Abstract
Purpose
We previously established a mechanistic rationale for Src inhibition as a novel therapeutic target in pancreatic cancer and have shown that activated STAT3 is a biomarker of resistance to Src inhibition. The purpose of this study was to translate the current understanding of complementary activated tyrosine kinase signaling pathways by targeting Src kinase and epidermal growth factor receptor (EGFR).
Experimental Design
IC50 values for dasatinib, a Src kinase inhibitor, erlotinib, an EGFR tyrosine kinase inhibitor and gemcitabine were determined and sensitive and resistant pancreatic cancer cell lines were identified. The in vitro and in vivo effects of these agents on multiple signaling pathways and tumorigenicity in pancreatic cancer were investigated.
Results
The combination of dasatinib, erlotinib and gemcitabine resulted in cooperative inhibition of cell migration and invasion of both sensitive and resistant pancreatic cancer cells as well as cooperative inhibition of multiple signaling pathways including FAK, AKT, ERK, JNK, MAPK and STAT3 at concentrations that were ineffective as individual agents or as double combinations of agents. The triple combination of agents was also most effective at inhibiting the growth of xenografts of both sensitive and resistant pancreatic cancer cells in vivo without increasing toxicity. Furthermore, combined inhibition of Src and EGFR with gemcitabine inhibited constitutively activated STAT3 in vitro and in vivo.
Conclusions
These results provide evidence that combined targeted biological therapy in addition to cytotoxic chemotherapy can overcome treatment resistance. Such treatment strategies may be used to tailor therapy based on identified biomarkers of resistance to targeted monotherapy.
Keywords: Pancreatic cancer, dasatinib, erlotinib, Src, STAT3
Introduction
Pancreatic cancer remains a major therapeutic challenge. Five-year survival remains abysmal, around 5%, and has not changed over the past 30 years (1). Cytotoxic chemotherapy based on the pyrimidine analogue, gemcitabine, remains the standard approach in the adjuvant and palliative setting, but results in minimal responses. The failure of conventional chemotherapeutic regimes to produce any meaningful impact on survival in patients with pancreatic cancer highlights a desperate need for novel treatment strategies.
Over the past decade, a large number of studies have shown that strategies targeting specific molecular abnormalities implicated in pancreatic oncogenesis may result in inhibition of pancreatic tumor growth in pre-clinical studies. However these results have failed to translate into clinical benefit in numerous Phase III trials of molecularly targeted therapies in patients with advanced pancreatic cancer even when combined with gemcitabine. Recently, a detailed, global, genomic analysis identified pancreatic tumors to be highly heterogenous, containing a large number of genetic alterations (average of 63) that affect a core set of 12 signaling pathways and processes that are genetically altered in 67–100% of cases (2). This suggests that treatment for pancreatic cancer should target these complex and overlapping signaling pathways, rather than just the products of a single gene. We sought to translate the current understanding of activated tyrosine kinase signaling pathways in pancreatic cancer into improved patient therapies by targeting two tyrosine kinases, the non-receptor Src kinase and epidermal growth factor receptor (EGFR), which acts in complementary pathways. We hypothesized that targeting multiple tumor-associated pathways will enhance the therapeutic effects of cancer treatment by overcoming the inherent and acquired resistance associated with targeted monotherapy.
The rationale for targeting Src and EGFR signaling is based on the fact that both EGFR and Src kinase specific activity are elevated in the majority of pancreatic cancers and involved in cancer progression and metastasis (3, 4). Moreover, high EGFR and Src activity is also an indicator of poor clinical prognosis (5, 6). EGFR and Src family members are involved in numerous signaling pathways involved in proliferation, migration, invasion, tumor adhesion, and angiogenesis (7, 8). Several current paradigms in tumor cell biology attribute growth promotion to EGFR activity, while Src activity is thought to promote invasion. As Src and EGFR are both commonly activated in pancreatic cancer, these two tyrosine kinases may act in concert to regulate tumor growth (9–11).
Erlotinib, a tyrosine kinase inhibitor (TKI) of EGFR, was recently approved for patients advanced stage, non-resectable, and metastatic pancreatic tumors based on a modest but significant survival benefit in combination with gemcitabine therapy (12). This limited clinical utility further emphasizes the heterogenous and complex nature of tumors that makes it extremely unlikely that all tumors within a particular subtype will harbor the same activating mutation, or that the tumor will be entirely dependent on the de-regulation of one particular signaling pathway. Preclinical studies have shown resistance to targeting the EGFR pathway, and hence failure of such therapies is likely due to cross-talk between pathways that would circumvent any inhibitory effects on downstream signaling (13).
Src, is one of nine members of the Src family of non-receptor protein tyrosine kinases. We have previously shown that Src kinase is over expressed with the progression of pancreatic neoplasia from normal pancreas to chronic pancreatitis to increasing grade of pancreatic adenocarcinoma (PDAC) (14). Under normal conditions, Src is a cytoplasmic protein that is maintained in an inactive form. It is not activated by a mutation, but plays a critical role in mediating multiple signal transduction pathways via its interactions with multiple proteins including G-protein coupled receptors, receptor tyrosine kinases, such as EGFR and integrins, making it an ideal target for therapeutic intervention (15).
Src directly modulates EGFR function through phosphorylation of tyrosine residues on EGFR that allows for coupling to downstream signaling events (16). Other interaction partners include signal transducers and activators of transcription (STATs), heterotrimeric G proteins, the mitogen-activated protein kinase (MAPK), extracellular signal regulated kinase (ERK) and focal adhesion kinase (FAK) (17, 18).
We and others have previously established a mechanistic rationale for Src inhibition as a therapeutic target in the treatment of pancreatic cancer (14, 19–21). In addition, we have identified that one of the mechanisms of resistance to Src inhibition appear to be related to a lack of inhibition of activated STAT3 signaling. This study focuses on a novel approach that shifts away from conventional chemotherapy combinations and instead focuses on combining targeted therapies to Src and EGFR in pancreatic cancer that can have direct clinical impact by overcoming the limited anti-tumor activity seen with individual targeted therapy.
Materials and Methods
Materials
Mouse monoclonal antibodies against STAT3 and Src; rabbit monoclonal antibodies against MAPK, pSrc (Tyr416), pSTAT3 (Ser727) and pSTAT3 (Tyr705); rabbit polyclonal antibodies against pFAK (Tyr925), pEGFR (Tyr845), pEGFR(Tyr1173), pAKT (Ser473), pMAPK (Thr202/Tyr204), AKT, STAT3 and FAK were purchased from Cell Signaling Technology, Inc. EGFR antibody was obtained from Upstate. Mouse polyclonal antibodies directed against pJNK, pERK1/2, and also Rabbit polyclonal antibodies directed against JNK and ERK were obtained from Santa Cruz Biotechnology, Inc. The secondary antibodies for western blots (anti-mouse and anti-rabbit IgG antibodies) were from Santa Cruz Biotechnology, Inc.
Dasatinib (BMS-354825) was kindly provided by Richard Smykla from Bristol-Myers Squibb Oncology. For in vitro study, stock solution of dasatinib or erlotinib in 100% DMSO was diluted directly into the medium to indicated concentrations and stored at −20°C. For in vivo oral gavage, dasatinib was prepared freshly as a suspension in 80 mM sodium citrate/citric acid buffer, pH 3.0. Erlotinib hydrochloride was purchased from LC laboratories and formulated as a fine suspension with sodium carboy methylcellulose and Tween 80 in water for oral gavage in vivo. Gemcitabine was purchased from Eli Lilly and Company and diluted the drug to 40 mg/ml normal saline (NS, preservative free 0.9% sodium chloride solution).
Cell culture and animals
Human pancreatic cancer cell lines BxPC3, PANC1, MiaPaca2, AsPC1, CEPAC, Capan1, Capan2, SW1990 and HPAC were obtained from American Type Culture Collection (ATCC). Tumor cells were maintained according to the ATCC procedures. Female Athymic Nude mice – Foxn1 nu/nu (4–5 weeks old) were purchased from Harlan Sprague Dawley, Inc. and maintained at the Vanderbilt University School of Medicine animal facility under protocols approved by the Vanderbilt Institutional Animal Care and Use Committee (IACUC).
Cell viability assay
Cells were treated with DMSO or dasatinib (0–5000 nmol/L) (14) or gemcitabine (0–300 nmol/L) or erlotinib (0–5000 nmol/L) for 48 hours and cell viability was determined by MTT (Sigma, St. Louis, MO, USA) assay according to the manufacturer’s direction. IC50 was calculated using Prism software package. The control vector shRNA, Src shRNA and EGFR shRNA cells were treated with or without erlotinib (100 nmol/L) or dasatinib (3 nmol/L) for 48 hours and the cell viability was determined as detailed above. Each condition was assayed in triplicate.
Wound-healing assay
Cells were treated with mitomycin C (0.5 μg/ml) for four hours prior to wounding. Wounds were made across the cell monolayer by a sterile pipette tip. After wounding, BxPC3 cells were treated with DMSO or dasatinib (5 nmol/L) and/or gemcitabine (50 nmol/L) and/or erlotinib (100 nmol/L); SW1990 cells were treated with DMSO or dasatinib (10 nmol/L) and/or gemcitabine (50 nmol/L) and/or erlotinib (200 nmol/L); PANC1 cells were treated with DMSO or dasatinib (100 nmol/L) and/or gemcitabine (200 nmol/L) and/or erlotinib (1000 nmol/L) for 40 hours. Phase contrast images were taken. After 40 hours of wound healing study, the cells were washed and treated with regular media for up to 15 days and observed for recovery post-wounding. Every second day removed the old media and changed to fresh media with 10% FBS. Days were counted to close the wound.
Migration and invasion assay
3×104 cells were seeded into the upper chamber of 8-μM pore transwells coated with collagen for migration and 50 μl (~100 μg) of diluted matrigel (BD Biosciences) solution for invasion assay. BxPC3 cells were treated with DMSO or dasatinib (5 nmol/L) and/or gemcitabine (50 nmol/L) and/or erlotinib (100 nmol/L); PANC1 cells were treated with DMSO or dasatinib (50 nmol/L) and/or gemcitabine (100 nmol/L) and/or erlotinib (500 nmol/L) was added to the lower chambers as a chemo attractants as indicated with medium containing 10% FBS. Cells were allowed to migrate for 5 hours or invade the matrigel for 24 hours. Migrated or invaded cells were fixed with 4% paraformaldehyde, stained with 1% crystal violet and counted from six random fields for each membrane at ×20 magnifications and averaged. The control vector shRNA, Src shRNA, EGFR shRNA and STAT3 shRNA cells were treated with or without erlotinib (100 nmol/L) or dasatinib (5 nmol/L) as described above. Cells were allowed to invade the matrigel for 24 hours, fixed, stained and calculated as indicated above. Each data point represents the average number from three wells.
Western blot analysis
Western blot analyses were done using standard methods (22). Cells were grown in complete media overnight and then treated with dasatinib and/or erlotinib and/or gemcitabine as required in each assay. Membranes were probed with total and phosphorylated antibodies as detailed above in materials.
Src and EGFR gene knockdown
We used Open Biosystems pGIPZ-based short hairpin RNA (shRNA) lentiviral vectors to deplete Src and EGFR expression. Human pancreatic cancer cell line BxPC3 was cultured in RPMI containing 10% FBS. Lentiviral shRNA vector pGIPZ with either targeting sequences for knocking-down human Src (Clone IDs: V2LHS_262793 and V2LHS_70230) or EGFR (Clone IDs: V2LHS_200678 and V2LHS_201187) or non-silencing control sequence was obtained from Vanderbilt University Microarray Core and transfected into BxPC3 cells with FuGENE 6 transfection reagent (Roche) following the manufacturer’s instruction. After 48 h, cells were cultured in 0.5 mg/ml puromycin containing media for selecting pGIPZ vector expressing cells. By gradually increasing the concentration of puromycin to 3 mg/ml and after 3–4 weeks of culture, vector encoded GFP expression was observed in all BxPC3 cells. Expression of Src and EGFR in the above vector transfected cells were characterized by Western Blot analysis.
In vivo tumorigenicity assay
Tumors were established by injecting 5×106 cells of BxPC3 or PANC1 into the flank of 6-week-old female athymic nude mice Fox1-nu/nu mice (n=5, in each group). Treatment was initiated when the tumors reached approximately 100mm3 size. Dasatinib (25 mg/kg) or erlotinib (50 mg/kg) or vehicle was administered by oral gavage; gemcitabine (20 mg/kg/3 days) was administered intra-peritoneal (IP). We administered dasatinib daily twice and erlotinib daily based on the substantially longerhalf-life of erlotinib [36 hours; ref.(23)] relative to dasatinib [3–5 hours; ref (24)]. The combination of dasatinib and/or gemcitabine and/or erlotinib was treated and tumor volume (V) was determined from caliper measurements obtained every two days and calculated by the equation V = L × W2 × 0.5, where L is length and W is width of a tumor. The percent body weight change for each mouse was calculated with the following formula: [(Wn–W0)/W0] × 100% (in which Wn is the mouse weight on dayn). At the end of the study, animals were sacrificed and their primary tumors were removed for further analysis. Growth curves for tumors were plotted as the mean volume ± SD of tumors of mice from each group. All experiments were done in compliance with the Vanderbilt IACUC guidelines.
Immunohistochemistry
Mice were euthanized and tumor tissues were collected for immunohistochemical analysis. Tissues were fixed and immunostained using antibodies against cleaved caspase-3, pSrc (Tyr527), pAKT (Ser473), pEGFR (Tyr1173), pSTAT3 (Tyr705) and Ki67 (Biocare). Cleaved caspase-3, Ki67, pSrc, pAKT, pEGFR and pSTAT3 were evaluated by an expert pathologist (M.K.W). For Ki67, caspase-3, pSrc and pAKT staining quantification, positive staining was quantified by using NIH image analysis software, Image J, and reported as percentage area of staining.
Statistical analysis
Descriptive statistics including mean values and SD were calculated using Microsoft Excel and Prism software (Graphpad). All data represent at least three independent experiments and are expressed as the means ± SD unless otherwise indicated. ANOVA was used to assess the differences between experimental groups unless otherwise indicated.
Results
Determination of sensitivity to dasatinib, erlotinib and gemcitabine in pancreatic cancer cell lines
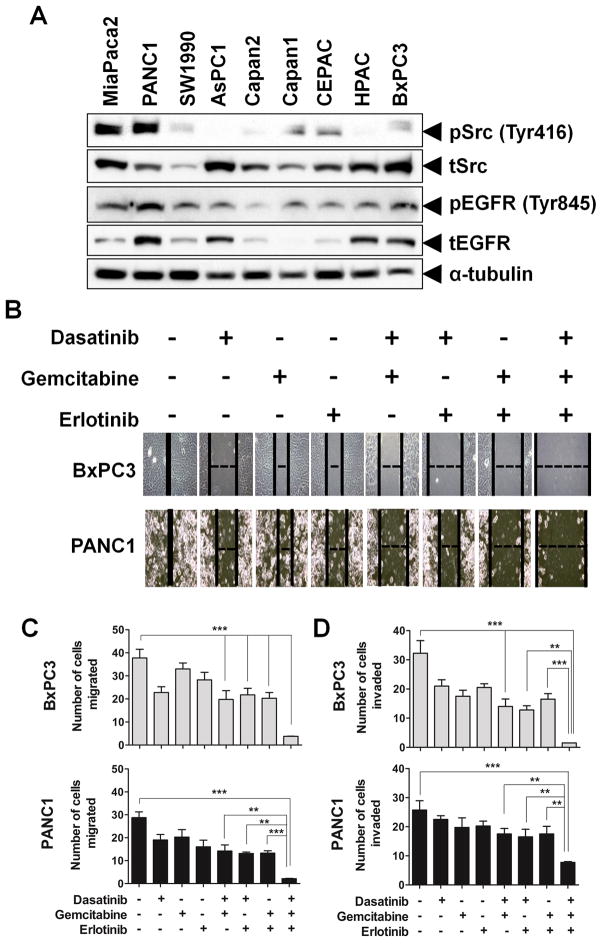
We screened nine human pancreatic cancer cell lines derived from different stages of tumor and with varied genetic backgrounds. IC50 values of dasatinib, erlotinib and gemcitabine treatment were determined in these cell lines and identified both sensitive and resistant lines (Table 1) (14). All three drugs showed antiproliferative activity at nanomolar concentrations in all cell lines studied. BxPC3 and HPAC cell lines, which showed the greatest sensitivity to dasatinib treatment, were also markedly more sensitive to both erlotinib and gemcitabine. Furthermore, MiaPaca2, PANC1 and AsPC1 cells which are least sensitive to dasatinib treatment were also more resistant to erlotinib or gemcitabine treatment. We assessed the expression of pSrc, Src, pEGFR and EGFR protein in all nine cell lines (Fig. 1A). pSrc and pEGFR expression was lower in BxPC3 when compared to PANC1 cells.
Table 1.
Pancreatic cancer cell lines characteristics and their IC50 values for dasatinib, erlotinib and gemcitabine
| Cell Line | Derivation | Genetic background | IC 50 (nmol/L) |
||
|---|---|---|---|---|---|
| Dasatinib* | Erlotinib | Gemcitabine | |||
| MiaPaca2 | Primary | Mutant K-ras , mutant p53 , wt smad4, wt EGFR |
51.28 | 122.3 | 49.99 |
| PANC1 | Primary | Mutant K-ras , mutant p53 , wt smad4, mutant EGFR |
45.68 | 1118 | 100.2 |
| AsPC1 | Metastasis (ascites) | Mutant K-ras , wt p53 , wt smad4, wt EGFR |
44.97 | 693.1 | 39.96 |
| CEPAC1 | Metastasis (liver) | Mutant K-ras , wt p53 , mutant smad4, ND EGFR |
24.99 | 431.6 | 50.55 |
| Capan1 | Metastasis (liver) | Mutant K-ras , ND p53 , mutant smad4, mutant EGFR |
9.6 | 317.2 | 30.12 |
| SW1990 | Metastasis (spleen) | Mutant K-ras , wt p53 , ND smad4, ND EGFR |
5.1 | 548.3 | 29.78 |
| Capan2 | Primary | Mutant K-ras , wt p53 , wt smad4, ND EGFR |
4.87 | 125 | 20.08 |
| HPAC | Primary | Mutant K-ras , wt p53 , wt smad4, mutant EGFR |
2.79 | 49.9 | 30.16 |
| BxPC3 | Primary | Wt K-ras , mutant p53 , mutant smad4, wt EGFR |
2.8 | 99.7 | 30.09 |
wt: wild type; ND: not determined;
: data from Nagaraj et al. (14)
Fig. 1.
Inhibition of cell motility, migration and invasion with dasatinib, erlotinib and gemcitabine in pancreatic cancer cells. (A) Expression of pSrc, Src, pEGFR and EGFR in human pancreatic cancer cell lines. Cells were grown to 70% to 80% confluence then cell lysates were prepared from nine human pancreatic cancer cell lines as indicated and immunoblotted for pSrc, Src, pEGFR and EGFR as described in Materials and Methods. The blots were subsequently stripped and reprobed for α-tubulin. BxPC3 and PANC1 cells were treated with their respective IC50 doses of dasatinib (BxPC3-5 nmol/L; PANC1-50 nmol/L), erlotinib (BxPC3-100 nmol/L; PANC1-1000 nmol/L) and gemcitabine (BxPC3-30 nmol/L; PANC1-100 nmol/L). Treatment with the combination of the three drugs significantly decreased (B) cell motility, (C) migration and (D) invasion compared with treatment of individual or double combinations of drugs. BxPC3 cells were significantly more sensitive with any agent studied when compared with more resistant PANC1 cells. Individual data points represent the mean ± SD of three independent experiments. **p<0.01; ***p<0.001.
We therefore sought to determine if combining these targeted therapies with gemcitabine would overcome the resistance seen with these individually agents alone. The combination of dasatinib, erlotinib and gemcitabine inhibited cell viability (Supplementary Fig. S1) and increased apoptotic activity (Supplementary Fig. S2) of pancreatic cancer cells in vitro to a greater degree than individual or double combination of agents.
Src and EGFR inhibition with gemcitabine augments attenuation of cell motility, migration and invasion of pancreatic cancer cells
We investigated the functional effects of dasatinib, erlotinib and gemcitabine on cell motility, migration and invasion (Fig. 1). The effect of these drugs on cell motility was evaluated initially using a monolayer wound-healingassay. Cells were treated with IC50 doses of these drugs for 40 hours. Optimal inhibition of wound closure was seen with the combined treatment of dasatinib, erlotinib and gemcitabine as compared with individual and two-agent combinations. Complete wound closure was seen in 30–35 hours in all of the untreated cells (Fig. 1B). Removal of the agents resulted in re-migration of cells into the wound (Supplementary Fig. S3).
Single agent treatment weakly affected both cell migration and invasion of BxPC3 and PANC1 cells. Double combination therapy of dasatinib, erlotinib or gemcitabine augmented the effect of cell migration and invasion compared with treatment of individual agents. However, the addition of gemcitabine to the combination of dasatinib and erlotinib resulted in the optimal inhibition of cell migration and invasion in both sensitive BxPC3 as well as resistant PANC1 cells (Figs. 1C and 1D). These data indicate that combined treatment of dasatinib and erlotinib with gemcitabine elicits the greatest anti-invasive properties of pancreatic cancer cells.
Co-operative effects of Src and EGFR inhibition
Since dasatinib is a multi-targeted kinase inhibitor, and erlotinib can affect other kinase pathways besides EGFR, we sought to confirm that the effects of dasatinib and erlotinib were specific to their activity on Src kinase and EGFR inhibition respectively. We stably inhibited the expression levels of Src and EGFR in BxPC3 cells using small interfering RNA (Lentiviral-shRNA vector) as previously described (Supplementary Fig. S4) (14). Cell viability (Fig. 2A) and invasion assays (Fig. 2B) were performed in non-silencing vector stably transfected control cells, and Src or EGFR knockdown cells. Src shRNA cells were treated with erlotinib and EGFR shRNA cells were treated with dasatinib to determine the cooperative effects of Src and EGFR inhibition. Erlotinib treatment resulted in a 54% growth inhibition at 48 hours in vehicle treated control (non-silencing) shRNA cells (P<0.05). The antiproliferative effects of erlotinib were significantly increased when added to Src shRNA cells (44.2% compared with erlotinib treated control shRNA cells, p<0.005). Dasatinib treatment resulted in a 24% growth inhibition at 48 hours in vehicle treated control shRNA cells (P<0.0001). The antiproliferative effects of dasatinib were increased significantly when combined with EGFR shRNA cells (62.2% compared with dasatinib treated control shRNA cells, p<0.001) (Fig. 2A). Similarly, Src shRNA and EGFR shRNA significantly reduced BxPC3 cell invasion when combined with erlotinib (78.2% compared with erlotinib treated control shRNA cells, p<0.001) or dasatinib (67.8% compared with dasatinib treated control shRNA cells, p<0.05), respectively, when compared with control shRNA cells treated with these agents alone (Fig. 2B). These results confirm that dasatinib and erlotinib augment inhibition of cell viability and invasion through Src and EGFR signaling.
Fig. 2.
Cooperative effect of Src and EGFR inhibition with gemcitabine on cell signaling. BxPC3 vector control-shRNA, Src shRNA and EGFR shRNA cells were investigated with dasatinib (5 nmol/L) or erlotinib (100 nmol/L) for anti-proliferative activity by MTT assay and anti-invasion by matrigel invasion assay. Significantly reduction in (A) cell viability and (B) invasion was seen with combined targeting of Src and EGFR signalling. Individual data point represents the mean ± SD of three independent wells. *p<0.05; **p<0.01; ***p<0.001. (C) BxPC3 cells were treated with dasatinib (5 nmol/L) and/or gemcitabine (30 nmol/L) and/or erlotinib (100 nmol/L); PANC1 cells were treated with dasatinib (25 nmol/L) and/or gemcitabine (75 nmol/L) and/or erlotinib (500 nmol/L) for 12 h, lysed, and analyzed by Western blotting with indicated antibodies. At doses that had minimal effects on inhibition of any signaling pathway, only the triple combination of dasatinib, erlotinib and gemcitabine showed inhibition of pSrc, pEGFR, pFAK, pAKT, pSTAT3, pERK, pJNK or pMAPK. Furthermore, only this triple combination overcomes constitutive activation of STAT3-mediating signaling seen in PANC1 cells. Blots are representative of at least two separate experiments with similar results.
Cooperative effect of dasatinib, erlotinib and gemcitabine on signal transduction in pancreatic cancer
We have previously shown that Src inhibition results in decreased phosphorylation of FAK, paxillin, AKT, STAT3, ERK, JNK and MAPK in a time and concentration-dependent manner in pancreatic cancer cell lines sensitive to dasatinib, whereas one of the mechanisms of resistance to Src inhibition appear to be related to a lack of inhibition of STAT3 signaling in resistant PANC1 cells (14). As revealed by immunoblotting of total cell lysates (Fig. 2C), at IC50 concentrations of dasatinib, erlotinib and gemcitabine for each cell line, there is minimal effect on cellular signaling in both BxPC3 and PANC1 cells. The combination of dasatinib and erlotinib enhances the inhibition of several signaling pathways, more so in sensitive BxPC3 cells compared with PANC1 cells. However, the addition of gemcitabine to dasatinib and erlotinib results in inhibition of pSrc, pEGFR, pFAK, pAKT, pERK and pJNK activity in both sensitive BxPC3 and resistant PANC1 cells. More importantly, the combination of these three agents overcomes the constitutive activation of STAT3-mediating signaling seen in PANC1 cells as previously described (14) (Fig. 2C).
Combined inhibition of Src kinase and EGFR with gemcitabine attenuates pancreatic tumor growth in vivo
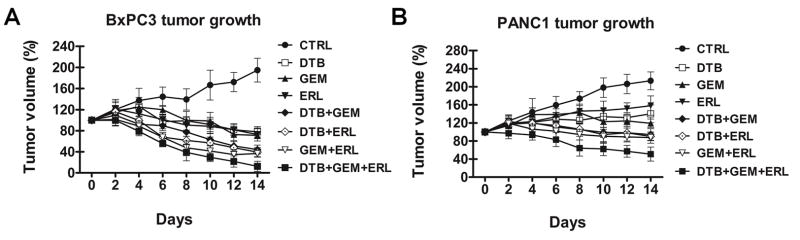
BxPC3 and PANC1 tumor bearing mice were treated with dasatinib (25 mg/kg), erlotinib (50 mg/kg) and gemcitabine (20 mg/kg) either individually or in double or triple combinations. Relatively low doses of these drugs were selected to enable us to determine a cooperative effect in combination. In BxPC3 xenografts (Fig. 3A), treatment with dasatinib, erlotinib or gemcitabine alone and with the combination of two agents inhibited tumor growth and caused some tumor regression. Compared with vehicle treated control tumors, tumor volume of BxPC3 xenografts treated with dasatinib and erlotinib treatment was decreased 79.8% (p<0.001), 77.3% (p<0.001) with dasatinib and gemcitabine treatment and 81.1% (p<0.001) with gemcitabine and erlotinib treatment. Optimal tumor regression, however, was seen with the triple combination of dasatinib, erlotinib and gemcitabine (93.6% compared with vehicle treated xenografts, p<0.001). In the more resistant PANC1 xenografts (Fig. 3B), treatment with dasatinib, erlotinib or gemcitabine alone and with the combination of two agents inhibited tumor growth, but did not result in any tumor regression. Only the combination of dasatinib, erlotinib and gemcitabine treatment resulted in tumor regression (75.9% compared with vehicle treated xenografts, p<0.001). The addition of dasatinib and erlotinib to gemcitabine produced minimal weight loss in BxPC3 and PANC1 tumor bearing mice, suggesting that these combinations did not produce significant in vivo toxicity (Supplementary Fig. S5). The length of time of the in vivo studies was limited due to the tumor-related morbidity of the control animals.
Fig. 3.
Combination of dasatinib, erlotinib and gemcitabine effectively inhibits tumor growth in vivo. Mice with subcutaneously established tumors from (A) BxPC3 (n=5) (B) or PANC1 (n=5) cells were treated with dasatinib (DTB) at 25 mg/kg daily and/or gemcitabine (GEM) at 20 mg/kg for every 3 days and/or erlotinib (ERL) at 50 mg/kg/daily or vehicle for 14 days. Growth curves for tumors are presented as the mean ± SD of five tumors in each data point.
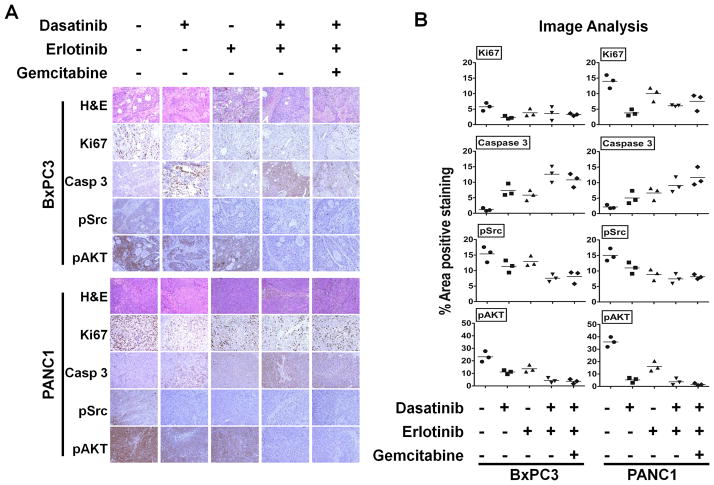
Combined inhibition of Src kinase and EGFR with gemcitabine correlates with in vivo inhibition of proliferation and increased apoptosis in pancreatic tumors
To correlate our in vitro finding of Src and EGFR inhibition with gemcitabine in vivo, immunohistochemical analysis of treated tumor xenografts of BxPC3 and PANC1 cells relative to vehicle-treated controls was measured. Tumor tissues were evaluated for Ki67, cleaved caspase 3 and phosphorylated Src and AKT immunoreactivity (Fig. 4A). Percent area of positive staining was analyzed by Image J software (Fig. 4B). BxPC3 and PANC1 tumors treated with dasatinib, erlotinib individually or in combination and with gemcitabine all showed a decrease in cell proliferation (Ki67). Compared with individual or combined targeted therapy of dasatinib and erlotinib, the addition of gemcitabine to this combination exhibited the optimal increase in apoptosis (cleaved caspase-3) and optimal down-regulation of pSrc Tyr527 and pAKT Ser473. The reduction in Ki67 staining with the combination of dasatinib, erlotinib and gemcitabine appears to be greater in PANC1 xenografts. Caspase 3, pAKT and pSrc staining are similar in BxPC3 and PANC1 xenografts. Taken together, these results show that in pancreatic cancer, the combination of dasatinib and erlotinib with gemcitabine results in optimal in vivo effects of apoptosis and inhibited tumor growth.
Fig. 4.
Effect of Src and EGFR inhibition on proliferation, apoptosis, angiogenesis and phosphorylation of Src and AKT in vivo. Representative examples of immunohistochemical analysis of (A) BxPC3 and PANC1 tumor tissues stained with H&E, Ki67, cleaved caspase 3, pSrc and pAKT antibodies are shown. There is no evidence of treatment-induced necrosis on H&E staining. Magnification ×20. (B) The percent area positive staining was determined using Image J image analysis. Compared with vehicle treated controls or any single or double agent treatment, mice treated with the triple combination of dasatinib, erlotinib and gemcitabine show reduced expression of pSrc and pAKT and increased cleaved caspase 3 staining in their tumors, showing successful target inhibition. Individual data point represents the mean ± SD of three independent tissue samples analyzed in each treatment.
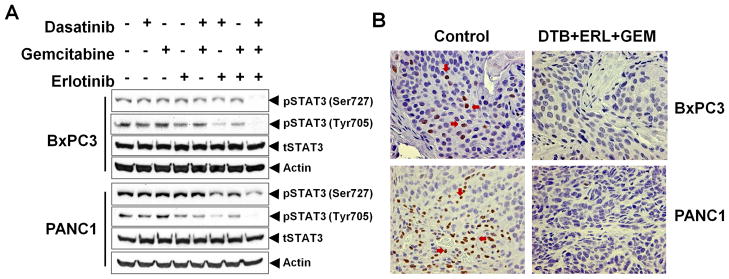
Combined inhibition of Src and EGFR with gemcitabine inhibits constitutively activated STAT3
We have previously shown that activated STAT3 signaling is associated with resistance to Src inhibition in resistant PANC1 pancreatic cancer cells (14). As analyzed by Western blot, treatment with dasatinib, erlotinib or gemcitabine as individual agents or in double combinations showed no decrease in pSTAT3 (both Ser727 and Tyr705) levels. Only the combination of dasatinib, erlotinib and gemcitabine showed substantially decreased inhibition of activated STAT3, even in resistant PANC1 xenografts tissues (Fig. 5A), suggesting that combining multi-targeted agents with cytotoxic agents can overcome resistance to targeted monotherapy. Both cytoplasmic and nuclear expression of phosphorylated STAT3 was seen in the control tumors and this expression was significantly decreased in tumors treated with dasatinib, erlotinib and gemcitabine (Fig. 5B).
Fig. 5.
Effect of Src and EGFR inhibition with gemcitabine on constitutively activated STAT3. (A) BxPC3 and PANC1 tumor tissue lysates were analyzed for pSTAT3 (Ser727 and Tyr705) and total STAT3. Only the combined treatment of dasatinib and erlotinib with gemcitabine inhibits constitutively activated STAT3 in both BxPC3 and PANC1 tumor xenografts in vivo. (B) Representative examples of immunohistochemical analysis of BxPC3 and PANC1 tumor tissues of vehicle (control) and dasatinib (DTB)+erlotinib (ERL)+gemcitabine (GEM) treated, and were stained for pSTAT3 (positive staining in brown is indicated by arrows).
Discussion
Treatment for pancreatic cancer remains a therapeutic challenge due to the lack of any effective therapy. Despite a greater understanding of the molecular pathways involved in pancreatic tumorigenesis, the use of individual targeted agents, have failed to provide meaningful improvements in survival. We investigated a strategy of targeting multiple tumor-associated pathways, namely, activated EGFR and Src tyrosine kinase signaling, which act as complementary pathways in pancreatic cancer to promote tumor growth. Nine human pancreatic cancer cell lines were characterized for their expression of Src and EGFR, phosphorylated Src and EGFR (Fig. 1A) and sensitivity to dasatinib, erlotinib and gemcitabine, identifying cell lines that were sensitive and resistant to these agents (Table 1). Interestingly, pancreatic cancer cell lines that were sensitive to dasatinib were also sensitive to erlotinib and gemcitabine treatment, while cell lines that were more resistant to dasatinib also showed greater resistance to erlotinib and gemcitabine therapy, suggesting an inherent resistance to individual cytotoxic or targeted therapies.
Numerous reasons exist to expect that targeting multiple tumor-associated pathways will enhance the therapeutic effects of cancer treatment. As stated above, even though the genetic alterations associated with pancreatic cancer can be associated with a core set of 12 signaling pathways, the processes that are genetically altered vary amongst each individual patient (2). In addition, the interplay between tumor cells and surrounding supportive cells such as vascular endothelial cells and pericytes, fibroblasts and immune cells adds to the complexity of altered cellular signaling to stimulate tumor growth, clearly suggesting that targeting a single component will not result in sustained inhibition of tumor growth. Moreover, even with the use of cytotoxic or targeted therapies, parallel and reciprocal signaling pathway activation can promote resistant cell clones to continue to expand, resulting in refractory tumor response to a given agent that affects a single mechanism of action. Therefore, targeting multiple signaling pathways involved in tumor growth has the potential to overcome either primary or acquired resistance to targeted monotherapy and increase the likelihood of sustained response by affecting different mechanisms of action associated with cancer development and enhance the effects of conventional cytotoxic chemotherapy. Our results clearly indicate that combined inhibition of Src and EGFR signaling results in improved inhibition of pancreatic tumorigenesis and potentiates the effect of gemcitabine chemotherapy, the addition of which further enhances the anti-tumor effects of EGFR and Src inhibition.
Resistance to cytotoxic chemotherapy has remained a major factor in the poor outcomes seen in patients with pancreatic cancer. Inhibition of Src signaling has been shown to restore sensitivity to gemcitabine and 5-flourouracil in human pancreatic cancer cells (19, 25, 26). Similarly, silencing of FAK expression in PANC1 cells restores sensitivity to gemcitabine treatment (27). In addition, Src inhibition has also been shown to increase sensitivity to other cytotoxic agents such as oxaliplatin in colon cancer (28). This implicates Src inhibition in restoring chemosensitivity and suggests a novel approach using dasatinib in combination with gemcitabine for the treatment of pancreatic cancer. However, we show that at IC50 doses of dasatinib, erlotinib and gemcitabine as individual agents or as double combinations of agents have minimal effects on inhibiting signaling of multiple tumor-associated pathways in both BxPC3 and PANC1 cells, whereas only the triple combination dasatinib and erlotinib with gemcitabine resulted in cooperative inhibition of these pathways (Fig. 2C). These results support the idea that modulation of chemoresistance may require the inhibition of both Src and EGFR signaling, requiring targeting of different pathways at multiple levels.
The heterogeneity of genetic alterations associated with pancreatic cancer, as with most solid tumors for that matter, makes it highly unlikely that a “one size fits all” approach will be effective and the need to identify biological markers that can predict treatment response (or lack of response) will be essential to optimize targeted treatment strategies. We have previously shown that activated STAT3 is a biomarker of resistance to Src inhibition with dasatinib in resistant PANC1 cells (14). STAT3 is activated by a variety of growth factors receptors such as EGFR via non-receptor tyrosine kinases such as Src family kinases (SFKs) (29). STAT3 activation regulates oncogenic signaling in many tumor types and leads to increased cell survival, proliferation and tumor growth (30). Constitutively active STAT3 is sensitive to both EGFR and Src inhibition (31). However, despite early suppression of aberrant STAT3 signaling, sustained EGFR and Src inhibition can result in Janus kinase (JAK)-mediated reciprocal activation of STAT3 signaling (30). This type of feedback and parallel signaling limits the effectiveness of individual targeted therapies as seen in our results in which dasatinib or erlotinib, either individually or in combination did not result in inhibition of activated STAT3 signaling. Only the combination of dasatinib and erlotinib with gemcitabine overcame STAT3-mediated resistance of EGFR and Src inhibition both in vitro and in vivo. These results implicate treatment strategies not only attacking multiple targets, but combining these multi-targeted approaches with cytotoxic chemotherapies.
Although our results show that this triple combination of agents optimizes pancreatic cancer therapy, the addition of multiple targeted or cytotoxic drugs can also add potentially toxicity when administered to patients in combination. This emphasizes the importance of identifying molecular markers of resistance, such as activated STAT3, to tailor therapies targeting specific resistant pathways. We confirmed the role of activated STAT3 as a biomarker of resistance to Src inhibition.
Our results clearly suggest that the combination of dasatinib and erlotinib with gemcitabine may be a potent treatment regimen for pancreatic cancer and overcomes STAT3 mediated resistance of inhibition of pancreatic tumorigenesis. Taken together, our findings provide compelling evidence establishing the role of combined targeted therapy with cytotoxic chemotherapy as a paradigm to overcome resistance associated with reciprocal and parallel signaling seen with biologically targeted monotherapy. In addition, identifying biomarkers of resistance to targeted therapy, such as activated STAT3 signaling, can result in tailoring treatment to target specific resistant pathways, thereby limiting the toxicity associated with delivery of multiple agents.
Translational Relevance.
The failure of conventional chemotherapeutic regimes to produce any meaningful impact on survival in patients with pancreatic cancer highlights a desperate need for novel treatment strategies. Src inhibitors represent a novel class of targeted drugs that have disease activity in several tumor types, however, have shown limited single-agent activity. One of the mechanisms of resistance to Src inhibition appears to be related to a lack of inhibition of activated STAT3 signaling. This study characterizes the in vitro and in vivo molecular effects of targeting two complementary tyrosine kinase pathways, Src with dasatinib and EGFR with erlotinib, combined with gemcitabine chemotherapy. Our results suggest that this combination of agents may be a potent treatment regimen for pancreatic cancer and provide evidence that combined targeted biological therapy in addition to cytotoxic chemotherapy can overcome treatment resistance. Such treatment strategies may be used to tailor therapy based on identified biomarkers of resistance to targeted monotherapy.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. R. Daniel Beauchamp and Wael El-Rafai for their helpful discussion and critical reading of the manuscript, Mr. Jason M. Herndon for assistance with the animal experiments and Mr. Frank Revetta for his technical assistance with immunohistochemical staining.
Grant Support: P50 95103 GI Special Program of Research Excellence Grant (SPORE Project 1), 5P30DK058404-08 Digestive Disease Research Center (DDRC) Translational Award, Vanderbilt-Ingram Cancer Center Support Grant 5P30 CA068485-1 and Core services performed through Vanderbilt University Medical Center’s DDRC supported by NIH grant P30DK058404 (N.B. Merchant). Special thanks to Mrs. Ginny Auschwitz and family for provision of funds to N.B. Merchant.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clinicalcancerres.aacrjournals.org/).
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992;90:1352–60. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutz MP, Esser IB, Flossmann-Kast BB, et al. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun. 1998;243:503–8. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–9. [PubMed] [Google Scholar]
- 6.Ueda S, Ogata S, Tsuda H, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 7.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5:203–20. doi: 10.1158/1541-7786.MCR-06-0404. [DOI] [PubMed] [Google Scholar]
- 9.Stover DR, Becker M, Liebetanz J, Lydon NB. Src Phosphorylation of the Epidermal Growth Factor Receptor at Novel Sites Mediates Receptor Interaction with Src and P85[IMAGE] J Biol Chem. 1995;270:15591–7. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 10.Biscardi J, Belsches A, Parsons S. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Molecular Carcinogenesis. 1998;21:261–72. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Biscardi JS, Tice DA, Parsons SJ. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 12.Rivera F, Lopez-Tarruella S, Vega-Villegas ME, Salcedo M. Treatment of advanced pancreatic cancer: from gemcitabine single agent to combinations and targeted therapy. Cancer Treat Rev. 2009;35:335–9. doi: 10.1016/j.ctrv.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Ali S, Banerjee S, Ahmad A, El-Rayes BF, Philip PA, Sarkar FH. Apoptosis-inducing effect of erlotinib is potentiated by 3,3′-diindolylmethane in vitro and in vivo using an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2008;7:1708–19. doi: 10.1158/1535-7163.MCT-08-0354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Nagaraj NS, Smith JJ, Revetta F, Washington MK, Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther. 2010;9:2322–32. doi: 10.1158/1535-7163.MCT-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–68. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 16.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Hecker TP, Grammer JR, Gillespie GY, Stewart J, Gladson CL. Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Research. 2002;62:2699–707. [PubMed] [Google Scholar]
- 18.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14:667–78. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yezhelyev MV, Koehl G, Guba M, et al. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10:8028–36. doi: 10.1158/1078-0432.CCR-04-0621. [DOI] [PubMed] [Google Scholar]
- 20.Trevino JG, Summy JM, Lesslie DP, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–72. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajeshkumar NV, Tan AC, De Oliveira E, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009;15:4138–46. doi: 10.1158/1078-0432.CCR-08-3021. [DOI] [PubMed] [Google Scholar]
- 22.Merchant NB, Voskresensky I, Rogers CM, et al. TACE/ADAM-17: a component of the epidermal growth factor receptor axis and a promising therapeutic target in colorectal cancer. Clin Cancer Res. 2008;14:1182–91. doi: 10.1158/1078-0432.CCR-07-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu JF, Eppler SM, Wolf J, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80:136–45. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Shah NP, Kantarjian HM, Kim D-W, et al. Intermittent Target Inhibition With Dasatinib 100 mg Once Daily Preserves Efficacy and Improves Tolerability in Imatinib-Resistant and -Intolerant Chronic-Phase Chronic Myeloid Leukemia. J Clin Oncol. 2008;26:3204–12. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 25.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. Journal of the American College of Surgeons. 2004;198:953–9. doi: 10.1016/j.jamcollsurg.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of Src tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clinical Cancer Research. 2004;10:2307–18. doi: 10.1158/1078-0432.ccr-1183-3. [DOI] [PubMed] [Google Scholar]
- 27.Ischenko I, Camaj P, Seeliger H, et al. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene. 2008;27:7212–22. doi: 10.1038/onc.2008.326. [DOI] [PubMed] [Google Scholar]
- 28.Kopetz S, Lesslie DP, Dallas NA, et al. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69:3842–9. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song LX, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–65. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 30.Byers LA, Sen B, Saigal B, et al. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res. 2009;15:6852–61. doi: 10.1158/1078-0432.CCR-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaganathan S, Yue P, Turkson J. Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant Stat3 and EGFR or Src. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.109.162669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.