Abstract
Despite the impact of chronic pain on the quality of life in patients, including changes to affective state and daily life activities, rodent preclinical models rarely address this aspect of chronic pain. To better understand the behavioral consequences of the tissue and nerve injuries typically used to model neuropathic and inflammatory pain in mice, we measured home cage and affective state behaviors in animals with spared nerve injury (SNI), chronic constriction injury (CCI) or intraplantar CFA. Mechanical hypersensitivity is prominent in each of these conditions and persists for many weeks. Home cage behavior was continuously monitored for 16 days in a system that measures locomotion, feeding and drinking and allows for precise analysis of circadian patterns. When monitored after injury, animals with SNI and CFA behaved no differently from controls in any aspect of daily life. Animals with CCI were initially less active, but the difference between CCI and controls disappeared by 2 weeks after injury. Further, in all pain models, there was no change in any measure of affective state. We conclude that in these standard models of persistent pain, despite the development of prolonged hypersensitivity, the mice do not have significantly altered “quality of life”. As alteration in daily life activities is the feature that is so disrupted in patients with chronic pain, our results suggest that the models used here do not fully reflect the human conditions and point to a need for development of a murine chronic pain model in which lifestyle changes are manifest.
Keywords: Animal model, Emotional behaviors, Quality of Life, Neuropathic pain, Inflammatory pain
Introduction
The perception of pain is not simply a sensory, nociceptive experience, but one that often disrupts a patient’s quality of life [45]. Despite this, animal studies have focused on measures of hypersensitivity in the setting of tissue or nerve injury [38]. Unquestionably, mechanical and thermal hypersensitivity are often associated with human chronic pain disorders [3,13,32], but often more disruptive to patients is spontaneous, ongoing pain [1]. The latter is difficult to document in animals, and thus rarely studied. Chronic pain disorders are also associated with changes in affective state, such as depression and anxiety [2,11,51], and overall quality of life measures [2,31,48]; again few of these conditions are studied in preclinical models.
Spontaneous pain is particularly difficult to measure in rodents. Animals occasionally display measurable signs of pain, such as flinching or guarding in some pain models, but this has proved hard to detect in persistent pain models [9,21,26,29,39,63]. Further, if the animal is indeed experiencing spontaneous pain similar to humans, there should be an impact on its daily life activity and affective state, behaviors we will herein refer to as “quality of life” measures. To date, such studies in rodents have concentrated on the rat and included measures of daily life such as sleep [15,24,28,40,55,60] and locomotion [6,16,27,33,61]. Results from these and other studies on affective state changes following chronic injury are mixed. For example, some studies only reported changes in depression-like behavior [12,19], others only found alterations of anxiety-like behavior [16,20,54,56], and still others found changes to both [30] or none [25]. There are even less data for the mouse, despite its utility in genetic studies. As in the rat, there are conflicting reports as to whether nerve injury-related pain leads to an increase in anxiety in mice [17,34,42,43,59] and only a handful of studies have focused on changes to daily life behavior with mixed results. A gastritis model revealed decreased locomotion, eating and drinking [46]. A sciatic nerve cuff model of neuropathic pain found no change in daily life activities, but did report alterations in measures of affect [4].
The paucity of studies monitoring the daily life of animals in pain undoubtedly reflects the complexity of long-term monitoring of behavior. Video recording of mice in their home cage has been used [18], but the time required to analyze the data limits this method. In the present study, we used a new approach that allows for automatic and detailed analysis of home cage activity of mice over an extended period [14]. Our objective was to determine the extent to which tests of daily activity and affective state can be used as surrogate measures of pain in mice using current standard models of persistent pain, spared nerve injury (SNI), chronic constriction injury (CCI) and intraplantar Complete Freund’s Adjuvant (CFA). Because of the known variability among mouse strains in their expression of pain behavior, we studied two inbred strains (Balb/c and C57Bl/6). We monitored daily activity in the home cage and also measured performance in a variety of behavioral tests of affective state, for up to 5 weeks following injury.
Methods
Animals
Adult male mice (Balb/c and C57Bl/6) were purchased from Jackson and Charles River laboratories, and arrived at least 2 weeks before testing began. All mice were housed in cages with corncob bedding and a cotton nestlet in groups of 3 to 5, unless separated due to fighting issues, which occurred occasionally in both strains. All cages were changed every two weeks, at least two days before the next behavioral test. Animals had freely available food and water under a standard 12-hour light/dark cycle with a regulated ambient temperature of 20–22°C. Experimental manipulation occurred at 7–10 weeks of age. All procedures were approved by the Institutional Animal Care and Use Committee at UCSF and the guidelines of the Committee for Research and Ethical Issues of IASP. For all experiments, animals were habituated to handling prior to testing.
Experimental models of pain
Surgeries were performed under isoflurane anesthesia. Spared nerve injury (SNI) was performed as described previously [57] with two of the three branches (sural and common peroneal) of the sciatic nerve ligated and cut. Sham controls received the same surgical procedure, except that the sciatic nerve remained undisturbed. Chronic constriction injury (CCI) was performed using a modification of the Bennett and Xie [5] procedure. Briefly, the sciatic nerve was exposed at mid-thigh, proximal to its trifurcation. Two ligatures of 6-0 chromic gut suture were tied loosely around the sciatic nerve, 1–2mm apart. As in the SNI sham procedure, the sciatic nerve was exposed but not disturbed in the sham group and, for all groups, overlying muscle was closed with 6-0 silk suture and the skin closed separately with wound clips. Naïve groups were exposed to isoflurane anesthesia for a similar length of time as sham, SNI, and CCI animals. After surgeries animals were returned to their home cages in a mixed environment (generally at least two different groups per cage.)
For the model of inflammatory persistent pain, animals received an intraplantar injection of 10μL Complete Freund’s Adjuvant (CFA) into the left hindpaw. To extend the length of time of the inflammation, a second 10μL intraplantar injection was given one week after the first. Controls received intraplantar injections of saline each time. For shorter duration persistent pain, animals received 5% formalin (in 20μL) intraplantarly. Sickness was induced by an injection of lipopolysaccharide (20μg in 200μL, i.p.). As the injuries are comparable to those that evoke inflammatory and neuropathic pain in humans, we refer to these conditions as models of inflammatory or neuropathic pain.
Mechanical hypersensitivity
For both home cage monitoring and affective behavior experiments, the mice were habituated to the testing chambers 2 to 3 times before baseline testing began. Testing chambers consisted of clear plexi-glass cylinders on a raised wire mesh grid. On each day of testing, the mice were first habituated to the testing cylinders 60–90 minutes before. We used von Frey filaments with the up-down method [7] to obtain the baseline mechanical threshold. For home cage monitoring groups, post-injury thresholds were obtained immediately before and after the monitoring period. Other animals were tested in weeks 1, 2 (3 for CFA experiments), and 5.
Home cage monitoring
To monitor behavior in the home cage, we used the automated monitoring system developed by Goulding, et al [14]. This system consists of 32 cages (with betachip bedding, plastic niche and cotton nestlet) each placed on a pivoting platform with two load beams calibrated to detect position of the mouse, with photobeams at the feeder and a lickometer at the water bottle to detect bouts of feeding and drinking. While this experiment required the mice to be individually housed in the system, they were group housed until placed into the system. For each round of monitoring, the 32 animals were evenly divided between experimental and control (naïve and sham or, for the CFA experiment, saline-injected) groups. Experiments were done in Balb/c mice with CCI (n=20–22, in two separate runs), SNI (n=10–11) or CFA (n=16) and SNI C57Bl/6 mice (n=10–11). For nerve injury experiments, animals were placed in the system at 48 hours after injury, to allow time for mechanical threshold testing. In the CFA run, monitoring began 48 hours after the second injection of CFA. In all experiments, monitoring proceeded continuously (except for 1.5–2 hours daily maintenance on the system) for 16 days. Using methods developed for the system, data were checked for errors and activity classified as inactive or active. Within the active state, mouse behavior was further classified based on location, movement, and feeder/lick spout data as feeding, drinking, moving, or other (which includes small movements and can be separated by location.)
Short-duration behavioral tests of locomotor and affective state behavior
Groups of mice were run in a battery of activity and affective tests (not all tests were performed in every group, supplemental figure 5). Each test was run with an interval of at least 3 days, which has been shown to be sufficient so that behavior on one test does not influence the next [35,47]. For all short-duration behavioral tests, animals were brought into the testing room at least half an hour prior to beginning the test. Between tests, the testing apparatuses were sprayed with dilute bleach and wiped dry.
Tests of anxiety-like behavior
For all tests of anxiety-like behavior, animals were only studied once on each apparatus. The open field (OF) test of activity and anxiety was performed under normal lab lighting (more than 100 lux). Mice were placed in the OF apparatus, which consists of four white chambers measuring 50 × 50 × 38 cm, allowing 4 animals to be tested concurrently. Mice were allowed to freely explore the chamber for 30 minutes. Each chamber was divided into the outer zone (15 cm from the walls) and the center zone. Activity was recorded by video and analyzed using the Ethovision software. Time spent in the center zone was used as the measure of anxiety. The elevated zero maze (EZM) is a modified elevated plus maze test of anxiety-like behavior that eliminates the ambiguous center square[36]. This maze consists of an elevated (42 cm), round platform (5.5-cm width) divided into 4 equal quadrants: 2 open areas without walls and 2 walled areas. Mice were placed in the closed area of the maze, and activity was recorded for 8 min by a video camera mounted above the maze. This test is performed under dim light (about 40 lux). Time spent in the open quadrants was later scored and indicated the level of state anxiety. To assess marble burying behavior, marbles were evenly spaced in a plastic cage with a 5-cm layer of bedding to which the animals had previously been habituated. Mice were placed in the cage and recorded by video camera for 20 minutes. At the end of the test, marbles that were more than 2/3 covered by bedding were considered “buried” and used as a measure of anxiety [44]. Videos from the test and habituations were scored for the time spent digging.
Tests of motor function
To test motor function, we used the rotarod test, climbing, and gait analysis. Rotarod was performed on an accelerating rod apparatus (Ugo Basile). For training, mice were run until all the animals stayed on for more than 200 sec (at least 3 training days). On subsequent testing days, animals were tested three times. In the untrained experiments, animals were run for three trials each testing day without any previous experience. To assess climbing behavior, animals were placed into a 40 cm high wire-mesh cylinder with a clear plastic top [10]. Behavior was recorded by video for 10 minutes and time spent climbing, defined by all four limbs off the floor, was measured. Gait analysis was performed using the Noldus CatWalk system[62]. Briefly, mice were trained to walk across a clear glass runway. After they could move through the apparatus without pauses, tracks are recorded and analyzed using the CatWalk software.
Other affective tests
Other affective tests were performed on some groups of mice. Forced swim test of despair-like depression behavior was performed before and after injury. For this test, animals were placed in a large beaker of water (about 25°C), in which animals could not reach the bottom. Behavior was recorded for 6 minutes, after which, time spent immobile (defined as not actively swimming) was counted, a measure of the time in “despair”-like behavior [49]. As a second measure of depression-like behavior, we used the sucrose preference test to document the presence of anhedonia. Mice were given the choice of drinking water or 2% sucrose for two consecutive nights. The position of the water and sucrose bottles was switched on the second night. Mice were trained once before obtaining two baseline measures. A preference score was given as a percent of sucrose liquid consumed over two nights compared to total liquid consumed. In normal mice, this is generally above 90%. Anhedonia was considered to have occurred if preference dropped to 65% [41,58]. Time spent in social interaction was recorded in a new clean cage with a novel animal of similar size and strain for 8 min. Social sniffing, exploration and physical contact were all counted as signs of interaction.
Experimental design and statistical analyses
Separate groups of animals were used for the home cage monitoring, motor behavior and affective behavior experiments. Supplemental figure 5 shows the timing and group distribution for HCM and affective behavior testing. Additional groups were used to test open field activity at days 3 or 7, followed by rotarod (untrained) and to test locomotor activity on the catwalk and rotarod (trained). Animals were allocated to groups in a block design, by the same researcher who also performed the surgeries and behavioral testing. No animals were excluded from the study once data were collected. After the surgeries, the identity of the cages was concealed from the researcher performing behavioral tests and not revealed until all data collection and analysis were completed. For animals in the home cage monitoring experiment, only the Von Frey testing before and after the monitoring period was done blind. As the monitoring system and subsequent analysis is automated, this part of the experiment was not blinded.
Results are expressed as mean±SEM and p values less than 0.05 were considered significant. Comparisons were analyzed with one-way or repeated measure analysis of variance (ANOVA). In experiments with only one control group, we used Student’s t-test, except in cases where data were non-parametric, for which the Mann-Whitney u-test was used. For ANOVAs, post-hoc analysis used Bonferroni tests. Data were analyzed using GraphPad Prism 5 for Mac.
Results
Mechanical hypersensitivity in different chronic pain models
With the exception of a few animals that underwent CCI or SNI and appeared to favor the uninjured hindlimb, gross observation of mice after injury rarely revealed notable signs of pain or discomfort in any of the injury models. Unquestionably, however, animals in each injury group had significant decreases in mechanical sensitivity (i.e. mechanical hypersensitivity) after injury (Fig. 1A and B). Mechanical withdrawal thresholds of naïve, saline and sham controls also decreased over time, albeit to a smaller extent. This drop might reflect the fact that animals undergo other tests during the post-surgical period, which might alter, to a small extent, their behavior in this test. The fact that mice were only tested weekly after injury, rather, that every other day for baseline testing, may also be relevant. However, regardless of the explanation, when data for the two nerve injury groups were normalized to the naïve control, both SNI and CCI groups still showed a significant decrease in threshold. Sham controls did not differ from naive at any time point (Fig. 1C). Nerve-injured and CFA-injected mice that underwent home cage monitoring were also tested for mechanical hypersensitivity before being introduced into the monitoring system. Animals in these injury groups all developed mechanical hypersensitivity, and this was still present when these mice were removed from the system after 16 days (Supplemental Figure 1A–D).
Figure 1.
Mechanical hypersensitivity after nerve injury or inflammation. 50% withdrawal thresholds (in grams) were obtained using the von Frey up-down method in mice (A) after SNI (red bars) or CCI (blue), and in sham (grey) and naïve (white) controls or (B) after saline (grey) or CFA (orange) injection. (C) Mechanical thresholds were normalized to the naïve groups for the SNI and CCI experiments. For all, repeated measures ANOVA showed a significant effect of treatment with Bonferroni post-test differences between naïve/sham and CCI groups indicated with: * <0.05, ** <0.01, *** <0.001; post-hoc differences between naïve/sham and SNI groups: + <0.05, ++ <0.01, +++ <0.001
Monitoring of home cage activity
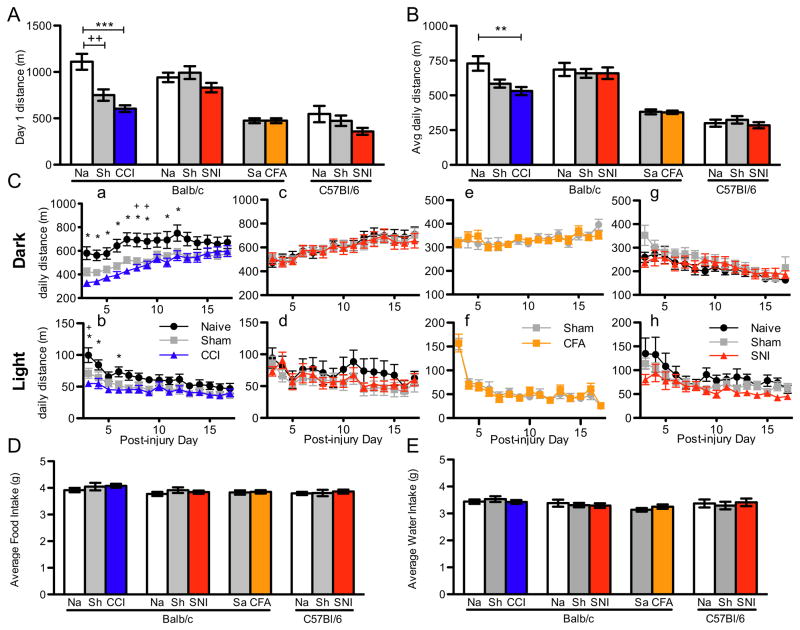
Animals were placed in the home cage monitoring (HCM) system on the second day after surgery or after CFA injection. The first day in the HCM is generally marked by increased activity, which is presumably a manifestation of the animal’s exploration of the new cage and its building of a new nest. This increased exploration is expressed as an elevated distance traveled on day one in the HCM compared to subsequent days. In fact, all animals with SNI, CCI or CFA and their respective controls displayed this elevated locomotion. On the other hand, although CCI animals showed a normal heightened response to the novel environment (157% greater on day 1 compared to day 3 of monitoring for CCI, 175% in Naïve controls, p=0.38, Supplemental Figure 1F), their total distance traveled on the first day of monitoring (corresponding to day 2 after injury) was significantly less than naïve controls (naïve vs. CCI, 1110±85m vs. 605±36m, F=17.5, p<0.0001, Fig. 2A). Animals with SNI or CFA did not have a similar significant reduction in locomotion on the first monitoring day compared to control groups. Interestingly, animals with CCI sham injury, like their full surgery counterparts, moved significantly less on the first day of monitoring compared to naives and were not different from CCI animals (Fig. 2A). Animals that underwent sham SNI surgery did not differ in distance traveled from mice with SNI or from their naïve controls, indicating that the two sham surgeries are not equivalent, possibly as a result of the more proximal site of injury in the CCI group. The locomotor difference between CCI and naïve controls was also seen in the average daily movement over days 3–17 after injury, although sham was not significantly different from either group. (Fig. 2B) Again, there was no difference between SNI or CFA and controls.
Figure 2.
Home cage movement, feeding and drinking in mice with SNI, CCI and CFA. (A) Total movement (in meters traveled) on day 1 of monitoring was significantly decreased in Balb/c mice with CCI (blue) and sham (grey) compared to naïve (white) controls, but not with either SNI (red) strain or Balb CFA (orange). (B) Average total movement over days 3–17 after injury was also significantly decreased in mice with CCI, but was similar to controls in all other experimental and sham groups. (C) Total daily movement in the dark (i.e. night; a, c, e, g) and light (b, d, f, h) cycles over days 3 to 17 was very similar to controls across the entire period for Balb/c mice with SNI (c, d) and CFA (e, f) and C57Bl/6 with SNI (g, h), but was significantly decreased in the early days after injury in the light cycle (b; repeated measures ANOVA, p=0.024) and for a longer period in the dark cycle (a; repeated measures ANOVA, p=0.002). Post-test differences are all indicated as less than 0.05 for ease of reading, though many are much smaller. Average daily food (D) and water (E) intake were no different in any group. Na: Naïve control; Sh: Sham control; Sa: Saline control. Post-test differences between CCI and Naive: *; differences between Sham and Naïve: +.
To observe better the activity differences among groups throughout the course of the monitoring period, we analyzed light and dark cycle locomotor activity separately, on each day (days 3–17 after injury.) There was no difference among animals with SNI, CFA injection, sham and naïve controls in the light and the dark cycle (Fig. 2Cc–h). In the CCI experiments, however, repeated measures ANOVA revealed a significant effect of treatment groups during both the light and dark cycles (dark, F=6.8, p=0.0023; light, F= 4.02, p=0.0235, Fig 2Ca–b). Thus post hoc analysis of the dark cycle activity indicated a significant difference between CCI and naïve groups during the first 12 days post-injury; the sham group was only significantly different from the naïve group on days 8 and 9 and did not differ from CCI on any day. In contrast to the active phase of the mouse, light cycle activity (i.e. inactive phase) in CCI animals was only reduced in the first week post-injury. It appears therefore that SNI and CFA did not alter overall activity, but CCI caused a significant, albeit transient (for 12 days) decrease in daily activity.
SNI, CCI and CCI sham injuries all resulted in significant loss of weight after the first 2 days of injury (measured just before monitoring began.) Moreover, all groups had similar weights upon removal from the monitoring system, i.e. at day 17 post-injury. Thus, injury groups gained slightly more weight during the monitoring period, though not significantly so (Supplemental Figure 1E). However, despite the small differences in weight gain/loss in these groups, there were no differences in average daily food and water intake among any experimental and control groups (Fig. 2D–E). Even during the early days after injury (day 3 to 7), a time when CCI animals moved considerably less than did the controls, the food and water intake of mice with CCI did not change (Supplemental Table 1).
Circadian patterns of activity in nerve-injured animals
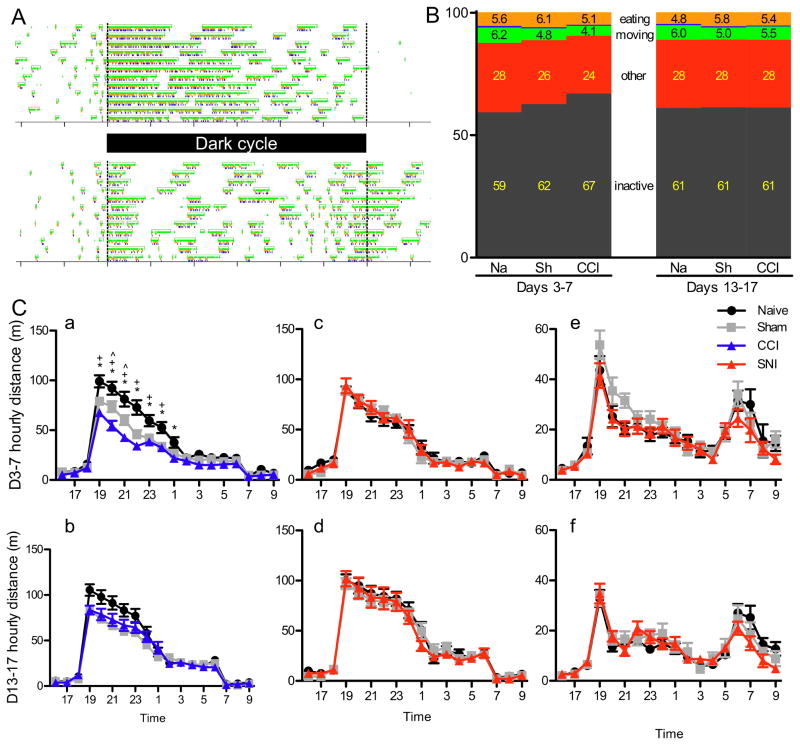
The circadian patterns of the two strains used in this study are very stereotyped and reproducible. Both Balb/c and Bl/6 mice have a peak of activity immediately after the dark cycle onset, and the Bl/6 mice have a second peak of activity around the end of the dark cycle (Fig. 3A). Because we initially hypothesized that the circadian pattern of animals experiencing persistent pain would become more fragmented[28], we focused our analysis on the Balb/c strain, expecting to observe disruption of the extended peak of activity in the first half of the dark cycle.
Figure 3.
Average distance traveled over circadian time. (A) Raster plots of a naïve mouse activity in Balb/c (top) and C57Bl/6 (bottom) mice over 12 days, with green representing periods of locomotor activity; orange, eating; and blue, drinking. (B) Time budgets of naïve, sham and CCI groups of mice in the early and late monitoring periods. Mouse time budgets include inactive (grey), locomotion (green), eating (orange), drinking (blue, less than 1% of the day, so not visible in the graph), and “other” (red, mouse is active, but not engaged in other activities). Inset numbers are percent of time in each activity. (C) Average movement (in distance traveled) binned by hour on days 3–7 (a, c, e) or days 13–17 (b, d, f). SNI Balb/c (c-d) and C57Bl/6 (e-f) animals did not show any differences in their circadian pattern in either the early or late days of the experiment. Average hourly distance traveled in the initial monitoring days by animals with CCI were significantly decreased from controls during the first half of the dark cycle (a; p< 0.0001), but not significantly different in the later days of monitoring (b; p=0.0505).
A great advantage of the HCM system is that it allows for precise temporal analysis of the animal’s activity over the course of the day. Because the decrease in activity in the CCI and sham groups only occurs in the early post-surgical period, we divided the circadian analysis into an early (days 3 to 7) and late (days 13 to 17) period. None of the injury groups displayed dramatic changes to the circadian pattern. In fact, for animals with SNI or CFA, there were no differences in distance traveled at any time of the day, in either the early or late monitoring period (Fig. 3Cc–d). As expected, during the early analysis period, animals in the CCI group moved significantly less than did animals in the naïve group, during the first half of the dark cycle (effect of surgery, F=14.5, p< 0.0001; post-hoc tests between CCI and Naïve are significant from 7pm to 1am, Fig. 3Ca). For animals with CCI sham injury, the amount of dark cycle activity fell between CCI and naïve group levels, being significantly different from naïve for the first half of the dark cycle (p<0.0001, post-hoc differences at 7pm–12am) and from CCI during only two hours early in the dark cycle (post-hoc differences at 8 and 9pm). By the final days of monitoring, however, there were no longer any significant differences in the distance traveled at any time of day for the CCI experiment (effect of surgery, F=3.1, p=0.0505, Fig. 3Cb), indicating that CCI and to a lesser extent CCI sham surgeries, lead to an initial decrease in activity, which recovered by 2 weeks.
In addition to using distance traveled at various times of day as an indication of circadian pattern, the HCM system can further define the state of an animal (active or inactive), allowing detailed analysis of a mouse’s daily life[14]. We found that the animals’ circadian rhythms did not change, using either a measure of distance traveled or the probability of being in an active state over circadian time. And, as when using distance moved, animals with CCI have a decreased active state probability during the dark cycle, but only early after injury (early, F=6.1, p=0.006; late, F=0.01, p=0.99, Supplemental Fig. 2A).
Bout and time budget analysis
Behavior within the active state can be further classified as feeding, drinking, moving or other (active, but not in one of the three main activities.) This is particularly useful as we had predicted that given the need to climb onto the feeder to eat, injured animals might have fewer, but longer feeding bouts. However, even in the early monitoring period, when there were changes in locomotion, animals with CCI had no significant difference in feeding bout number, size or duration. Nor was there a difference in the SNI or CFA groups (Supplemental table 1, 2). We did observe that movement bouts of animals with CCI were both fewer in number and shorter (in terms of distance per bout) during days 3 to 7, but this recovered by the later period of monitoring (Days 3–7: 4150 vs. 2710 bouts per day in naïve vs. CCI, p=0.0006; Days 13–17: 4183 vs. 3902, p=0.297; Supplemental Table 1). Importantly, movement bout averages were not different from controls in any other injured group. Taken together these data indicate that it is the combination of less time spent in the active state as well as a dramatic reduction in movement bouts during this state that likely contribute to the decrease in overall activity seen in animals with CCI in the first week of monitoring.
Examining the time budgets of the animals is particularly helpful for appreciating the differences in the CCI group of animals in early vs. late monitoring periods. To this end, we measured and compared time spent inactive, moving, feeding, drinking, or other (i.e. stopped, but active). While SNI and CFA did not alter any parameter of daily activity (Supplemental Figure 2 and Supplemental Table 2), CCI animals, during the initial monitoring days, spent significantly less of the day engaged in locomotion (6.3% vs. 4.1%, p=0.0004) and more of the day inactive (59% vs. 67%, p=0.002, Fig. 3B). This difference was no longer present during the late monitoring period.
Note that although we focused our analysis on Balb/c animals, we also studied C57Bl/6 animals with SNI or sham and naïve controls. Much like Balb/c mice that underwent SNI, we found no changes in the movement and feeding bout properties, and time budgets of injured animals did not differ, when compared to either sham or naïve controls (Supplemental Figure 2 and Supplemental Table 2).
Other tests of daily life activity
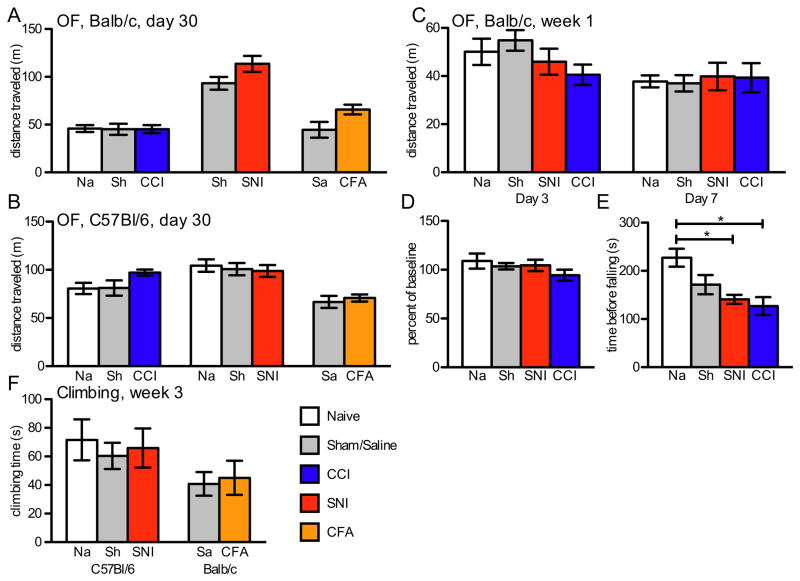
Because animals with CCI displayed reduced locomotion in the home cage, we also tested separate groups of Balb/c mice in an open field test on days 3 or 7 after CCI, SNI or CCI sham surgery (naïve controls received anesthesia only). On neither day did animals with SNI show a decrease in distance traveled; and perhaps more surprisingly, this was also true for the mice with CCI (Fig. 4C, day 3 p=0.23, day 7 p=0.88). We also tested animals for open field activity one month after injury, in each of the pain models and in both Balb/c and C57Bl/6 mice. No pain model resulted in significant decreases in distance traveled (Fig. 4A,B).
Figure 4.
Short-duration tests of daily activity: (A) Distance traveled in the open field one month after injury was not different in any pain model in Balb/c mice (CCI n=8–9, p=0.99; SNI n=11–12, p=0.12; CFA n=6–7, p=0.07) or (B) Bl/6 mice (CCI n=8–9, p=0.09; SNI n=18–19, p=0.82; CFA n=8–9, p=1.0). (C) Distance traveled in the open field on days 3 and 7 after surgery did not differ among any of the experimental and control groups (n=8, Day 3 p=0.23, Day 7 p=0.97). (D) Mice with SNI or CCI performed as well after injury as before on the rotarod test when previously trained (n=5, p=0.62), (E) but significantly worse 3 days after surgery (n=8, p=0.006), if untrained. (E) Time spent climbing at 3 weeks after injury in Bl/6 mice with SNI or Balb/c mice with CFA did not differ from controls (SNI n=7–8, p=0.82; CFA n=10, p=1.0).
As the open field only measures horizontal movement, we also examined vertical movement. In this test, the animals were allowed to explore a climbing apparatus, in which they had an opportunity to climb on a wire mesh grid, an activity that requires the use of all 4 limbs. At 3 weeks post-injury, animals with SNI and CFA injections climbed for the same amount of time (10 min test) as did control animals (Fig. 4F). We also counted the number of rearing events in the elevated maze and found no difference between injured and control animals in any group. (Supplemental Fig. 3A)
As noted above, occasionally we observed animals in the nerve-injured groups that appeared to have an altered stance. Thus, to assay for possible motor deficits, we tested sham, SNI or CCI groups of mice on the accelerating rotarod. All animals were trained and performed equally well prior to surgery, and this did not change on days 4 to 11 after surgery (naïve, 109±8% baseline; SNI, 104±6%; CCI, 94±6%, p=0.62, Fig. 4D, Supplemental Figure 3B). Interestingly, however, when animals with no prior experience on the rotarod were tested 3 days after injury, those in the CCI and SNI groups stayed on the apparatus for a significantly shorter time than compared to untrained naïve controls (p= 0.0059, Fig. 4E). When these animals were retested on days 5 and 9 after surgery, the apparent motor deficit was still present (Supplemental Figure 3C). By contrast, when untrained animals had their first training day a full week post-injury, we found no significant difference among groups (Supplemental Figure 3D). Finally, we analyzed the locomotor ability of CCI injured animals using the CatWalk system, which allows for gait analysis. Even in this test, there were no changes in parameters of ipsilateral vs. contralateral hindpaw pressure or timing of the stride (Supplemental Figure 3E), indicating that there is no change in the use of the injured paw when the animal is in motion. However, we cannot rule out alterations in weight distribution in stationary animals.
Behavioral tests of affective state
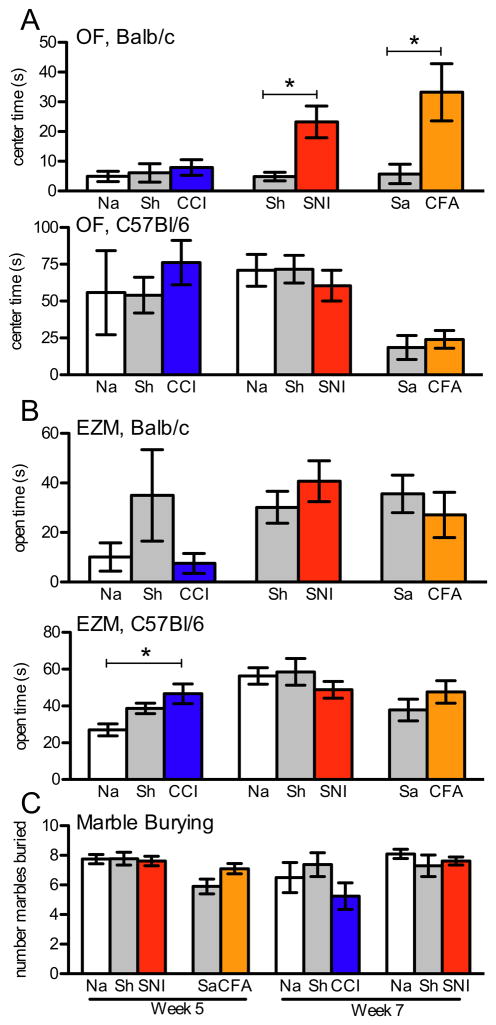
To determine if any of the pain models are associated with a change in affective state, we next performed a battery of behavioral tests of emotion. To assay for the level of anxiety, we used the open field, elevated maze and marble burying tests. At time points soon after surgery (days 3 and 7) and later (one month), we found little difference in the open field test of anxiety, i.e. time spent in the center area, among any of the injury groups and controls (Fig. 5A and Supplemental Fig. 4). Likewise, elevated zero maze time in the open areas revealed little difference among injury and control groups (Fig. 5B). On the other hand, although none of the injury groups showed consistent differences in either measures of anxiety, a few groups did show an unexpected decrease in anxiety-like behavior on one of the measures. Thus, Balb/c animals with SNI or CFA injury spent more time in the center area of the open field (SNI, p= 0.0173; CFA, p= 0.0278); and C57Bl/6 animals with CCI spent more time in the open areas of the elevated maze (p= 0.0169). In other words, while there was no consistent change in the two related measures, these injured animals may, if anything, have reduced anxiety. However, we do not rule out the possibility that there was a floor effect in the naïve animals, especially in the case of Balb/c mice, which prevented us from observing any further changes in injured animals.
Figure 5.
Behavioral measures of affective state at one month after injury. (A) Time spent in the center area of the open field in C57Bl/6 mice (bottom) with CCI (n=8–9, p=0.67), SNI (n=18–19, p=0.69) and CFA (n=8–9, p=0.58) was not different from controls. Balb/c animals (top) with CCI (n=8–9, p=0.71) did not differ from controls, but there was a significant increase in the time spent in the center in Balb/c mice with SNI (n=11–12, p=0.017) and CFA (n=6–7, p=0.073). (B) There was also little difference between experimental groups and controls in the time spent in the open areas of the elevated zero maze, in Balb/c (top) animals (CCI p=0.18, SNI p=0.33, CFA p=0.48) and in C57Bl/6 (bottom) animals (SNI p=0.44, CFA p=0.58) except for those with CCI that spent significantly more time in the open areas (p=0.017). (C) The number of marbles buried on week 5 in Bl/6 SNI animals or Balb CFA animals did not differ from control nor did Balb CCI or Bl/6 SNI differ from their controls at week 7.
Additionally, we observed no difference in anxiety-like behavior in the marble burying test. Thus, when tested at both 5 and 7 weeks after SNI surgery, C57Bl/6 animals buried the same number of marbles as did controls. And Balb/c mice with CCI or CFA injection did not display any difference in anxiety state in the marble-burying task (Fig. 5C). Furthermore, in the testing cage, we found no difference in the time the injured groups of mice spent digging compared to controls (data not shown), with or without the presence of the marbles. So even using a different test of anxiety, injured animals showed no change in their anxiety-like state.
Although anxiety was the focus of the affective state behavior tests, we also tested some groups of animals using the forced swim test of depression-like behavior, sucrose preference test of anhedonia and a social interaction test (Supplemental Fig. 4). In the forced swim test, neither CFA nor SNI significantly altered the amount of time animals spent immobile, i.e. we found no evidence for an increased depressive state. The same conclusion was drawn in the sucrose preference test, where C57Bl/6 animals with SNI or CFA, drank similar amounts of sweetened water compared to their respective sham or saline injected controls and never fell below 65% preference. Lastly, we found that there was no change in the time spent exploring a novel mouse, which is presumed to test a more complex affective state. Here too, injury did not appear to interfere with normal social interaction.
Open field behavior after short-term persistent pain
Having found that our standard models of chronic pain did not alter behavior in tests of locomotion and affective behavior, we also tested animals in a shorter duration persistent pain model in the open field test. We used a model of pain that is associated with very overt signs of discomfort (compared to that observed in CFA-injected animals). In these tests, C57Bl/6 and Balb/c mice received intraplantar injections of formalin and were tested two hours later. Because we did not want to confound the analysis during the time the animal licked its hindpaw in response to the formalin, we performed the open field test in the period immediately after licking behavior had ended. As for the longer-term persistent pain models, we again found no difference in the overall distance traveled in the open field, for either strain (Fig. 6A, B). And while there was no significant difference between saline- and formalin-injected animals in the time spent in the anxiety-related center area, Balb/c animals with formalin did show a trend to less time in the center, i.e. appeared to display more anxiety (Fig. 6C, D).
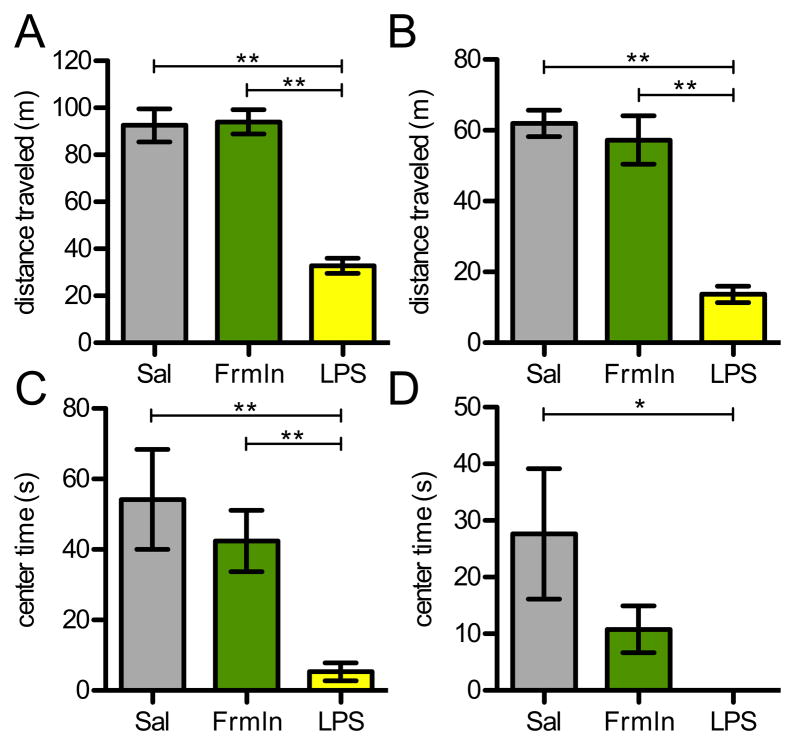
Figure 6.
Open field behavior in short-term models of sickness and pain. (A-B) Distance traveled in the 30-minute open field test 2 hours after injection was severely decreased in animals injected with LPS, but not in those with intraplantar formalin in either (A) C57Bl/6 or (B) Balb/c animals (n=8/grp). (C-D) Sick animals also spent very little time in the center area compared to saline injected animals in (C) Bl/6 or (D) Balb/c strains. While formalin injected Balb/c animals showed a trend to less time in the center, the difference was not statistically significant (p=0.1). Sal: saline control; Frmln: formalin-injected group.
Finally, as a positive control for the open field experiments, we also tested animals two hours after sickness was induced with an injection of LPS. Animals with LPS were fully capable of ambulating, as they reacted by running from the researchers hand as did control mice. In the open field, however, LPS-injected mice showed a profound reduction in both distance traveled and time spent in the center, indicating an increased anxiety state and an overall decrease in activity (Fig. 6). Since the lack of movement likely confounds activity measured in the center, when we normalized the distance in the center to the total distance moved, we also found that C57Bl/6 animals with LPS still showed significant anxiety-like behavior (5% vs. 0.8%, data not shown). We conclude that the test is sufficiently sensitive to detect changes in behaviors after sickness, but that pain alone is not sufficient to reveal differences.
Discussion
Humans with chronic pain often have higher measures of depression and anxiety [11,51] and score lower on quality of life inventories [31], which typically measure the impact of pain on daily activity, overall mood, sleep, social function, etc. [45]. Here we addressed the behavioral impact of persistent pain in animals by monitoring the daily life of a mouse using three different standard mouse models of chronic pain. We also studied the mice using a battery of tests of affective state. In the home cage, we found that none of the injury models had a lasting impact on basic parameters of daily life activity, such as daily food intake and locomotion. Only mice with CCI showed an early, but short-lived decrease of activity and only this group had significant alterations in other patterns of home cage daily life (e.g. increased time spent in the inactive state.) All groups showed similar behavior in tests of affective state. Taken together, these results indicate that despite the profound and prolonged mechanical hypersensitivity characteristic of these different “pain” models (inflammatory and neuropathic), there is minimal to no alteration in what we define as quality of life measures. Our results suggest that these standard models, at least when used in the mouse, do not adequately incorporate important features of the human chronic pain condition, raising questions as to whether mice experience significant ongoing pain with these injuries.
Home cage behavior is altered only early after CCI
Despite the findings of early postoperative changes in the CCI group, we found no changes in the SNI group of mice, even though the two nerve injury models are presumed to have similar etiology and both are associated with mechanical hypersensitivity. Comparison of results with sham-treated animals suggests that much of the difference is attributable to differences in the surgical procedures. For example, incisions for the CCI procedure are more proximal, which might be more disruptive to the animals. In effect, the initial decrease seen in the CCI groups could reflect differences in the time to fully recover from the surgery. On the other hand, neither sham group showed a significant development of hypersensitivity compared to naïve animals, demonstrating an important dissociation between the level of hypersensitivity and daily behavior in the home cage.
Somewhat surprisingly, however, the short-term reduction in home cage behavior in animals with CCI was not recapitulated in short-duration locomotor tests performed during the same time window. Indeed, we found very little motor deficit in either nerve injury group, despite the large denervation that occurs. This discrepancy suggests that the HCM may be more sensitive for detecting pain-related behavior than are these short duration tests of locomotor activity. Conceivably, decreases in home cage activity result from a summation of many small bouts of spontaneous pain, which the shorter open field tests miss. Contrary to this explanation, however, mice with persistent pain caused by intraplantar formalin, where there are measurable signs of discomfort, also did not alter activity in the open field. It is also possible that the HCM, which uses a more normal, familiar, environment, facilitates detection of behavioral differences that are otherwise masked by the novelty of the open field test.
There are, however, two limitations of the HCM method that merit discussion. First, we cannot directly measure sleep, disruption of which is an often-reported problem in chronic pain patients [48] and has been observed in some studies of rats with nerve injury or experimental arthritis [15,28,40,60]. By documenting periods and patterns of inactivity, the HCM system can, to some extent, provide a reasonable estimate of sleep. However, we have no information as to possible disruptions of sleep architecture. A second major limitation of the HCM is that the mice must be individually housed. Although our short-duration social interaction tests showed no differences, there could be significant persistent pain-associated disruption of normal social interaction among cage mates. Indeed, it is our impression that immediately after surgery, injured animals, in both the sham and nerve injury groups, sleep apart, not huddling, as do intact mice.
Alterations of affective state in standard chronic pain models
Previous studies using persistent pain models found conflicting results as to whether injured mice show changes in anxiety- and depression-like behaviors [4,17,42,59]. In the present study, we found that mice in the SNI, CCI or CFA groups did not differ in measures of affective state using an array of tests; this was true for weeks after injury. If anything, the few significant differences detected were all opposite to what we initially predicted, given previous studies in the rat. That is, mice appeared less, rather than more anxious. However, these few changes were neither consistent across all tests of anxiety state nor across mouse strain. Indeed, our results fit well with a previous study in mice that reported that nerve injury did not alter open field or elevated plus maze behavior at 1, 2 or 4 weeks after injury[17]. Moreover, our results are consistent between strain and injury type as well as with the absence of a long-term change in home cage behavior.
In contrast to our findings, there is a sizable body of work on rats showing changes in anxiety- and depression-like behaviors occurring after nerve injury and inflammation [12,16,19,20,25,30,54]. If CFA, CCI or SNI in mice produces pain that models the full human condition, or even replicates the behavior of a rat, we would expect an impact on the emotional state of a mouse. Our results could be due to the particular methods that we used or to the species itself. As a prey animal, mice do not show signs of weakness, including overt signs of persistent pain. Regarding the latter, it is of interest that we did observe changes in the LPS injected animal, indicating that mice have the capacity to display sickness behavior. There are, of course, other examples of rat behaviors do not translate to the mouse, such as the positive and negative affect of the different vocalization frequencies in the rat [22,50]. Finally, as some of these post-injury changes are age [30] and gender [23,37,46] dependent, it is also possible that our failure to replicate the rat data is simply a consequence of our focus on adult male mice.
These standard tests of affective behavior have two key problems in their use in pain studies. First, as for tests of mechanical hypersensitivity, the tests of emotional state are provocative, i.e. require an external stimulus. Evoked behavior might alter or mask the presence of spontaneous pain. This further highlights the importance of measuring daily home cage behavior, which, as a passive observation, is minimally disruptive of the animal’s life. The second limitation stems from the validity of these tests themselves. Indeed, there has been extensive discussion in the affective science literature on the use of open field, elevated maze and forced swim test to reliably assess “anxiety” and “depression” [8,52,53].
Dissociation of mechanical hypersensitivity and quality of life measures
The most common endpoint in pre-clinical trials in rodent chronic pain models is evoked hypersensitivity. At present there are few other surrogates for chronic pain in rodents. The failure of some drugs in clinical trials (after extensive preclinical validation) may thus reflect the limitation of hypersensitivity as the only endpoint for monitoring of pain. Although hypersensitivity measures may be very useful for understanding mechanisms of nociception and defining potential targets for treating hypersensitivity, they are likely inadequate for the study of persistent, spontaneous pain. Indeed, by their very nature, measures of evoked hypersensitivity ignore the emotional and spontaneous components of the human chronic pain experience. Only operant models have successfully addressed the emotional component, albeit still an evoked response. Here we found a complete dissociation of hypersensitivity and quality of life measures. The mechanisms that drive the development of hypersensitivity, therefore, might be independent or simply insufficiently severe to drive changes in the daily life of a mouse.
As noted above, behavioral manifestations of quality of life changes have, to some extent, been demonstrated in the rat, but, given the value of genetic manipulation, the lack of a quality of life surrogate measure of pain in mice is unfortunate. In fact, our results raise the possibility that mice do not experience the persistent, ongoing pain that would affect what we have defined as quality of life measures. Indeed, a recent study reported that CCI did not alter behavior in short-duration tests of locomotion nor in any measurable outward sign of pain when observed at 2 or 4 weeks after injury [39]. Our data, along with this report, suggest that in these commonly used models of “chronic pain” there are no reliable measures of spontaneous pain even if the animal does experience it. If we are to continue to use mice in chronic pain studies, it is clearly essential that we develop new pain models that more accurately mimic the key characteristics of chronic pain experienced by humans.
Supplementary Material
Acknowledgments
This work was supported by NIH grants NS14627 and T32 MH020006 and the Pell Family Foundation.
Footnotes
The authors declare no conflict of interest in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rochelle Urban, Department of Anatomy, University of California, San Francisco, San Francisco, CA 94158.
Gregory Scherrer, Department of Physiology and Cellular Biophysics, Columbia University, New York, NY 11032.
Evan H. Goulding, Department of Psychiatry, University of California, San Francisco, CA 94143
Laurence H. Tecott, Department of Psychiatry, University of California, San Francisco, CA 94143
Allan I. Basbaum, Department of Anatomy, University of California, San Francisco, San Francisco, CA 94158
References
- 1.Backonja M-M, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Bailey R, Kaskutas V, Fox I, Baum CM, Mackinnon SE. Effect of upper extremity nerve damage on activity participation, pain, depression, and quality of life. YJHSU. 2010;34:1682–1688. doi: 10.1016/j.jhsa.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146:34–40. doi: 10.1016/j.pain.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, Freund-Mercier MJ, Barrot M. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain. 2008;12:591–599. doi: 10.1016/j.ejpain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 6.Cain CK, Francis JM, Plone MA, Emerich DF, Lindner MD. Pain-related disability and effects of chronic morphine in the adjuvant-induced arthritis model of chronic pain. Physiol Behav. 1997;62:199–205. doi: 10.1016/s0031-9384(97)00158-3. [DOI] [PubMed] [Google Scholar]
- 7.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nature reviews Drug discovery. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 9.D’Almeida JA, de Castro-Costa CM, Frota CH, Severo JF, Rocha TD, Nogueira TF. Behavioral changes of Wistar rats with experimentally-induced painful diabetic neuropathy. Arq Neuropsiquiatr. 1999;57:746–752. doi: 10.1590/s0004-282x1999000500004. [DOI] [PubMed] [Google Scholar]
- 10.Deacon RMJ, Rawlins JNP. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav Brain Res. 2005;156:241–249. doi: 10.1016/j.bbr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Geisser ME, Roth RS, Theisen ME, Robinson ME, Riley JL., 3rd Negative affect, self-report of depressive symptoms, and clinical depression: relation to the experience of chronic pain. Clin J Pain. 2000;16:110–120. doi: 10.1097/00002508-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves L, Silva R, Pinto-Ribeiro F, Pêgo JM, Bessa JM, Pertovaara A, Sousa N, Almeida A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol. 2008;213:48–56. doi: 10.1016/j.expneurol.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Gottrup H, Nielsen J, Arendt-Nielsen L, Jensen TS. The relationship between sensory thresholds and mechanical hyperalgesia in nerve injury. Pain. 1998;75:321–329. doi: 10.1016/s0304-3959(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 14.Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH. A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci USA. 2008;105:20575–20582. doi: 10.1073/pnas.0809053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guevara-López U, Ayala-Guerrero F, Covarrubias-Gómez A, López-Muñoz FJ, Torres-González R. Effect of acute gouty arthritis on sleep patterns: a preclinical study. Eur J Pain. 2009;13:146–153. doi: 10.1016/j.ejpain.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, Kinchington PR, Dickenson AH, Pheby T, Rice ASC. Further characterization of a rat model of varicella zoster virus-associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasnie FS, Wallace VCJ, Hefner K, Holmes A, Rice ASC. Mechanical and cold hypersensitivity in nerve-injured C57BL/6J mice is not associated with fear-avoidance- and depression-related behaviour. Br J Anaesth. 2007;98:816–822. doi: 10.1093/bja/aem087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houghton AK, Kadura S, Westlund KN. Dorsal column lesions reverse the reduction of homecage activity in rats with pancreatitis. Neuroreport. 1997;8:3795–3800. doi: 10.1097/00001756-199712010-00028. [DOI] [PubMed] [Google Scholar]
- 19.Hu B, Doods H, Treede R-D, Ceci A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 2009;143:206–212. doi: 10.1016/j.pain.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain. 2007;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jourdan D, Ardid D, Eschalier A. Analysis of ultrasonic vocalisation does not allow chronic pain to be evaluated in rats. Pain. 2002;95:165–173. doi: 10.1016/s0304-3959(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 22.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 23.Koehl M, Battle S, Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29:1224–1231. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- 24.Kontinen VK, Ahnaou A, Drinkenburg WHIM, Meert TF. Sleep and EEG patterns in the chronic constriction injury model of neuropathic pain. Physiol Behav. 2003;78:241–246. doi: 10.1016/s0031-9384(02)00966-6. [DOI] [PubMed] [Google Scholar]
- 25.Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80:341–346. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- 26.Kupers RC, Nuytten D, De Castro-Costa M, Gybels JM. A time course analysis of the changes in spontaneous and evoked behaviour in a rat model of neuropathic pain. Pain. 1992;50:101–111. doi: 10.1016/0304-3959(92)90117-T. [DOI] [PubMed] [Google Scholar]
- 27.LaBuda CJ, Fuchs PN. A comparison of chronic aspartame exposure to aspirin on inflammation, hyperalgesia and open field activity following carrageenan-induced monoarthritis. Life Sci. 2001;69:443–454. doi: 10.1016/s0024-3205(01)01136-5. [DOI] [PubMed] [Google Scholar]
- 28.Landis CA, Robinson CR, Levine JD. Sleep fragmentation in the arthritic rat. Pain. 1988;34:93–99. doi: 10.1016/0304-3959(88)90186-8. [DOI] [PubMed] [Google Scholar]
- 29.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, Maagdenberg AMJMvd, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010:1–6. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 30.Leite-Almeida H, Almeida-Torres L, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ, Almeida A. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain. 2009;144:57–65. doi: 10.1016/j.pain.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Liedberg GM, Vrethem M. Polyneuropathy, with and without neurogenic pain, and its impact on daily life activities – A descriptive study. Disabil Rehabil. 2009;31:1402–1408. doi: 10.1080/09638280802621382. [DOI] [PubMed] [Google Scholar]
- 32.Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Üçeyler N, Valet M, Wasner G, Treede R-D. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzawa-Yanagida K, Narita M, Nakajima M, Kuzumaki N, Niikura K, Nozaki H, Takagi T, Tamai E, Hareyama N, Terada M, Yamazaki M, Suzuki T. Usefulness of Antidepressants for Improving the Neuropathic Pain-Like State and Pain-Induced Anxiety through Actions at Different Brain Sites. Neuropsychopharmacology. 2008;33:1952–1965. doi: 10.1038/sj.npp.1301590. [DOI] [PubMed] [Google Scholar]
- 35.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 36.Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 37.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 38.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 39.Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin J-S, Schorscher-Petcu A, Langford DJ, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain. 2010;6:34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monassi CR, Bandler R, Keay KA. A subpopulation of rats show social and sleep-waking changes typical of chronic neuropathic pain following peripheral nerve injury. Eur J Neurosci. 2003;17:1907–1920. doi: 10.1046/j.1460-9568.2003.02627.x. [DOI] [PubMed] [Google Scholar]
- 41.Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 42.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 43.Narita M, Kuzumaki N, Narita M, Kaneko C, Hareyama N, Miyatake M, Shindo K, Miyoshi K, Nakajima M, Nagumo Y, Sato F, Wachi H, Seyama Y, Suzuki T. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J Neurochem. 2006;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- 44.Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- 46.Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, Bock E, Pabst MA, Thoeringer CK, Huber HP, Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Pilowsky I, Crettenden I, Townley M. Sleep disturbance in pain clinic patients. Pain. 1985;23:27–33. doi: 10.1016/0304-3959(85)90227-1. [DOI] [PubMed] [Google Scholar]
- 49.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 50.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 51.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 52.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 53.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Roeska K, Doods H, Arndt K, Treede R-D, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139:349–357. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Schütz TCB, Andersen ML, Tufik S. Sleep alterations in an experimental orofacial pain model in rats. Brain Res. 2003;993:164–171. doi: 10.1016/j.brainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Seminowicz DA, Laferriere AL, Millecamps M, Yu JSC, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shields SD, Eckert WA, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 58.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki T, Amata M, Sakaue G, Nishimura S, Inoue T, Shibata M, Mashimo T. Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg. 2007;104:1570–1577. doi: 10.1213/01.ane.0000261514.19946.66. table of contents. [DOI] [PubMed] [Google Scholar]
- 60.Tokunaga S, Takeda Y, Shinomiya K, Yamamoto W, Utsu Y, Toide K, Kamei C. Changes of sleep patterns in rats with chronic constriction injury under aversive conditions. Biol Pharm Bull. 2007;30:2088–2090. doi: 10.1248/bpb.30.2088. [DOI] [PubMed] [Google Scholar]
- 61.Vonsy JL, Ghandehari J, Dickenson AH. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain. 2009;13:786–793. doi: 10.1016/j.ejpain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Vrinten DH, Hamers FFT. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102:203–209. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]
- 63.Wallace VCJ, Norbury TA, Rice ASC. Ultrasound vocalisation by rodents does not correlate with behavioural measures of persistent pain. Eur J Pain. 2005;9:445–452. doi: 10.1016/j.ejpain.2004.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.