extracts and sera. Life Sci. 1991;49(24):1793–1801. doi: 10.1016/0024-3205(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Benichou G., Kanellopoulos J. M., Wallon C., Boue F., Delfraissy J. F. Interferon-gamma restores T lymphocyte proliferation of nonresponders to IgG1 anti-CD3 via the induction of Fc gamma 1 receptors on monocytes. Eur J Immunol. 1987 Aug;17(8):1175–1181. doi: 10.1002/eji.1830170815. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crooke R. M. In vitro toxicology and pharmacokinetics of antisense oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):609–646. [PubMed] [Google Scholar]
- Gamper H. B., Reed M. W., Cox T., Virosco J. S., Adams A. D., Gall A. A., Scholler J. K., Meyer R. B., Jr Facile preparation of nuclease resistant 3' modified oligodeoxynucleotides. Nucleic Acids Res. 1993 Jan 11;21(1):145–150. doi: 10.1093/nar/21.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Harel-Bellan A., Ferris D. K., Vinocour M., Holt J. T., Farrar W. L. Specific inhibition of c-myc protein biosynthesis using an antisense synthetic deoxy-oligonucleotide in human T lymphocytes. J Immunol. 1988 Apr 1;140(7):2431–2435. [PubMed] [Google Scholar]
- Hoke G. D., Draper K., Freier S. M., Gonzalez C., Driver V. B., Zounes M. C., Ecker D. J. Effects of phosphorothioate capping on antisense oligonucleotide stability, hybridization and antiviral efficacy versus herpes simplex virus infection. Nucleic Acids Res. 1991 Oct 25;19(20):5743–5748. doi: 10.1093/nar/19.20.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Iversen P. L., Zhu S., Meyer A., Zon G. Cellular uptake and subcellular distribution of phosphorothioate oligonucleotides into cultured cells. Antisense Res Dev. 1992 Fall;2(3):211–222. doi: 10.1089/ard.1992.2.211. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Gmelig-Meyling F., Gourley M. F., Kisch W. J., Chrisey L. A., Steinberg A. D. Uptake of oligodeoxyribonucleotides by lymphoid cells is heterogeneous and inducible. Antisense Res Dev. 1991 Summer;1(2):161–171. doi: 10.1089/ard.1991.1.161. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Tonkinson J., Matson S., Zhao Q., Saxon M., Zhang L. M., Bhanja U., Yakubov L., Stein C. A. Modification of antisense phosphodiester oligodeoxynucleotides by a 5' cholesteryl moiety increases cellular association and improves efficacy. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1048–1052. doi: 10.1073/pnas.90.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti G., Egan W., Noguchi P., Zon G., Matsukura M., Broder S. Oligodeoxyribonucleotide phosphorothioate fluxes and localization in hematopoietic cells. Antisense Res Dev. 1992 Spring;2(1):27–39. doi: 10.1089/ard.1992.2.27. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]
- Orson F. M., Thomas D. W., McShan W. M., Kessler D. J., Hogan M. E. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991 Jun 25;19(12):3435–3441. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Stein C., Subasinghe C., Haldar S., Croce C. M., Yum S., Cohen J. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990 Oct 15;50(20):6565–6570. [PubMed] [Google Scholar]
- Rosa F. M., Fellous M. Regulation of HLA-DR gene by IFN-gamma. Transcriptional and post-transcriptional control. J Immunol. 1988 Mar 1;140(5):1660–1664. [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. K., Lui G. M. Propagation of fetal human RPE cells: preservation of original culture morphology after serial passage. J Cell Physiol. 1990 Apr;143(1):196–203. doi: 10.1002/jcp.1041430127. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry A. R., Dritschilo A. Intracellular availability of unmodified, phosphorothioated and liposomally encapsulated oligodeoxynucleotides for antisense activity. Nucleic Acids Res. 1992 Nov 11;20(21):5691–5698. doi: 10.1093/nar/20.21.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendegui J. G., Vasquez K. M., Tinsley J. H., Kessler D. J., Hogan M. E. In vivo stability and kinetics of absorption and disposition of 3' phosphopropyl amine oligonucleotides. Nucleic Acids Res. 1992 Jan 25;20(2):307–314. doi: 10.1093/nar/20.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Matson S., Herrera C. J., Fisher E., Yu H., Krieg A. M. Comparison of cellular binding and uptake of antisense phosphodiester, phosphorothioate, and mixed phosphorothioate and methylphosphonate oligonucleotides. Antisense Res Dev. 1993 Spring;3(1):53–66. doi: 10.1089/ard.1993.3.53. [DOI] [PubMed] [Google Scholar]