Abstract
Aims
To compare the effects of lifestyle modification programs that prescribe low-glycemic load (GL) vs. low-fat diets in a randomized trial.
Methods
Seventy-nine obese adults with type 2 diabetes received low-fat or low-GL dietary instruction, delivered in 40-week lifestyle modification programs with identical goals for calorie intake and physical activity. Changes in weight, HbA1c, and other metabolic parameters were compared at weeks 20 and 40.
Results
Weight loss did not differ between groups at week 20 (low-fat: −5.7 ± 3.7%, low-GL: −6.7 ± 4.4%, p = .26) or week 40 (low-fat: −4.5 ± 7.5%, low-GL: −6.4 ± 8.2%, p = .28). Adjusting for changes in antidiabetic medications, subjects on the low-GL diet had larger reductions in HbA1c than those on the low-fat diet at week 20 (low-fat: −0.3 ± 0.6%, low-GL: −0.7 ± 0.6%, p = .01), and week 40 (low-fat: −0.1 ± 1.2%, low-GL: −0.8 ± 1.3%, p = .01). Groups did not differ significantly on any other metabolic outcomes (p ≥ .06).
Conclusions
Results suggest that targeting GL, rather than dietary fat, in a low-calorie diet can significantly enhance the effect of weight loss on HbA1c in patients with type 2 diabetes.
Keywords: Glycemic Load, Dietary Fat, Obesity, Type 2 Diabetes, Lifestyle Modification, Diet
Introduction
Current guidelines recommend a program of diet, exercise, and behavior therapy (i.e., lifestyle modification) for the treatment of obesity [1]. Such programs typically instruct patients to consume a low-calorie diet that provides ~50-60% of energy from carbohydrate, ≤ 30% from fat, and the remainder from protein. Although the abundance of carbohydrate may be of concern to persons with diabetes and their care providers, numerous studies have shown that diabetic participants in standard lifestyle modification programs (which typically prescribe a low-fat, high-carbohydrate diet) achieve significant mean improvements in blood glucose and glycated hemoglobin (HbA1c) [2-3].
Dietary approaches to weight loss and diabetes control may target glycemic index (GI) or glycemic load (GL) as an alternative to standard low-fat diet prescriptions. The findings regarding the health effects of these alternative approaches, however, are mixed. Some laboratory tests have found foods higher in GI or GL to be related to poorer short-term metabolic outcomes, as well as greater hunger, less satiety, and greater subsequent food intake [4-6]. Others have produced null findings [7-8] or inverse associations [9].
Results of individual clinical trials and epidemiological studies also have produced equivocal findings regarding the relationship of GI and GL to metabolic parameters [10-13], cardiovascular disease risk factors [13-15], and weight loss [16-19]. A recent meta-analysis of randomized controlled trials found a statistically significant, though modest, difference favoring low-GI/GL diets over higher-GI/GL alternatives for weight loss in non-diabetic participants [20]. An earlier meta-analysis found that subjects with type 2 diabetes achieved a 6% greater reduction in glycated proteins (HbA1c and fructosamine) on low-GI vs. high-GI diets [21]. These studies were typically short-term and were not intended to induce weight loss through lifestyle modification.
The present study compared the effects of low-fat and low-GL diets, delivered in the context of a lifestyle modification program for weight loss in participants with type 2 diabetes mellitus. Co-primary outcomes were changes in weight and HbA1c, and secondary outcomes included changes in several additional metabolic parameters and dietary intake variables.
Methods
Trial Design and Study Setting
This was a single-site parallel-group study with balanced (1:1) randomization. Given the nature of the interventions, blinding to treatment condition was not possible. The study was conducted at the University of Pennsylvania Center for Weight and Eating Disorders in Philadelphia, PA, from September 2006 to July 2009.
Participants
Men and women, ages 18-65 years, with a diagnosis of type 2 diabetes and a BMI of 27 to 45 kg/m2 (maximum weight of 136 kg) were eligible to participate. Individuals with type 1 diabetes, uncontrolled hypertension (> 160/100 mm Hg) or thyroid disease, unstable angina, malignant arrhythmias, myocardial infarction in the past year, cancer (active or in remission < 5 years), clinically significant psychosocial impairment, or any history of cerebrovascular, renal, hepatic, or protein-wasting diseases were excluded. Additionally, women who were pregnant or lactating were excluded from the trial. The research was approved by the Institutional Review Board at the University of Pennsylvania School of Medicine and is registered with ClinicalTrials.gov (NCT00729196). Participants were not charged for treatment, and they were not paid for their participation in the trial.
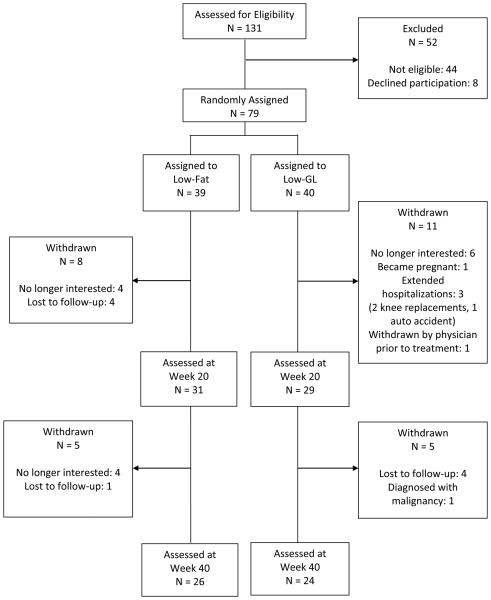
Seventy-nine individuals (63 women, 16 men) were enrolled in six cohorts and randomly assigned, using a computer-generated scheme, to a lifestyle modification program that included a low-fat or low-GL diet prescription. Twenty-nine participants did not complete treatment (See Figure 1 for participant flow). Attrition did not differ between groups (p = .54).
Figure 1.
Participant flow through the trial.
Interventions
For both the low-fat and low-GL conditions, the dietary prescription was delivered as a component of a lifestyle modification program, which also included an exercise prescription and group-based behavior therapy sessions. Behavior therapy sessions included four to eight participants, lasted approximately 90 minutes each, and were held weekly for 20 weeks and biweekly for 20 additional weeks. Both conditions were taught the same set of behavioral and cognitive skills (e.g., self-monitoring, stimulus control, problem-solving, challenging dysfunctional thoughts). Goals for energy intake were 5024-6280 kJ/d and 6280-7536 kJ/d for participants who weighed < 113.4 kg and ≥ 113.4 kg, respectively. Participants were provided with a calorie-counting guide [22] to assist in meeting energy intake goals. The physical activity prescription was identical in the two conditions; participants initially were instructed to complete at least 50 minutes of moderate-intensity activity (e.g., brisk walking) per week, and to increase to at least 175 minutes per week over the first 20 weeks of treatment. Interventionists had doctoral- or masters-level training in clinical psychology. (Masters-level clinicians were supervised by a licensed psychologist with extensive experience in lifestyle modification for weight loss.) Within each randomization cohort, the same interventionist provided treatment to the low-fat and low-GL conditions.
Low-fat condition
Participants randomized to the low-fat condition received instruction on identifying sources of dietary fat and were encouraged to model their diet on a “Low-Fat Pyramid” (similar to the Food Guide Pyramid). They were prescribed a goal of consuming ≤ 30% of energy from fat (i.e., 40-50 g/d and 50-60 g/d for participants in the 5024-6280 kJ/d and 6280-7536 kJ/d ranges, respectively). Participants recorded calorie and fat gram intake in their daily self-monitoring logs. Low-fat recipes were distributed and low-fat items and meals were sampled in session. Participants also received a low-fat eating plan (i.e., menus, recipes, and grocery lists) for 2 weeks' worth of meals and snacks at an average of ~6280 kJ and 30 grams of fat per day.
Low-GL condition
Participants in this condition received instruction on the glycemic effects of food. They were given a “Low-GL Pyramid” and were encouraged to structure their diets accordingly. Rather than computing GL values for the foods they consumed, they were taught several guidelines for identifying low-, moderate-, and high-GL items [23]. Participants in this condition were prescribed goals of consuming ≤ 3 and ≤ 1 serving per day of moderate-GL and high-GL items, respectively. They recorded servings of moderate- and high-GL foods, as well as calorie intake, in their daily self-monitoring logs. Participants received recipes and sampled foods that were consistent with the dietary goals of the low-GL condition. In addition, participants were given a low-GL eating plan that provided an average of ~6280 kJ, 3 servings of moderate-GL foods, and < 1 serving of high-GL foods per day over 2 weeks.
Outcome Measures
Study outcomes were assessed at baseline, week 20, and week 40. (In addition, weight was measured, for feedback purposes, at each lifestyle modification session.)
Anthropometric measures
Weight was measured on a calibrated electronic scale (Tanita BEB-800, Tokyo, Japan) with participants in light clothing and no shoes. Height was measured (at baseline) with a wall-mounted stadiometer. Waist circumference was measured at the umbilicus with the participant standing and the tape measure parallel to the floor. Blood pressure was measured with an automated sphygmomanometer (Dinamap Pro 100, GE Healthcare, Waukesha, WI), after participants were seated for at least 5 minutes. Height, waist circumference, and blood pressure were each measured in duplicate and recorded as the mean of the two values.
Biochemical measures
Blood samples were collected after an overnight fast and were analyzed at the Clinical and Translational Research Center (CTRC) at the University of Pennsylvania School of Medicine using standard procedures for: HbA1c; glucose; insulin; c-peptide; total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol; triglycerides; and high-sensitivity C-reactive protein. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin levels [24]. Medication use was tracked throughout the study and changes in antidiabetic medications were quantified as follows: new medication or increased dosage from baseline (+1); no change in medications or dosages from baseline (0); or discontinued medication or decreased dosage from baseline (−1).
Dietary measures
Participants were instructed to keep 3-day food records (2 weekdays and 1 weekend day) prior to each assessment visit. A research dietitian at the CTRC Bionutrition Core reviewed records for plausibility, queried participants when appropriate, and analyzed records using Nutrition Data Systems for Research software [25].
Statistical Analyses
Descriptive statistics were generated to identify the distribution of scores and the need for transformation. Unless noted otherwise, the distribution of all variables was sufficiently normal. Groups were compared at baseline using t-tests for continuous data and chi-square tests for categorical data. The co-primary outcome variables were changes in weight and HbA1c, which were compared between groups at weeks 20 and 40. Weight change was defined as a percentage of baseline body weight.
Hierarchical linear modeling (HLM) with full maximum likelihood estimation was used to investigate changes in weight and HbA1c, as well as other metabolic parameters and dietary intake variables over time. This approach allows for participants with at least two longitudinal observations to be included in the analysis, thereby allowing participants with partial data to contribute to the analysis. Unconditional models were fitted to test for linear and non-linear (quadratic) patterns in the outcomes, and to determine whether slopes should be treated as random effects. Covariates (i.e., treatment group, age, and gender) were then added to these models to evaluate their effect. The model of HbA1c change also included change in diabetes medication (increase, decrease, or no change) as a time-varying covariate. Continuous predictors were mean-centered (around the group mean) before entry into the analysis. Modeled means and standard errors were used to evaluate differences in outcomes between treatment groups at 20 and 40 weeks. SPSS version 17 [26] was used to complete all analyses.
Results
Baseline demographic, clinical, and dietary intake characteristics for participants in the low-fat and low-GL conditions are shown in Table 1. Groups did not differ significantly on any variables. Values for continuously distributed data are presented as group mean ± standard error.
Table 1.
Baseline characteristics of participants.
| Low-Fat (n = 39) | Low-GL (n = 40) | p | |
|---|---|---|---|
| Female sex – n (%) | 31 (79.5%) | 32 (80.0%) | .96 |
| Ethnicity – n (%) | .60 | ||
| Caucasian | 16 (41.0%) | 17 (42.5%) | |
| African American | 17 (43.6%) | 21 (52.5%) | |
| Other/Not Reported | 6 (15.4%) | 2 (5.0%) | |
| Age (y) | 52.5 ± 1.3 | 52.8 ± 1.4 | .87 |
| Weight (kg) | 99.1 ± 2.3 | 102.3 ± 2.7 | .37 |
| BMI (kg/m2) | 35.8 ± 0.7 | 36.7 ± 0.8 | .44 |
| HbA1c (%) | 7.0 ± 0.2 | 6.6 ± 0.2 | .20 |
| Waist circumference (cm) | 111.6 ± 1.6 | 114.2 ± 1.7 | .25 |
| Glucose (mg/dl) | 124.8 ± 7.3 | 112.2 ± 6.4 | .20 |
| Insulin (mIU/L) | 13.2 ± 1.5 | 15.8 ± 1.7 | .26 |
| HOMA | 4.2 ± 0.6 | 4.4 ± 0.5 | .83 |
| Total cholesterol (mg/dl) | 171.2 ± 5.1 | 183.9 ± 5.6 | .10 |
| LDL cholesterol (mg/dl) | 90.7 ± 4.4 | 99.9 ± 5.1 | .18 |
| HDL cholesterol (mg/dl) | 54.3 ± 2.2 | 56.7 ± 2.7 | .49 |
| Triglycerides (mg/dl) | 131.9 ± 10.0 | 141.8 ± 15.8 | .60 |
| Systolic blood pressure (mm Hg) | 128.6 ± 2.3 | 130.1 ± 2.4 | .66 |
| Diastolic blood pressure (mm Hg) | 71.2 ± 1.5 | 71.4 ± 1.6 | .93 |
| High sensitivity C-reactive protein (mg/L) | 7.5 ± 1.8 | 8.0 ± 1.3 | .83 |
| c-peptide (ng/mL) | 2.8 ± 0.3 | 3.3 ± 0.2 | .15 |
| Total energy (kJ) | 7812.2 ± 404.0 | 8403.7 ± 416.6 | .33 |
| Energy from fat (% kJ) | 37.0 ± 1.0 | 38.2 ± 1.1 | .44 |
| Energy from carbohydrate (% kJ) | 46.0 ± 1.1 | 44.0 ± 1.2 | .22 |
| Energy from protein (% kJ) | 17.6 ± 0.6 | 18.8 ± 1.1 | .35 |
| Total sugar (g) | 82.4 ± 6.2 | 78.4 ± 5.6 | .65 |
| Added sugar (g) | 57.7 ± 5.8 | 52.2 ± 5.4 | .51 |
| Fiber (g) | 16.1 ± 1.2 | 16.4 ± 1.1 | .86 |
| Dietary glycemic index | 63.4 ± 0.7 | 63.6 ± 0.8 | .81 |
| Dietary glycemic load | 129.3 ± 8.0 | 129.0 ± 7.2 | .98 |
For continuous variables, cells contain group means ± standard error. P-values are for the between-groups difference.
Weight Change
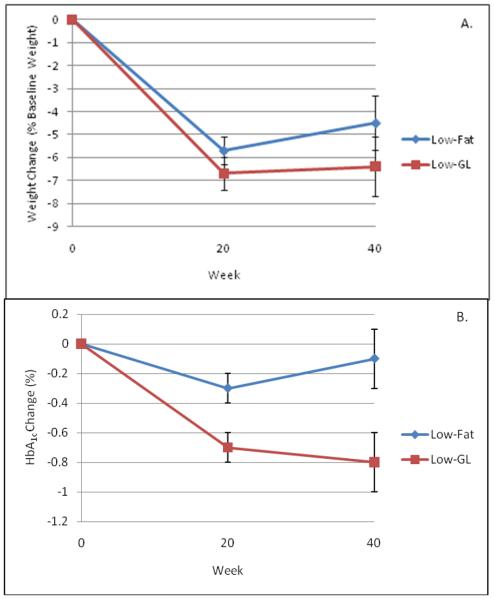
As shown in Figure 2 (Panel A), mean reductions in body weight were not significantly different between groups. Participants in the low-fat and low-GL groups lost 5.7 ± 0.6% and 6.7 ± 0.7% of initial weight, respectively, at week 20 (p = .26). At week 40, reductions were 4.5 ± 1.2% and 6.4 ± 1.3%, respectively (p = .28).
Figure 2.
Mixed model estimates showing effects of low-fat and low-GL diets on weight change (Panel A) and HbA1c (Panel B). Differences in weight loss were not statistically significant at week 20 or week 40. However, the changes in HbA1c were significantly different at both times.
Glycemic Control
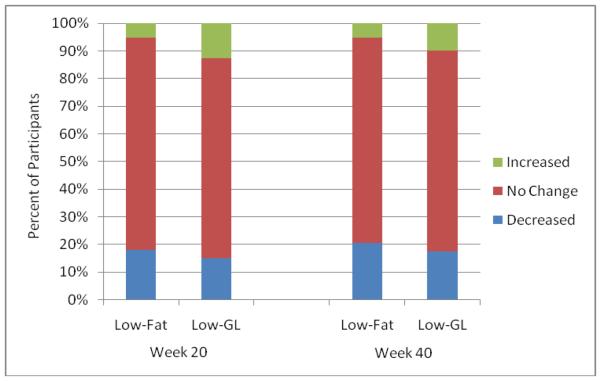
Comparisons of between-groups changes in HbA1c, controlling for changes that participants' physicians made to their diabetes medications, are shown in Figure 1 (Panel B). At week 20, participants in the low-fat and low-GL groups achieved reductions in HbA1c of 0.3 ± 0.1% and 0.7 ± 0.1%, respectively (p = .01). The difference between groups was greater at week 40, with participants in the low-fat and low-GL groups achieving reductions of 0.1 ± 0.2% and 0.8 ± 0.2%, respectively (p = .01). As shown in Figure 2, the percentage of participants who increased, decreased, or did not change the intensity of their diabetes medication regimen did not differ between groups at week 20 (p = .51) or week 40 (p = .70).
Metabolic Outcomes
Changes in metabolic markers also were compared at weeks 20 and 40. As shown in Table 2, changes in systolic and diastolic blood pressure were marginally more favorable among participants in the low-fat vs. low-GL group at weeks 20 and 40 (p = .06 to .08). There were no between-groups differences in changes in fasting glucose, insulin, HOMA-IR, c-peptide, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, or high-sensitivity C-reactive protein at week 20 or week 40 (p ≥ .15).
Table 2.
Changes in Metabolic Outcomes at Weeks 20 and 40.
| Variable | Low-Fat (n = 39) | Low-GL (n = 40) | p |
|---|---|---|---|
| Waist Circumference (cm) | |||
| Week 20 | −6.2 ± 0.7 | −6.6 ± 0.7 | .662 |
| Week 40 | −6.4 ± 1.4 | −7.1 ± 1.4 | .721 |
| Glucose (mg/dl) | |||
| Week 20 | −7.9 ± 4.4 | −10.8 ± 4.6 | .624 |
| Week 40 | −10.4 ± 8.2 | −17.0 ± 8.5 | .570 |
| Insulin (mIU/L) | |||
| Week 20 | −3.2 ± 1.2 | −3.6 ± 1.2 | .801 |
| Week 40 | −4.6 ± 2.2 | −5.5 ± 2.3 | .770 |
| HOMA | |||
| Week 20 | −1.2 ± 0.4 | −1.2 ± 0.4 | .979 |
| Week 40 | −1.7 ± 0.7 | −1.8 ± 0.7 | .969 |
| Systolic Blood Pressure (mmHg) | |||
| Week 20 | −4.2 ± 1.9 | 0.6 ± 2.0 | .068 |
| Week 40 | −4.5 ± 3.6 | 4.6 ± 3.7 | .078 |
| Diastolic Blood Pressure (mmHg) | |||
| Week 20 | −2.7 ± 1.1 | 0.2 ± 1.2 | .059 |
| Week 40 | −3.0 ± 2.1 | 2.8 ± 2.2 | .057 |
| Triglycerides (mg/dl) | |||
| Week 20 | −17.2 ± 8.0 | −29.9 ± 8.3 | .246 |
| Week 40 | −3.0 ± 15.0 | −29.3 ± 15.6 | .223 |
| Total Cholesterol (mg/dl) | |||
| Week 20 | −9.4 ± 4.0 | −17.3 ± 4.2 | .147 |
| Week 40 | 2.7 ± 7.5 | −10.8 ± 7.8 | .209 |
| LDL Cholesterol (mg/dl) | |||
| Week 20 | −1.3 ± 3.3 | −6.4 ± 3.5 | .256 |
| Week 40 | 3.1 ± 6.2 | −5.5 ± 6.3 | .328 |
| HDL Cholesterol (mg/dl) | |||
| Week 20 | −3.9 ± 1.0 | −4.8 ± 1.1 | .544 |
| Week 40 | 0.0 ± 1.9 | −1.3 ± 2.0 | .641 |
| High Sensitivity C-Reactive Protein (mg/L) | |||
| Week 20 | −3.3 ± 1.2 | −2.8 ± 1.2 | .744 |
| Week 40 | −3.3 ± 2.2 | −2.6 ± 2.3 | .828 |
| C-Peptide (ng/mL) | |||
| Week 20 | −0.1 ± 0.2 | −0.2 ± 0.2 | .739 |
| Week 40 | −0.4 ± 0.3 | −0.6 ± 0.3 | .581 |
Cells contain group means ± standard error. P-values are for the between-groups difference.
Dietary Intake
Changes in dietary intake are presented in Table 3. Reductions in energy from fat were significantly greater among those in the low-fat group at weeks 20 and 40 (p ≤ .01). By contrast, those in the low-GL group had significantly greater reductions in energy from carbohydrate (p ≤ .01), and significantly greater reductions in dietary GI (p ≤ .003) and GL (p ≤ .03), at both time points. Changes on other measured dietary variables did not differ significantly between groups at week 20 or 40.
Table 3.
Changes in Dietary Intake at Weeks 20 and 40
| Variable | Low-Fat (n = 39) | Low-GL (n = 40) | p |
|---|---|---|---|
| Total energy (kJ/d) | |||
| Week 20 | −2223.2 ± 316.5 | −2745.7 ± 319.5 | .208 |
| Week 40 | −800.1 ± 562.3 | −1810.0 ± 586.2 | .215 |
| Energy from fat (% kJ) | |||
| Week 20 | −7.4 ± 0.9 | −4.3 ± 0.9 | .007 |
| Week 40 | −4.1 ± 1.5 | 1.6 ± 1.6 | .010 |
| Energy from carbohydrate (% kJ) | |||
| Week 20 | 5.6 ± 1.0 | 2.0 ± 1.1 | .007 |
| Week 40 | 3.8 ± 1.7 | −2.7 ± 1.8 | .010 |
| Energy from protein (% kJ) | |||
| Week 20 | 2.3 ± 0.9 | 2.5 ± 0.9 | .903 |
| Week 40 | 1.3 ± 1.7 | 1.6 ± 1.7 | .917 |
| Total Sugar (g) | |||
| Week 20 | −12.1 ± 4.5 | −17.0 ± 4.5 | .417 |
| Week 40 | 4.2 ± 8.3 | −8.0 ± 8.6 | .308 |
| Added Sugar (g) | |||
| Week 20 | −17.0 ± 4.0 | −22.3 ± 4.1 | .311 |
| Week 40 | −2.3 ± 7.2 | −14.9 ± 7.5 | .229 |
| Fiber (g) | |||
| Week 20 | 0.0 ±1.1 | 0.6 ± 1.1 | .676 |
| Week 40 | 0.2 ± 2.1 | 1.6 ± 2.2 | .648 |
| Dietary glycemic index | |||
| Week 20 | −2.6 ± 0.9 | −6.5 ± 1.0 | .002 |
| Week 40 | 1.3 ± 1.7 | −6.2 ± 1.7 | .003 |
| Dietary glycemic load | |||
| Week 20 | −33.0 ± 5.7 | −49.9 ± 5.7 | .027 |
| Week 40 | −8.0 ± 10.2 | −40.4 ± 10.6 | .031 |
Cells contain group means ± standard error. P-values are for the between-groups difference.
Discussion
This study found significantly larger reductions in HbA1c in overweight and obese patients with type 2 diabetes who were prescribed a low-GL diet, versus an isoenergetic low-fat diet, as part of a lifestyle modification program. The advantage for diabetes control was observed despite lack of significant differences in body weight, fasting glucose, and dietary fiber between the two groups. This finding suggests that the quality and quantity of carbohydrate consumed play an important role in broad glycemic exposure among patients with type 2 diabetes, even in a state of negative energy balance necessary for concomitant weight loss.
The effect of reducing GI or GL on glycemic control in persons with diabetes has been summarized in several meta-analyses [21, 27-28]. However, the studies included in those analyses differed notably from the present trial. In the Cochrane review by Thomas and Elliot, for instance, only 5 of the 11 included studies tested diets for at least 12 weeks [28]. Of those, only two were of participants with type 2 diabetes; they were both cross-over studies with a total of only 22 subjects between them. The meta-analysis found that low-GI or low-GL diets were associated with reductions in HbA1c that were approximately 0.3% to 0.5% greater than with higher-GI or GL alternatives [28]. In the present study, we found that the advantage for the low-GL diet was 0.4% at the midpoint of treatment (i.e., week 20) and 0.7% at the conclusion. The widening gap between groups was more attributable to an increase in HbA1c (from week 20 to 40) among those in the low-fat group, than to a continued reduction in the low-GL group.
A recent 12-month randomized controlled comparison of low-GI, high-GI, and low-carbohydrate diets in patients with type 2 diabetes found no differences among groups in HbA1c [29]. Furthermore, HbA1c was higher in all groups at study's end than at baseline. Similarly, another trial found no significant change in HbA1c at 1 year among patients with type 2 diabetes who were assigned to follow a low-carbohydrate (and by extension, low-GL) or low-fat diet [30]. Both of those studies, however, offered far less frequent and intense clinical contacts than the level of treatment provided in the present study.
Studies of lifestyle modification in participants with type 2 diabetes typically show that changes in HbA1c track with changes in weight in this population [31]. Thus, diminished improvements in HbA1c are expected when patients with type 2 diabetes reach a weight loss plateau or begin to regain weight. The low-calorie diet that is typically prescribed, however, is a low-fat diet. Without explicit instruction to choose low-GI carbohydrates, participants who follow a low-fat (i.e., high-carbohydrate) diet may be inadvertently following an eating plan that puts them at risk for increasing glycemic exposure when they cease to create an energy deficit. That participants in our low-GL group maintained their HbA1c reduction in full, in the absence of additional weight loss in the second half of the intervention, suggests that they continued to adhere to the principles of low-GL eating, even as treatment became less frequent.
In contrast to the significant HbA1c advantage for the low-GL diet, the between-groups differences in weight loss (~ 1 and 2 kg at weeks 20 and 40, respectively) were not statistically significant and were consistent with those reported previously. A meta-analysis of six randomized controlled trials found that low-GI/GL diets induced a mean weight loss that was 1 kg greater than that achieved with higher-GI/GL alternatives in healthy participants [20]. We had hypothesized a significant difference in weight loss, in part, due to findings that the reduction in metabolic rate after a 10% weight loss was significantly but modestly smaller with a low-GL diet, compared with a low-fat diet [32]. Additionally, feeding laboratory-based findings that lower-GL meals are associated with greater satiety and less energy intake at subsequent meals suggested that consumption of low-GL foods would facilitate adherence to a low-calorie diet [4-6]. Whether those mechanisms were engaged in the present study is unknown. However, the study is underpowered to detect changes in body weight that might result from relatively subtle effects on metabolic rate, hunger, or satiety, especially when calorie prescriptions were identical in both groups.
At least two studies, published after the launch of the present one, found that insulin secretion rates – as assessed by insulin concentration 30 minutes after oral glucose consumption – moderated the effect of a low-GL diet on weight loss. Pittas et al. compared weight loss achieved with isocaloric low- and high-GL provided diets in healthy overweight participants [33]. Although there was no effect of diet on weight loss for those with low insulin secretion, the low-GL diet produced a significantly greater reduction in weight for those with a high insulin response. Similarly, Ebbeling et al. found no main effect of diet (low-GL vs. low-fat) on weight loss among obese young adults [34]. However, a significant effect was apparent among those with high baseline insulin secretion; the low-GL and low-fat diets produced weight losses of 5.8 kg and 1.2 kg, respectively, at 18 months. In both of the studies described above, participants were overweight or obese, but with no history of type 2 diabetes. It is unclear whether a similar moderating effect would have been found in the present study had we conducted an oral glucose tolerance test at baseline.
Several previous studies that examined the effects of low-GL diets provided diet-consistent food to participants. We instead opted to instruct participants in how to reduce the GL of diets they selected autonomously. This approach has the advantage of external validity, as long-term food provision is not practical for the long-term management of obesity and type 2 diabetes. The disadvantage of providing instruction, rather than food, is that adherence to the diet is likely less complete. We note, however, that between-groups differences in dietary intake (as measured by analysis of 3-day food records) were consistent with expectations based on the dietary instruction that each group received. That is, participants in the low-fat group reduced fat intake to a greater extent than did those in the low-GL group, who in turn, reduced carbohydrate intake, GI, and GL to a greater degree than their low-fat counterparts.
Aside from the significant advantage of the low-GL diet for reducing HbA1c, there were no other significant differences between groups in other metabolic outcomes, including fasting glucose. This finding highlights the contribution of postprandial glycemic exposure to overall diabetes control. Both systolic and diastolic blood pressure improved to a marginally greater degree in the low-fat group at weeks 20 and 40, compared to the low-GL group. Examination of group means revealed a modest increase in blood pressure among participants in the low-GL group. This finding is difficult to interpret. We did not control for changes in antihypertensive medications, sodium intake, or other factors that may explain the observed differences in blood pressure.
A weakness of this study is the attrition rate of 36.7%. We suspect that dissatisfaction with treatment (including with initial weight loss) contributed to attrition, particularly among those who dropped out before week 20 (24.1%) or were lost to follow-up (16.5%). In addition, the lack of financial compensation provided little incentive for participants who achieved unsatisfactory results to return for assessments. Our greatest protection against the effects of attrition was the use of mixed models for analyses. This approach is considered superior to alternative methods of handing missing data such as including only participants with complete data or assigning a pre-specified score to the missing data. A major strength of the trial includes the long duration and large sample size relative to most previous comparably designed studies. An additional strength was the incorporation of each diet into a standard-format lifestyle modification program with identical energy intake goals, exercise prescriptions, and behavioral interventions in both conditions.
The current position of the American Diabetes Association [35] is that “the use of the glycemic index and glycemic load may provide a modest additional benefit for glycemic control over that observed when total carbohydrate is considered alone” (Evidence grade B: supportive evidence from well-conducted cohort studies.) The present study was not designed to address this issue directly, as the comparison diet was a higher-GL low-fat diet. Future investigations should compare low- and high-GI/GL diets that control for total carbohydrate intake.
This study demonstrated that prescribing a calorie-restricted low-GL diet to overweight and obese adults with type 2 diabetes resulted in greater glycemic control than was achieved with an isoenergetic low-fat prescription. The advantage of the low-GL diet for improving HbA1c was apparently not attributable to weight change or calorie-restriction, as these were equivalent between groups. These results add to a growing literature on the benefits of following a low-GL diet for diabetes control.
Figure 3.
Comparisons of the percentage of participants in each condition who increased, did not change, or decreased the number or dosage of medications used to treat diabetes during the course of the study. Chi-square analyses showed no differences between groups at week 20 or week 40.
Acknowledgements
This study was funded by grant K23DK070777 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to Dr. Fabricatore. In addition, this project was supported by grant K24DK065018 from NIDDK to Dr. Wadden, by grant K24DK082730 from NIDDK to Dr. Ludwig, and by grant UL1 RR024134 from the National Center for Research Resources. Calorie guides were donated by Calorie King.
The authors gratefully acknowledge the contributions of Drs. Kelly Nabal, Carla Moore, and Joseph Giorgio, who served as interventionists for this study, as well as Jennifer Krasucki, Elizabeth Gravallese, and Allison Higginbotham, who served as research coordinators.
Dr. Fabricatore has received research funding from Merck, and has received consulting fees from Merck, Pfizer, and Allergan. Although he was employed full-time by the University of Pennsylvania when this study was conceived and completed, Dr. Fabricatore is currently employed by Nutrisystem, Inc. Dr. Wadden has received research support from Novo Nordisk and serves on the Advisory Board of Novo Nordisk, Orexigen Therapeutics, and Vivus. Dr. Schwartz has received honoraria in the past year from Medtronic, Takeda, Novo Nordisk, Lilly, Merck, and Amylin for advisory board participation, and from Lilly, Amylin, Sanofi-Aventis, BMS/Astra-Zeneca, Novo Nordisk, Merck, and Takeda for speaker bureau participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registry: ClinicalTrials.gov NCT00729196
Conflict of Interest
The authors have a competing interest to declare.
References
- 1.National Heart Lung and Blood Institute, North American Association for the Study of Obesity . The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health; Bethesda: 2000. [Google Scholar]
- 2.Anderson JW, Kendall CWC, Jenkins DJA. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22:331–339. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- 3.The Look AHEAD Research Group Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:e26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 5.Warren JM, Henry JK, Simonite V. Low glycemic index breakfasts and reduced food intake in preadolescent children. Pediatrics. 2003;112:e414. doi: 10.1542/peds.112.5.e414. [DOI] [PubMed] [Google Scholar]
- 6.Ball SD, Keller KR, Moyer-Mileur LJ, Ding Y, Donaldson D, Jackson WD. Prolongation of satiety after low versus moderately high glycemic index of meals in obese adolescents. Pediatrics. 2003;111:488–494. doi: 10.1542/peds.111.3.488. [DOI] [PubMed] [Google Scholar]
- 7.Soenen S, Westerterp-Plantenga MS. No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads. Am J Clin Nutr. 2007;86:1586–1594. doi: 10.1093/ajcn/86.5.1586. [DOI] [PubMed] [Google Scholar]
- 8.Aston LM, Stokes CS, Jebb SA. No effect of a diet with a reduced glycaemic index on satiety, energy intake and body weight in overweight and obese women. Int J Obes. 2008;32:160–165. doi: 10.1038/sj.ijo.0803717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson GH, Catherine NL, Woodend DM, Wolever TM. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr. 2002;76:1023–1030. doi: 10.1093/ajcn/76.5.1023. [DOI] [PubMed] [Google Scholar]
- 10.Mayer-Davis EJ, Dhawan A, Liese A, Teff K, Schulz M. Towards understanding of glycemic index and glycemic load in habitual diet: associations with measures of glycemia in the Insulin Resistance Atherosclerosis Study. Br J Nutr. 2006;95:397–405. doi: 10.1079/bjn20051636. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan S, Rosenberg L, Singer M, Hu FB, Djoussé L, Cupples LA, et al. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch Intern Med. 2007;167:2304–2309. doi: 10.1001/archinte.167.21.2304. [DOI] [PubMed] [Google Scholar]
- 12.Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007;167:2310–2316. doi: 10.1001/archinte.167.21.2310. [DOI] [PubMed] [Google Scholar]
- 13.Mosdol A, Witte DR, Frost G, Marmot MG, Brunner EJ. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am J Clin Nutr. 2007;86:988–994. doi: 10.1093/ajcn/86.4.988. [DOI] [PubMed] [Google Scholar]
- 14.Levitan EB, Mittleman MA, Hakansson N, Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr. 2007;5:1521–1526. doi: 10.1093/ajcn/85.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, et al. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007;50:14–21. doi: 10.1016/j.jacc.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 16.Raatz SK, Torkelson CJ, Redmon JB, Reck KP, Kwong CA, Swanson JE, et al. Reduced glycemic index and glycemic load diets do not increase the effects of energy restriction on weight loss and insulin sensitivity in obese men and women. J Nutr. 2005;135:2387–2391. doi: 10.1093/jn/135.10.2387. [DOI] [PubMed] [Google Scholar]
- 17.Hare-Bruun H, Flint A, Heitmann BL. Glycemic index and glycemic load in relation to changes in body weight, body fat distribution, and body composition in adult Danes. Am J Clin Nutr. 2006;84:871–879. doi: 10.1093/ajcn/84.4.871. [DOI] [PubMed] [Google Scholar]
- 18.Maki KC, Rains TM, Kaden VN, Raneri KR, Davidson MH. Effects of a reduced-glycemic-load diet on body weight, body composition, and cardiovascular disease risk markers in overweight and obese adults. Am J Clin Nutr. 2007;85:724–734. doi: 10.1093/ajcn/85.3.724. [DOI] [PubMed] [Google Scholar]
- 19.McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, et al. Comparison of four diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2007;166:1466–1475. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- 20.Thomas D, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Systematic Reviews. 2007 doi: 10.1002/14651858.CD005105.pub2. Issue 3. Art. No.:CD005105. DOI: 10.1002/14651858.CD005105.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 22.Borushek A. The Calorie King Calorie, Fat, & Carbohydrate Counter. Family Health Publications; Costa Mesa: 2006. [Google Scholar]
- 23.Ebbeling CB, Ludwig DS. Dietary approaches for obesity treatment and prevention in children and adolescents. In: Goran MI, Sothern MS, editors. Handbook of Pediatric Obesity: Epidemiology, Etiology and Prevention. Marcel Dekker Inc; New York: 2005. [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Nutrition Data System for Research . University of Minnesota Nutrition Coordinating Center; Minneapolis: 2007. [Google Scholar]
- 26.Statistical Package for the Social Sciences, version 17.0. SPSS Inc; Chicago: 2008. [Google Scholar]
- 27.Opperman AM, Venter CS, Oosthuizen W, Thompson RL, Vorster HH. Meta-analysis of the health effects of using the glycaemic index in meal-planning. British Journal of Nutrition. 2004;92:367–381. doi: 10.1079/bjn20041203. [DOI] [PubMed] [Google Scholar]
- 28.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database of Systematic Reviews. 2009 doi: 10.1002/14651858.CD006296.pub2. Issue1. Art. No.:CD006296. DOI: 10.1002/14651858.CD006296.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolever TMS, Gibbs AL, Mehling C, Chiasson JL, Connelly PW, Josse RG, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008;87:114–125. doi: 10.1093/ajcn/87.1.114. [DOI] [PubMed] [Google Scholar]
- 30.Davis NJ, Tomuta N, Schechter C, Isasi CR, Segal-Isaacson CJ, Stein D, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a lowfat diet on weight and glycemic control in type 2 diabetes. Diabetes Care. 2009;32:1147–1152. doi: 10.2337/dc08-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med. 1987;147:1749–1753. [PubMed] [Google Scholar]
- 32.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 33.Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28:2939–2941. doi: 10.2337/diacare.28.12.2939. [DOI] [PubMed] [Google Scholar]
- 34.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a lowglycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl. 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]