Abstract
This protocol describes a simplified method for inducing a chronic depression-like state in mice that is based on the repeated open-space forced swim method for rats originally developed by Sun and Alkon (2003). The method consists of swimming mice daily in lukewarm water (32-34°C) in rat tub cages 24 × 43 × 23 cm w × h × l, for 15 min/day for 4 days, and thereafter once per week. This procedure produces a progressive decrease in distance swum and a concomitant increase in immobility (floating) in about 70 percent of the mice (Swiss Webster males), both of which persist unaltered for weeks and generalize to other tests of depression (tail suspension). The model has predictive, face and construct validity in that it is responsive to chronic antidepressants and coping responses but not to anxiolytics or antipsychotics, represents an inescapable stress that produces generalized passivity, and is accompanied by changes in neural activity and brain cell proliferation that are characteristic of depression and believed to contribute to the disorder. It is less effective in producing anhedonia than other models probably because it is less stressful. The model has a number of advantages over previous methods in that it utilizes very mild stress, is short in duration, is easily standardized, requires only a video camera and either a manual or automatic behavioral scoring system to measure immobility and distance swum, and can be readily used for time course studies of onset of drug action. Moreover, since it utilizes a greater swimming area than the traditional (Porsolt) method it can be used to study interactions of depressive behavior with behavioral flexibility and perseveration. Finally, its use of mice makes it readily amenable to genetic and molecular analyses.
Keywords: depression, open space, forced swim, model, mouse, immobility
INTRODUCTION
A number of animal models of depression are currently available. In general, these can be divided into those producing acute and chronic depression. Examples of the acute kind are the forced swim (Porsolt et al., 1977) and tail suspension tests (Steru et al., 1985); while of the chronic type are chronic mild stress (CMS) (Willner, 1997), learned helplessness (LH) (Seligman, 1976;Weiss et al., 1975) and prolonged subordination stress (Blanchard et al., 1993). The acute models are induced rapidly in one or two sessions and can respond to acute or subchronic antidepressant drug administration (Kitada et al., 1981). In contrast, the chronic models are induced either over a period of weeks (CMS and subordination) and/or require exposure to traumatic stress (LH and subordination) and only respond to prolonged treatment with antidepressants. Because depressed patients also only respond to chronic treatment, the chronic depression models are deemed to possess greater face validity than the acute ones with respect to human depression. However, the long induction periods or severe stress requirement of the chronic models have represented significant drawbacks. Consequently, there has been a need to develop more rapid and milder methods for the induction of chronic depression. To meet this need Sun and Alkon (2003) developed the repeated open-space forced swim model for rats in which the animals are subjected to short daily swims for three days. They found that this procedure produces a long lasting syndrome of passive, inactive water behavior which has high predictive validity in terms of its responsiveness to chronic and not acute antidepressants and lack of responsiveness to non-antidepressants. The Sun and Alkon model, however, has the drawback of requiring the use of a large swimming tank for rats. This prompted the authors to adapt the procedure to mice and use desktop-sized rat tub cages 24 × 43 × 23 cm w × h × l for swimming. The resulting mouse model has been found to be an efficient and valid method for inducing a chronic depression-like state described here.
Materials
Sink
Thermometer
Rat tub cages (24 × 43 × 23 cm) with no holes in wall placed on on a surface of a color contrasting with that of the mouse fur
Video camera mounted above tub cages
Lever-activated mechanical stop clock (e.g., The Science Fair, P.O. Box 934, St. Augustine, FL 32085)
Hand tally counter or video tracking system (Fisher Scientific; Flash)
Osmotic minipumps (Alzet #1002)
Isoflurane
Isoflurane and anesthesia mask
Surgical equipment for subcutaneous implantation
Non-linear regression software such as Prism (see Unit 7.5)
Habituate mice to laboratory
-
1
House mice in standard mouse cages singly with nesting material under a 12 h light/dark cycle at an ambient temperature of 22° C.
-
2
Allow animals to habituate to the animal facility for three days and then handle briefly on the morning of the fourth day by picking the mouse up by the tail and holding it momentarily against the labcoat. This simple procedure markedly reduces subsequent defensive and escape behavior.
Perform swim procedure
-
3
Fill tubs with tap water at 32-34° C to a depth of 13 cm.
-
4Transport each cage to the lab and place mouse gently in the water (do not drop in) for 15 min (Fig 9.36.1).
- Ambient noise and light levels are irrelevant.
-
5After the swim, approach the animal slowly, capture with the index and third fingers and place in the home cage. Do not dry as it will rapidly dry itself.
- At 30 min intervals maintain the temperature of the water in the tubs by replacing 2L with 2L of hot water (45-52°C). After 8 mice have swum change the water in the tubs completely.
-
6
OPTIONAL: If an escape coping response is to be investigated, place a 12 cm high inverted flower pot in the tub opposite to the animal and permit the mouse to learn to climb out of the water. Remove 30 sec later by picking the mouse up by the tail and transferring to its home cage.
-
7
Repeat swim procedure (steps 3-6) for two additional days which need not be consecutive.
Fig 9.36.1.

Two cage setup for mouse repeated open space forced swim method. Rat tub cages with no watering holes are placed beneath a video camera on a surface that has contrasting color to the mouse fur.
Record and rate behavior
-
8Videotape the fourth swim and rate the recordings at 4X actual speed for distance swum and immobility times as follows:
- Rate the distance swum as the number of quadrants of the tub entered or as the actual distance moved as detected by the video tracking system.
- Time the duration of immobility from the total time the animal is observed to float which is defined as drifting with the tail fully extended and no motion observed in the tail or limbs.
- Use a lever-activated mechanical stop-clock rather than a computer keyboard to time immobility since it will alternate rapidly with periods of activity and can be most easily scored by rapid lever actions.
- Match the animals primarily on immobility and secondarily on distance swum into control and experimental groups.
Treat mice with drugs
-
9Institute treatment at 24 h after the fourth swim (if the effects of drugs are to be studied).
- For chronic treatment, either use 14-day osmotic minipumps (Alzet #1002) or administer daily i.p. or per os.
- For minipumps, insert subcutaneously in the interscapular region.under isoflurane anesthesia (1-2%) and stitch the wound closed allowing 24 h for recovery.
-
10
Determine time of onset of drug action by swimming the mice at days 2, 4, 7, 10, and 14 post pump implantation or at 1, 4, 7, 10 and 14 days after initiation of i.p.or p.o. administration.
-
11
Fit the resulting time-course curves of distance swum and immobility for each animal to an exponential growth and decay curve, respectively (Figure 9.36.2), by a non-linear regression program and calculate the half-times and maximum response for each behavior.
Fig 9.36.2.
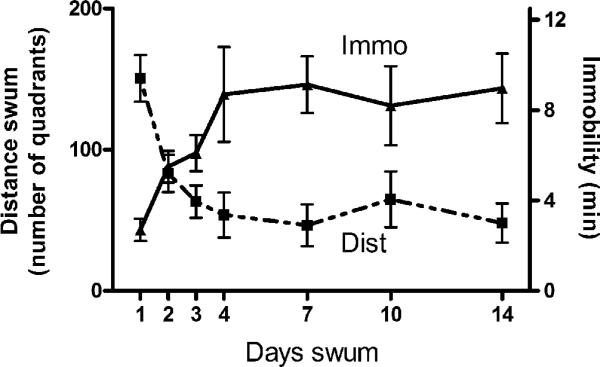
Time course of immobility and distance swum with mouse repeated open space swim method. N = 6-7 group. Note that changes in both behaviors asymptote at approximately the fourth swim and remain relatively constant thereafter. Drug treatment is typically begun after this swim.
COMMENTARY
Background Information
The present model is based on the well established finding that repeated exposure to an inescapable stressor for which no active coping responses are available will lead to depressive behavior (Wagner et al., 1977;Weiss et al., 1970). In the above open-space forced swim procedure, as the mice cannot escape from the tubs, they show a progressive extinction of most active behavioral responses and increased periods of immobile floating as the trials proceed. While it has been claimed that learned passive responses are as adaptive as learned active responses (Nishimura et al., 1988), passive responding has been found to be aversive and associated with negative mood and physiological signs of stress in humans and animals (Matsuda et al., 1996;Mercado et al., 2005;Stone et al., 2007). In support of these latter findings, we have shown with Fos immunohistochemistry that the open space swim procedure produces an activation of the paraventricular nucleus of the hypothalamus and one that shows minimal habituation with repeated swims (Stone et al., 2007). Furthermore, if mice are permitted to learn an active coping response in the form of climbing on a presented platform at the end of each swim they develop much less depressive behavior in terms of both distance swum and time floating in agreement with the protective nature of active coping responses on depression (Stone et al., 2008;Wagner et al., 1977). Similar results have been found in the Morris water maze with rats during extinction of a previously learned spatial memory response (Schulz et al., 2007). It is reasonable to conclude therefore that the repeated forced passivity in the presence of a stressful stimulus of the present model is sufficient to induce a depressive-like state in rodents.
Figure 9.36.2 shows the progressive decrease of distance swum and the concomitant increase in time floating in mice subjected to the procedure. Both changes appear to asymptote by about the fourth swim and to remain constant for at least 2 weeks. From about the fourth swim onward neither behavior appears to be influenced one way or the other by subsequent swims. Consistent with the learned nature of the changes, there is an incubation effect such that if animals are swum for four days and then given a few days to a week rest, they typically show an enhanced immobility and a further reduction in distance swum when swum again after this break.
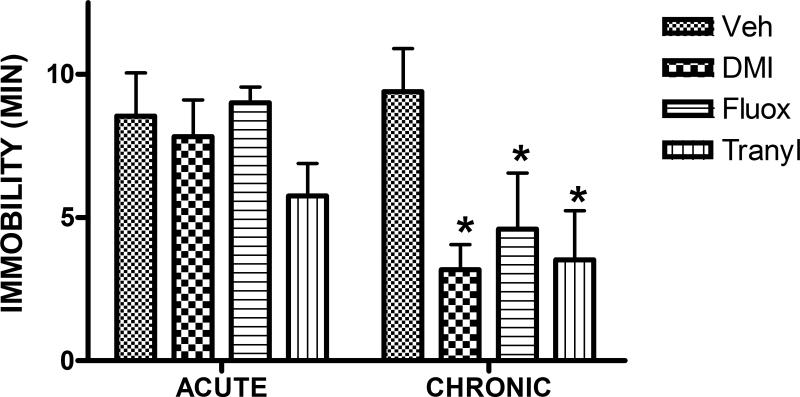
The procedure appears to have good predictive, face and construct validity as a model of depression. With respect to predictive validity, drugs and procedures that are effective antidepressants are also effective in reversing the high immobility and low distance swum, whereas non-antidepressants are not effective on either measure. Moreover, unlike the acute forced swim test, which responds to subchronic more than acute antidepressant treatment (Kitada et al., 1981;Porsolt et al., 1978), the mouse open space model is resistant to both acute and subchronic drug administration, and responds only to chronic treatment (Figure 9.36.3). Effective antidepressants tested include desmethylimipramine (DMI; norepinephrine selective), fluoxetine (serotonin selective), and tranylcypromine (monoamine oxidase inhibitor), as well as the behavioral coping response described above (escape training). Ineffective drugs have included diazepam (minor tranquilizer) and haloperidol (antipsychotic) (Stone et al., 2007;2008). This profile compares favorably with the responsiveness of the rat open-space model to imipramine (nonselective tricyclic), alaprocate (serotonin-selective) and iproniazid (monoamine oxidase inhibitor), and lack of responsiveness to buspirone (minor tranquilizer) (Sun and Alkon, 2003;2004). However, preliminary experiments have suggested that chronic amphetamine, which gives a false positive in the original Porsolt procedure, also gives a weak false positive in this test as well.
Fig 9.36.3.
Effect of acute and chronic administration of antidepressants on immobility in open-space test. Mice were implanted subcutaneously after the fourth swim with osmotic minipumps dispensing either vehicle, DMI (10mg/kg/d), fluoxetine (5 mg/kg/d) or tranylcypromine (10 mg/kg/d) and were swum at intervals thereafter until 14 d post implantation. The acute panel represents the second day after implantation whereas the chronic panel shows the results on day 14. The clear resistance of the test to acute treatment makes it useful for measures of time of onset of antidepressant action. *p < 0.05 versus vehicle.
As for face validity, the procedure increases passivity of most mice as reflected by significant generalization to reduced struggling in the tail suspension test (Stone et al., 2008). We have also anecdotally observed that singly-housed mice exposed to the procedure frequently become easier to handle. The model can also result in anhedonia as reflected by a reduced preference for sucrose (Stone et al., 2008), although, this effect tends to be less reliable than with other depression models suggesting that the stress is too mild to induce this symptom routinely.
With regard to construct validity, repeated open space swims produce a similar pattern of central neural changes that occur in other depression models and that is believed to underlie depressive behavior in humans (Stone et al., 2008). This includes a sustained activation of brain regions responsive to stress such as the paraventricular hypothalamus, locus coeruleus and bed nucleus of the stria terminalis, along with a deactivation of regions involved in motivation and behavioral performance such as the motor and piriform cortex, the lateral septal nucleus and nucleus accumbens (Mayberg, 2007;Price and Drevets, 2010;Steciuk et al., 1999;Stone et al., 2007). Furthermore, the procedure reduces the rate of cell proliferation in the subventricular zone, a phenomenon believed to underlie the loss of behavioral plasticity in depression (David et al., 2009).
In addition to providing a valid model of depression, the open space forced swim method may also permit studies of the relationship of depression to other types of behavioral strategies and coping responses. Its use of an increased swimming area in comparison with the original Porsolt forced swim method changes the active behavioral reactions of the animal from frantic escape and climbing responses to wide-ranged swimming and searching responses, and thereby permits a more detailed study of the relationship between immobility and other behavioral strategies such as perseveration and flexibility. This interplay has been particularly evident in a small subgroup of mice that remain swimming actively in a single corner of the tub for the full 15 min with virtually no immobility and no exploration. Although depression is usually conceptualized in terms of its maladaptive consequences, such animals may be displaying an obsessive behavioral style in which the absence of depression is maladaptive and interferes with the ability to abandon an unsuccessful behavioral strategy (Huston et al., 2009).
Critical Parameters and Troubleshooting
Any unexpected stress or strong stimulus that is given just before the test swim after the animals have developed the passive response will disrupt the passive behavior. Thus, if animals are unaccustomed to being held and injected, a simple i.p. saline injection given 30 min prior to the test swim will significantly reduce immobility and increase swimming distance. Therefore, it is necessary to adapt mice for several days prior to the test to any significant new stimulus that will be present on the test day. This property suggests that the depression produced by the repeated swim procedure, although it can generalize to other situations such as tail suspension, is also partially situation-specific and is weakened by a change in the contextual stimuli. The degree to which the depression is general versus specific remains to be determined.
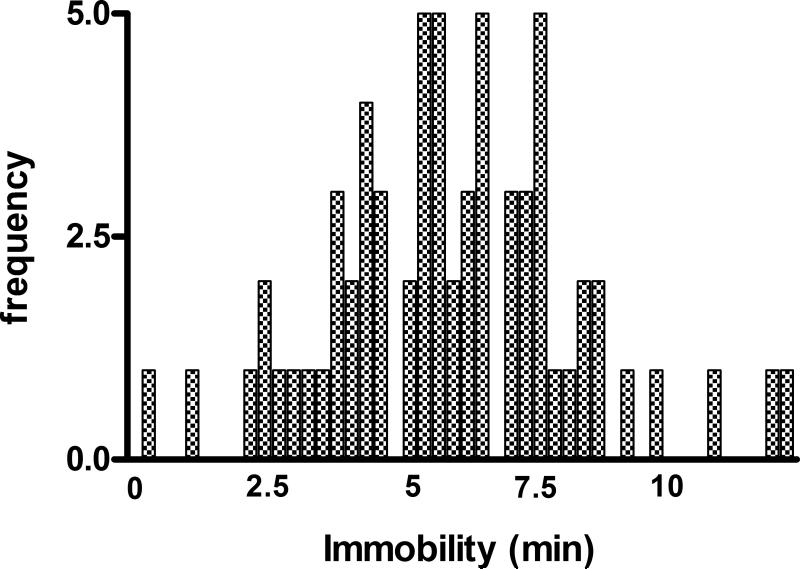
Another critical parameter concerns individual differences in vulnerability. There are large differences between mice in susceptibility to the behavior produced by this method. This can be seen from a distribution of immobility times of 65 naive mice on the fifth swim shown in Figure 9.36.4. The distribution is clearly multimodal and exhibits a nadir at 5 min with approximately 65% of the animals above this dividing line. We generally restrict our experiments to this vulnerable upper 65% and always match subjects in control and experimental groups on both distance and immobility scores. At present, it is unclear whether the animals in the lower 35% are resilient to depression or are showing agitated behavior. It may be possible to resolve this in future research by determining if there is a paradoxical “calming” effect of antidepressants in this subgroup or if they show anhedonia, which can occur with agitation produced by other depression models, e.g. olfactory bulbectomy (Slattery et al., 2007) or chronic mild stress (Harro et al., 1999;Shi et al., 2010).
Fig 9.36.4.
Distribution of immobility times in 65 mice on the fifth swim. Note the multimodal nature of the distribution with a nadir at 5 min. The group above 5 min, which represents 65-70% of the population, is arbitrarily defined as “vulnerable.”
Another important individual difference has to do with the swimming style. While most of the animals float on the surface of the water (“floaters”) and do not get their dorsal fur wet (Fig 9.36.5), about 10-20% of the mice are “sinkers” who swim with only their snouts above the surface of the water aided by their tails touching the bottoms of the tubs. Although at present we have not tested these two populations for differences in distance swum and immobility levels, we routinely also match experimental and control groups on sinkers and floaters.
Fig 9.36.5.

Most mice swim on the surface of the water, while a smaller number swim submerged up to the snout with the tail touching the bottom. Both types of swimming can be accompanied by immobility although experimental and control groups are generally matched on “floaters” and “sinkers” to control for possible differences in vulnerability.
A final critical variable has to do with drugs and procedures that interfere with swimming and which should be avoided or used with extreme caution. Although virtually all intact mice tolerate the procedure well and swim unaided, certain experimental manipulations such as anti-dopaminergic compounds (haloperidol) and brain lesions can reduce the motivation to swim or produce disorientation and dystonic movements that can cause drowning.
Anticipated results
It should be noted that while immobility and distance swum are inversely correlated, the correlation is far from perfect (r = .43, N = 65, p < 0.01) and it is not presently clear which measure is the most sensitive to antidepressant treatment. Sun and Alkon have utilized distance swum with rats because it is the more objective measure and can be automated. However, we have found that for mice, immobility tends to respond more consistently to antidepressants across different experiments (Stone et al., 2008) and represents the most commonly used measure in various other motoric-based antidepressant tests.
Time considerations
The open-space method for mice is economical in terms of time considerations. For example, to run a three group experiment with each group having an N = 6 and using a three tub setup, time requirements will be approximately 1.5 h/d of swimming and approximately 2 h/d for video rating of both immobility and distance swum.
Acknowledgements
Supported in part by NIMH/5RO1MH045265 and NCRR/MORR00096.
Literature Cited
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress; behavioral, brain, and neuroendocrine correlates. Behav. Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and - independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro J, Pähkla R, Modiri AR, Harro M, Kask A, Oreland L. Dose-dependent effects of noradrenergic denervation by DSP-4 treatment on forced swimming and •-adrenoceptor binding in the rat. J. Neural Transm. 1999;106:619–629. doi: 10.1007/s007020050184. [DOI] [PubMed] [Google Scholar]
- Huston JP, Schulz D, Topic B. Toward an animal model of extinction-induced despair: focus on aging and physiological indices. J. Neural Transm. 2009;0:0. doi: 10.1007/s00702-009-0210-4. [DOI] [PubMed] [Google Scholar]
- Kitada Y, Miyauchi T, Satoh A, Satoh S. Effects of antidepressants in the rat forced swimming test. Eur. J Pharmacol. 1981;72:145–152. doi: 10.1016/0014-2999(81)90269-7. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci. Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol. Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117:51–57. doi: 10.1016/j.pain.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Tsuda A, Oguchi M, Ida Y, Tanaka M. Is immobility of rats in the forced swim test “behavioral despair?”. Physiol. Behav. 1988;42:93–95. doi: 10.1016/0031-9384(88)90266-1. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–381. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch. int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D, Buddenberg T, Huston JP. Extinction-induced “despair” in the water maze, exploratory behavior and fear: effects of chronic antidepressant treatment. Neurobiol. Learn. Mem. 2007;87:624–634. doi: 10.1016/j.nlm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. Learned helplessness and depression in animals and man. In: Spence JT, Carson R, Thibaut J, editors. Behavioral Approaches to Therapy. General Learning Press; Morristown, NJ.s.: 1976. pp. 111–126. [Google Scholar]
- Shi M, Wang JY, Luo F. Depression shows divergent effects on evoked and spontaneous pain behaviors in rats. J Pain. 2010;11:219–229. doi: 10.1016/j.jpain.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Cryan JF. Evaluation of reward processes in an animal model of depression. Psychopharmacology. 2007;190:555–568. doi: 10.1007/s00213-006-0630-x. [DOI] [PubMed] [Google Scholar]
- Steciuk M, Kram M, Kramer GL, Petty F. Decrease in stress-induced c-Fos-like immunoreactivity in the lateral septal nucleus of learned helpless rats. Brain Res. 1999;822:256–259. doi: 10.1016/s0006-8993(99)01134-8. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiat. 2007;31:1196–1207. doi: 10.1016/j.pnpbp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Quartermain D. Evaluation of the repeated open-space swim model of depression in the mouse. Pharmacol. Biochem. Behav. 2008;91:190–195. doi: 10.1016/j.pbb.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. Open space swimming test to index antidepressant activity. J. Neurosci. Methods. 2003;126:35–40. doi: 10.1016/s0165-0270(03)00068-2. [DOI] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. Induced depressive behavior impairs learning and memory in rats. Neuroscience. 2004;129:129–139. doi: 10.1016/j.neuroscience.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Wagner HR, Hall TL, Cote IL. The applicability of inescapable shock as a source of animal depression. J. Gen. Psychol. 1977;96:313–8. doi: 10.1080/00221309.1977.9920828. [DOI] [PubMed] [Google Scholar]
- Weiss J, Glazer HI, Pohorecky LA, Brick J, Miller NE. Effects of chronic exposure to stressors on avoidance-escape behavior and on brain norepinephrine. Psychosom. Med. 1975;37:522–534. doi: 10.1097/00006842-197511000-00006. [DOI] [PubMed] [Google Scholar]
- Weiss J, Stone EA, Harrell N. Coping behavior and brain norepinephrine in rats. J. comp. physiol. Psychol. 1970;72:153–160. doi: 10.1037/h0029311. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]