Abstract
Background & Aims
A previous study demonstrated the presence of protease-activated receptor (PAR) 1 and 2 in the dorsal motor nucleus of vagus (DMV). The aim of this study is to characterize the effect of thrombin on the apoptosis of DMV neurons.
Methods
DMV neurons were isolated from neonatal rat brainstems using micro-dissection and enzymatic digestion and cultured. Apoptosis of DMV neurons were examined in cultured neurons. Apoptotic neuron was examined by Tunel and ELISA. Data were analyzed using ANOVA and Student’s t-test.
Results
Exposure of cultured DMV neurons to thrombin (0.1 to 10U ml−1) for 24h significantly increased apoptosis. Pre-treatment of DMV neurons with hirudin attenuated the apoptotic effect of thrombin. Similar induction of apoptosis was observed for the PAR1 receptor agonist SFLLR, but not for the PAR3 agonist TFRGAP, nor for the PAR4 agonist YAPGKF. PAR1 receptor antagonist Mpr(Cha) abolished the apoptotic effect of thrombin, while YPGKF, a specific antagonist for PAR4, demonstrated no effect. After administration of thrombin, phosphorylation of JNK and P38 occurred as early as 15 min, and remained elevated for up to 45 min. Pretreatment of DMV neurons with SP600125, a specific inhibitor for JNK, or SB203580, a specific inhibitor for P38, significantly inhibited apoptosis induced by thrombin.
Conclusions
Thrombin induces apoptosis in DMV neurons through a mechanism involving the JNK and P38 signaling pathways.
Keywords: Dorsal motor nucleus of vagus, protease–activated receptor, apoptosis, inflammatory bowel disease
Introduction
Thrombin is a multifunctional serine protease which has a role in neuronal injury produced by disorders such as stroke and Alzheimer’s disease. Two sources account for the presence of thrombin in the central nervous system (CNS). Thrombin can extravasate from blood into the brain when the blood-brain barrier is impaired, as observed in inflammation (1). Thrombin can also be synthesized in situ within the CNS, and the thrombin precursor, prothrombin, has been demonstrated in a wide range of CNS tissues (2–3). In addition, specific receptors for thrombin, protease activated receptors (PAR1, PAR3 and PAR4) (4), and the endogenous specific inhibitor, protease nexin-1(5), are widely expressed in the brain. These findings suggest that thrombin may alter the structure and function of the CNS.
The dorsal motor nucleus of vagus (DMV), together with visceral sensory nuclei of the solitary tract and the area postrema, compose the dorsal vagal complex. The dorsal vagal complex is a circumventricular organ which may be affected by vascularly-derived factors evoked by systemic inflammation (6). Previous studies have demonstrated the presence of functional PAR receptors in DMV neurons, and that PAR receptor expression is altered in animal models of intestinal inflammation (7). The present study examines the effect of thrombin on neuronal apoptosis in the DMV. We report that thrombin induces apoptosis in cultured DMV neurons derived from neonatal rats through a mechanism involving JNK and P38 signalling pathways.
Materials and Methods
Chemicals and solutions
Neurobasal medium, phosphate buffer solution (PBS), B27 supplement, L-glutamine, penicillin and streptomycin were purchased from Gibco (Grand Island, NY). β fibroblast growth factor (βFGF) was from Invitrogen (Carlsbad, CA). Poly-L-lysine, Triton X-100, thrombin, trypsin and hirudin were purchased from Sigma-Aldrich (St. Louis, MO). PAR-1, PAR-3 and PAR-4 agonist peptides (SFLLR, TFRGAP and YAPGKF), PAR-1and PAR4 antagonist peptides (Mpr(cha) and YPGKF) were from Peptides International (Louisville, KY). SP600125 and SB 203580 were purchased from Calbiochem (San Diego, CA).
Neuronal culture of dorsal motor nucleus
Dorsal motor nucleus neurons were isolated from neonatal Sprague-Dawley rats (Taconic, Hudson, NY) as described previously (8). The procedures used for the care and euthanasia of the animals were approved by the University of Michigan Committee on Use and Care of Animals. Briefly, rats were euthanized. The brainstem was rapidly removed and chilled at 0°C in a dissection solution containing: NaCl 138 mM, KCl 4 mM, MgCl2 1 mM, CaCl2 2 mM, glucose 20 mM and HEPES 10 mM. Tissue blocks were prepared and sectioned transversely into 400 μm slices at the level of the obex using a Vibratome 3000 (Redding, CA). The DMV area was identified under a dissecting microscope as the area immediately ventral to the nucleus of the solitary tract and dorsal to the XII nucleus. DMV tissue was excised and then digested in an enzyme solution containing protease type XIV (0.6 mg ml−1) and trypsin type I (0.4 mg ml−1) at 32 °C for 30 minutes. The tissue was then dissociated by gentle trituration with pipettes. Cells were plated onto poly-L-lysine coated culture dishes and chamber slides. Neurons were maintained at 37°C in an atmosphere of 5% CO2 in serum-free culture media containing Neurobasal medium containing 2% B27 supplement, 2 mM glutamine, 1% penicillin and streptomycin, and 5 ng ml−1βFGF. After 4 days, one-half of the medium was replaced and experiments were conducted at 7 days.
Enzyme-linked immunosorbent assay (ELISA)
Apoptosis was measured with Cell Death Detection ELISA Plus (Roche Diagnostics, Indianapolis, IN) following the manufacturer’s instructions. Neurons were plated at a density of 10,000 cells per well in 24-well plates for a week and exposed to experimental conditions or control for 24 hrs. Experimental conditions consisted of fresh media without βFGF in addition to thrombin with or without other reagents, while the control condition was fresh media. Subsequently, 200 μl lysis buffer was added and incubated for 30 min at room temperature. Lysates were centrifuged at 200 × g for 10min. 20 μl of supernatant was transferred into streptavidin coated wells and incubated with anti-histone-biotin and anti-DNA POD for 2 hrs at room temperature under gently shaking. Wells were washed, 100 μl of ABTS substrate was added and incubated for 20 min. After infusing 100 μl of ABTS Stop Solution, absorbance was determined at 405 nm. Apoptosis was calculated as the ratio of the absorbance of the sample cells/absorbance of control cells. The enrichment factor was used as a parameter of apoptosis. At least 6 repetitions were performed for each experimental condition.
Terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick-end labeling (TUNEL) assay
The amount of apoptosis in thrombin-treated neurons was also determined by TUNEL assay. Neurons were identified with specific anti-Hu (a neuronal marker) antibody. TUNEL assays were performed using the In Situ Cell Death Detection Kit, TMR red (Roche Diagnostics, Indianapolis, IN), which detects the 3′-OH termini of DNA strand breaks occurred during apoptosis. Briefly, DMV neuron cultures were fixed for 1hr with 4% paraformaldehyde in PBS, pH 7.4, then incubated for 2 min in a permeabilisation solution (0.1% Triton X-100 and 0.1% sodium citrate in PBS). TUNEL reaction mixture was added. DMV neurons were incubated in a humidified chamber for 1 hr at 37°C in the dark, and washed in PBS. Negative controls were incubated with label solution without terminal transferase, while positive controls were incubated with DNase I recombinant (300U ml−1 in 50 mM Tris-HCl, pH 7.5, 1mg ml−1 BSA) for 10 min at room temperature prior to labeling procedures. Cells were subsequently treated with blocking solution (10% normal goat serum, 3% BSA and 0.2% Triton X-100 in PBS, pH 7.4) at room temperature for 1 hr. After being incubated with anti-Hu antibodies (1:100) at 4°C overnight, cultures were washed and incubated with FITC-labeled anti-mouse IgG (1:100) for 1 hr at room temperature. Finally, cultured slides were mounted with ProLong® Gold antifade reagent with DAPI (Invitrogen) and viewed with fluorescent microscope (Nikon Eclipse Ti-U). Images of TUNEL staining with Hu immunofluorescence were created from the same cultures and merged using NIS-element software. Cells positive for TUNEL staining were counted from 5 fields of each slide and expressed as the percentage of total cells stained positive for Hu. Each experiment was repeated 3 to 5 times using cells harvested from 8–12 neonatal rats.
Double immunofluorescent staining
DMV neuronal cultures were fixed with 4% paraformaldehyde in PBS for 1 hr and treated with blocking solution for 1 hr at room temperature. The slides were then incubated at 4°C overnight with mouse anti-Hu (1:100, Invitrogen) and one of the following primary antibodies: goat anti-PAR-1 (C-18, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), goat anti PAR-3 (M-20, 1:100, Santa Cruz Biotechnology), goat anti PAR-4 (C-19, 1:100, Santa Cruz Biotechnology), rabbit anti-cleaved caspase3 (1:100, Cell Signaling Technology, Danvers, MA); Neurons were then incubated at room temperature for 1 hr with secondary antibodies: donkey anti-mouse FITC-conjugated IgG (1:100) and donkey anti-goat TRITC-conjugated IgG (1:100) or goat anti-mouse FITC-conjugated IgG (1:100) and goat anti-rabbit TRITC-conjugated IgG (1:100) (Jackson ImmunoResearch Laboratories). Negative controls included preincubating the PAR antibodies with specific immunizing blocking peptide (sc-8202P, sc-8209P, sc-1249P; Santa Cruz Biotechnology) or substituting primary antibodies with mouse IgG or rabbit IgG.
Western blotting
After treatment, DMV neuronal cultures were homogenized in 60 μl ice-cold lysis buffer (Cell Signaling Technology) containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg ml−1 leupeptin, 1 mM phenylmethylsulfonyl fluoride (PMSF), then centrifuged at 12,000 g at 4°C for 20 min. Equal amounts of protein (30~40 μg) from each sample were subjected to electrophoretic separation on a 10% polyacrylamide gel (BioRad), then transferred onto Immobilon™ PVDF membrane (BioRad). Membrane blots were blocked at room temperature for 1 hr in 5% milk in TBS–Tween 20 (0.05%), then incubated overnight in primary antibodies against JNK, phospho-JNK, P38 or phospho-P38 from Cell Signaling Technology (1:1,000 dilution). Membranes were washed three times in TBS–Tween (0.05%), then incubated for 1 hr with secondary antibody diluted 1/4000 in 5% TBS–Tween (0.05%). Detection was performed using Lumi-Light Blotting Substrate (Roche, Indianapolis, IN) or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific).
Data analysis
Results are expressed as mean ± SEM. Data were analyzed using ANOVA and Student’s t-test as appropriate. Significance was accepted as P < 0.05.
Results
Thrombin-induced DMN neuronal apoptosis
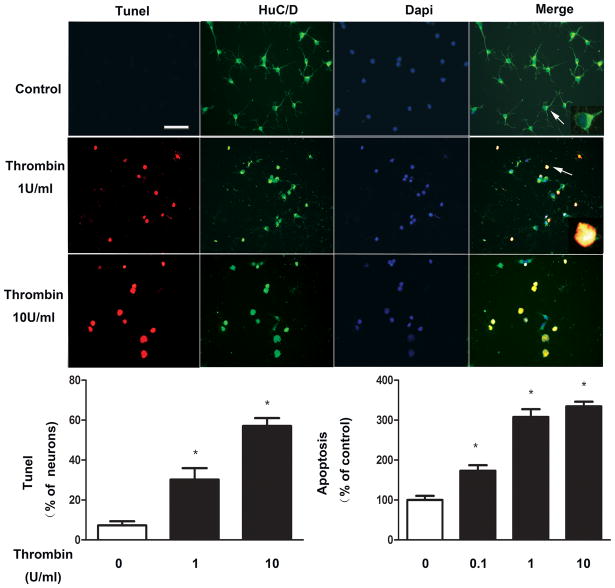
As shown in Figure 1, cultured DMV neurons exposed to concentrations of thrombin ranging from 0.1Uml−1 to 10U ml−1 for 24 hrs demonstrated significant increases in apoptosis measured either by Tunel assay or ELISA assay. Virtually all apoptotic cells detected by Tunel assay were positive for Hu, a neuronal marker, suggesting that thrombin-induced apoptosis occurs in neuronal cells.
Figure 1. Thrombin-induced apoptosis in cultured DMV neurons.
Thrombin at a dose from 0.1 to 10 U/ml significantly increases apoptosis in cultured DMV neurons. Cultured DMV neurons were exposed to varying concentrations of thrombin for 24 hrs. Apoptosis was assayed by Tunel and ELISA assay. Cells were stained positive for Hu, a neuronal marker. Results are expressed as mean±SEM. * p<0.05. Bar represents 100 μm. Cell marked with arrow is shown with higher magnification in the insert.
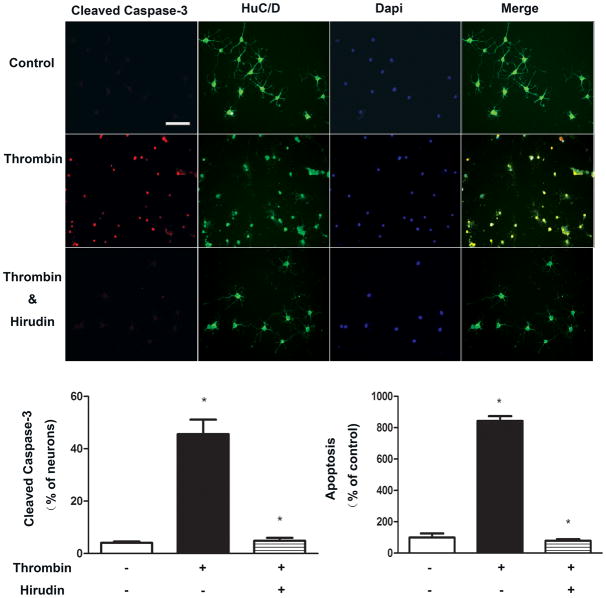
To investigate whether thrombin-induced apoptosis is specific, hirudin, a thrombin-specific protease inhibitor was used. As shown in Figure 2, cells pretreated with hirudin (10U ml−1) for 30 min demonstrated a significant reduction of apoptosis resulting from subsequent exposure to thrombin (10U ml−1).
Figure 2. Inhibition of thromin-induced apoptosis by hirudin.
Hirudin significantly reduces apoptosis resulting from subsequent exposure to thrombin. DMV neurons were pretreated with hirudin (10U ml−1) for 30 min before exposure to thrombin (10U/ml). A. Representative. B. Mean change in cleavage of caspase-3. C. Mean change as measured by ELISA. Results are expressed as mean±SEM. * p<0.05. Bar represents 100 μm
PAR receptor mediated effect
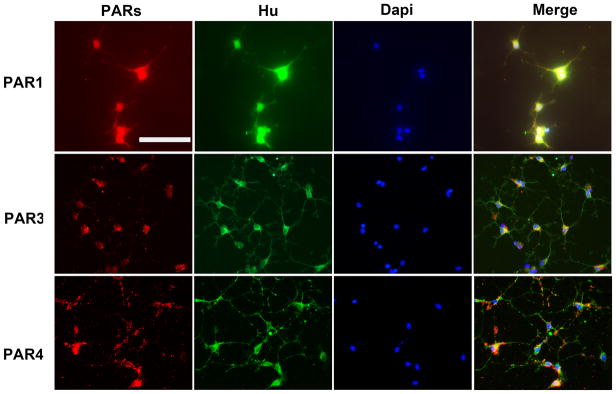
To examine PAR1, PAR3 and PAR4 protein expression in primary DMV cultures, cells were incubated with the relevant primary and secondary antibodies and visualized by fluorescent microscopy (Figure 3). To verify the neuronal nature of PAR-positive cells, cultures were stained for the neuronal marker Hu. As seen in Figure 3, the vast majority of cells staining positively for PAR1, PAR3 or PAR4 were also positive for Hu, suggesting of the neuronal nature of PAR-expressing cells. No staining was observed in control preparations.
Figure 3. Expression of PAR receptors in DMV neurons.
Majority of cultured DMV neurons stains positively for PAR1, PAR3 or PAR4. Cultured DMV neurons were stained with antibodies recognizing specific PAR receptor 1,3 or 4. Neurons cells were identified via Hu staining. Bar represents 100 μm
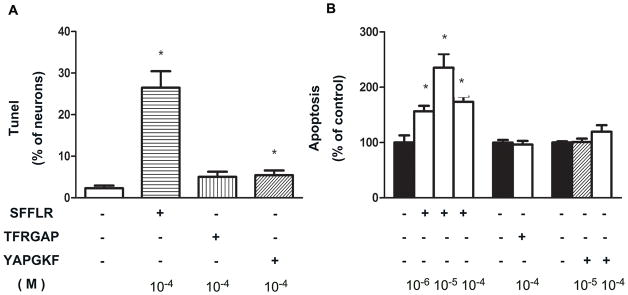
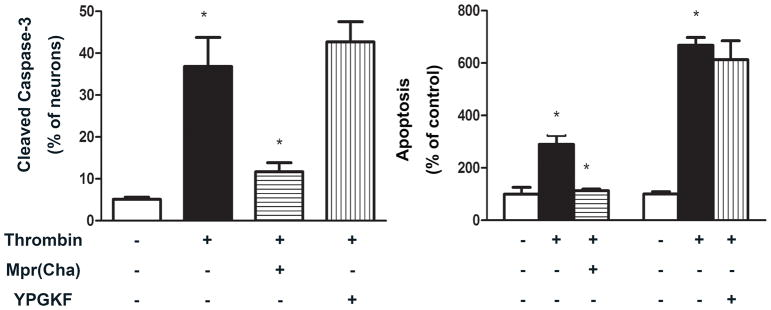
To examine the effect of PAR receptor activation on apoptosis, cultured DMV neurons were exposed to agonists or antagonists specific for individual PAR receptors. As shown in Figure 4, the PAR1 receptor specific agonist, SFLLR, induced a significant increase in apoptosis relative to control as measured by either Tunel assay or ELISA assay. Neither TFRGAP, a specific agonist for PAR3, nor YAPGKF, a specific agonist for PAR4, demonstrated a significant effect. These results suggest that thrombin causes apoptosis in DMV neurons through activation of PAR1 receptors. This interpretation is further supported by experiments using PAR antagonists. As shown in Figure 5, Mpr(Cha), a specific antagonist for PAR1 receptor, almost completely abolished the apoptotic effect of thrombin, while YPGKF, a specific antagonist for PAR4, demonstrated no effect.
Figure 4. Apoptosis of DMV neurons induced by PAR agonists.
Apoptosis is induced by PAR1 agonist only. Cultured DMV neurons were treated with agonists specific for PAR1 (SFLLR) ((10−6~10−4M), PAR3 (TFRGAP) (10−4M) or PAR4 (YAPGKF) (10−5 M and 10−4M) for 24 hrs. Apoptosis was analyzed by Tunel (A) and ELISA assay (B). Hu positive cells were counted. Results are expressed as mean±SEM. * p<0.05. Significant difference (P<0.05) was also observed between cells treated with 10−6 M and 10−5 M of SFLLR.
Figure 5. Effect of PAR antagonism on thrombin-induced apoptosis.
PAR1 antagonism abolishes the apoptotic effect of thrombin. Cultured DMV neurons were treated with antagonists for PAR1 (Mpr(Cha)) (10−4M) or PAR4 (YPGKF) (10−4M) 30 min before administration of thrombin (10 U ml−1). Cells were harvested and analyzed for apoptosis using cleaved caspase 3 immunofluorescent staining (left panel) and ELISA (right panel). Apoptotic signal in Hu positive cells were counted. Results are expressed as mean±SEM. * p<0.05.
Involvement of JNK and P38 signaling pathways
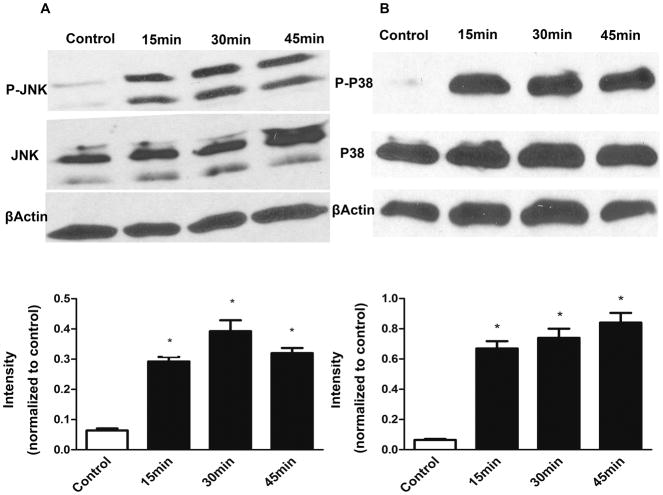
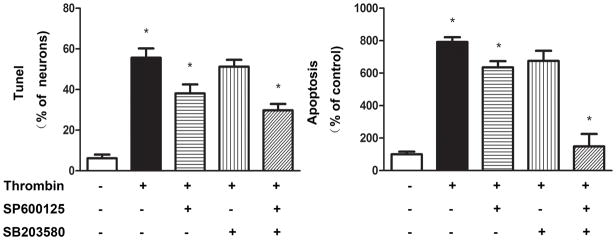
A variety of apoptotic stimuli affect the JNK and p38 signaling pathways and subsequently activate caspase-3. Accordingly, we examined whether JNK and P38 activation is involved in thrombin-induced neuronal apoptosis. As shown in Figure 6, phosphorylation of both JNK and P38 occurred as early as 15 min, and remained elevated for up to 45 min, after administration of thrombin with no alteration in the levels of total JNK and P38 proteins in cultured DMNV neurons. Pretreatment of cultured DMNV neurons with either SP600125, a specific inhibitor for JNK, or SB203580, a specific inhibitor for P38, significantly inhibited the apoptotic effect of thrombin. Combination of these two inhibitors to block both JNK and P38 signaling pathways demonstrated an additive effect to prevent thrombin-induced apoptosis in DMV neurons (Figure 7).
Figure 6. Activation of JNK and P38 by thrombin.
JNK and P38 are activated by thrombin. Cultured DMV neurons were treated with thrombin (10 U ml−1). Phosphorylation of JNK and P38 was detected using specific antibodies recognized phosphorylated JNK and P38.
Figure 7. Effect of JNK or P38 inhibitors on thrombin-induced apoptosis.
Thrombin-induced apoptosis is attenuated by JNK or P38 inhibitors. Cultured DMV neurons were treated with inhibitors specific for JNK(SP600125 10−5M), P38 (SB203580 10−5M) or both before exposure to thrombin (10 U ml−1). Apoptosis was evaluated by Tunel (left panel) and ELISA(right panel). Results are expressed as mean±SEM. * p<0.05.
Discussion
The current study demonstrates that thrombin induces apoptosis in cultured DMV neurons via a mechanism that is mediated by JNK and P38 signaling pathways activated by PAR1 receptor. This general conclusion is supported by five distinct observations: (1 thrombin evoked apoptosis inDMV neurons in a dose-dependent manner; (2 this effect was attenuated by pretreatment of cells with hirudin, a thrombin-specific protease inhibitor; (3 although all PAR receptor subtypes specific for thrombin are present in DMV neurons, only PAR1-specific receptor agonists and antagonists exhibited activity; (4 activation of both JNK and P38 was demonstrated in cultured DMV neurons exposed to thrombin; (5 pretreatment of cultured DMV neurons with inhibitors of JNK, or P38 inhibited apoptotic effects of thrombin, with a combination of inhibitors having additive effects.
Thrombin is known to generate intracellular signalling via PAR receptors, a family of G-protein-couple receptors. PARs are activated by proteolytic cleavage of their N-terminus to unmask an amino acid sequence, which then acts as a tethered receptor ligand. Among the four members of PAR family (PAR1 to PAR4), three receptor subtypes (PAR1, PAR3, and PAR4) are activated by thrombin. All four PARs are expressed by neurons and glial cells and have variously been implicated in a number of distinct physiological and pathological processes in the CNS (4, 9–10). For example, thrombin acts to protect neurons from a variety of insults, either directly (11) or through activation of neighboring astrocytes (12). These effects involve activation of PAR receptors at subnanomolar agonist concentrations. In contrast, high concentrations of thrombin induce neurodegeneration in vivo, and in vitro cause retraction of neurites, cell body shrinkage and apoptosis (13–14).
Althought all four PAR receptor subtypes are present in DMV neurons (7), our data suggest that thrombin evokes apoptosis in cultured DMV neurons mainly through activation of the PAR1 receptor. Hirudin, a thrombin-specific protease inhibitor, blocked the apoptotic effect of thrombin. PAR1 receptor antagonism using a short peptide inhibitor decreased apoptotic death in cultured DMV neurons induced by thrombin, while the PAR1 receptor-specific agonist, SFLLR, dose-dependently increased apoptosis. Exposure of DMV neurons to PAR3 and PAR4 synthetic agonists demonstrated no effects. Specific antagonism of the PAR4 receptor has no significant effect on thrombin-induced apoptosis in cultured DMV neurons.
The dorsal motor nucleus of vagus, together with visceral sensory nuclei of the solitary tract and the area postrema, composes the dorsal vagal complex. The dorsal vagal complex is a circumventricular organ with fenestrated capillaries and enlarged perivascular spaces (15). Dendrites penetrate the ependymal layer to reach the floor of the fourth ventricle(6). Because of this structure, the DMV may be affected by vascularly derived factors to evoke gastrointestinal dysfunction. For example, peripherally produced tumor necrosis factor can act on the dorsal vagal complex to cause gastric stasis (16–17).
Several studies suggest that circulating thrombin may enter the midbrain during states of systemic inflammation. Permeability through the blood-brain-barrier has been reported to be increased during systemic inflammation (18). Thrombin has also been demonstrated to compromise the integrity of the blood–brain barrier (19–20). Elevated levels of thrombin derived from vascular sources have been reported in cerebrospinal fluid (CSF) (21) and brain tissue (22).
The activity of thrombin and of other proteinases is significantly increased in the colon, intestine and circulation of patients with inflammatory bowel disease (IBD) (23–27). Circulating thrombin, as measured by endogenous thrombin potential, is significantly increased in patients with IBD (28). In addition, up-regulation of PAR receptor transcripts has been demonstrated in DMV neurons derived from animals with experimental models of IBD. Taken together, it seems likely that DMV neurons are exposed to thrombinderived from the circulatorysystemduring IBD, althought the possibility that thrombin may derive from microglia in situ cannot be exclused.
The histological hallmark of IBD is infiltration of immune cells including neutrophils, macrophages, lymphocytes and mast cells in the mucosa and submucosa of the intestine. In experimental models of IBD, such as trinitrobenzene sulfonic acid (TNBS)-induced colitis, activated CD4 helper T cells promote production of inflammatory cytokines and chemokines. These inflammatory mediators have been demonstrated to contribute to changes in neurochemical coding and in excitability of enteric neurons. While changes in the enteric nervous system have been well-demonstrated in IBD, less is known of the effects of inflammation on the extrinsic autonomic nervous system. Our previous studies demonstrated the presence of functional PAR receptors in the DMV. This study extends those observations to identify thrombin as an inflammatory mediator causing neuronal apoptosis in the DMV.
Several recent studies support the concept that thrombin can induce neurodegeneration in the central nervous system(13). In cerebral ischemia, activation of PAR1 receptor by thrombin causes neuronal death (29). Thrombin has also been linked via activation of PAR receptors to neurodegeneration associated with Alzheimer’s disease and stroke. In animals, intracortical injection of thrombin induces neuronal death in a dose-dependent manner. Neuronal apoptosis has been demonstrated in cultured hippocampal neurons and spinal motoneurons.
The signaling pathways involved in the induction of apoptosis by thrombin are diverse. Dependent upon the cell types, these include JAK (Janus kinase)/STAT (signal transducer and activator of transcription), RhoA, myosin light chain kinase, ERK1/2, phosphoinositide 3′-kinase (PI3K), c-Jun N-terminal kinase (JNK)/p38 MAP kinase, and various Bcl-2 family members. These pathways converge on caspase 3 which cleaves specific cellular substrates to cause the morphological and biochemical features of apoptotic cell death. Our study demonstrates that activation of PAR1 receptor by thrombin is linked to both JNK and P38 signaling pathways in DMV neurons. Inhibition of either JNK or P38 signaling pathway attenuated thrombin-induced apoptotic death in cultured DMV neurons, while inhibition of both pathways prevented thrombin-induced apoptosis.
In conclusion, this study provides the first evidence that thrombin causes apoptotic death in DMV neurons through activation of PAR 1 receptor linked to both JNK and P38 signaling pathways. These results imply that thrombin may serve as an endogenous neurotoxin associated with systemic inflammation.
Acknowledgments
This study was supported by National Institutes of Health Grant 4R37DK043225-20 and 2R01DK054032-11A1.
Abbreviations
- DMV
the dorsal motor nucleus of vagus
- IBD
inflammatory bowel disease
- JNK
c-Jun N-terminal kinase
- PAR
protease activated receptors
- TNBS
trinitrobenzene sulfonic acid
Footnotes
Conflict of interest
The authors have no other relevant disclosures or conflicts of interests.
Authors’ Contributions
Xiaobin Wu conducted the experiment, analyzed the data, made the figures and helped in drafting the manuscript; Weizhen Zhang designed the study, analyzed the results, supervised for all statistics, figures, interpretation of data, and wrote the manuscript; Ji-Yao Li, Biao-Xin Chai, Junsheng Peng, and Hui Wang assisted in analysis of results and drafting the manuscript; Michael W. Mulholland was involved in supervising and completing the manuscript.
References
- 1.Rohatgi T, Henrich-Noack P, Sedehizade F, et al. Transient focal ischemia in rat brain differentially regulates mRNA expression of protease-activated receptors 1 to 4. J Neurosci Res. 2004;75:273–279. doi: 10.1002/jnr.10847. [DOI] [PubMed] [Google Scholar]
- 2.Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6:575–581. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- 3.Kim S. Characterization of the prothrombin gene expression during nerve differentiation. Biochim Biophys Acta. 2004;1679:1–9. doi: 10.1016/j.bbaexp.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Striggow F, Riek-Burchardt M, Kiesel A, et al. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- 5.Niclou SP, Suidan HS, Pavlik A, Vejsada R, Monard D. Changes in the expression of protease-activated receptor 1 and protease nexin-1 mRNA during rat nervous system development and after nerve lesion. Eur J Neurosci. 1998;10:1590–1607. doi: 10.1046/j.1460-9568.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst. 1993;42:119–130. doi: 10.1016/0165-1838(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Wu X, Li JY, et al. Functional protease-activated receptors in the dorsal motor nucleus of the vagus. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Hu Y, Newman EA, Mulholland MW. Serum-free culture of rat postnatal neurons derived from the dorsal motor nucleus of the vagus. Journal of neuroscience methods. 2006;150:1–7. doi: 10.1016/j.jneumeth.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37:53–63. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- 10.Rohatgi T, Sedehizade F, Reymann KG, Reiser G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in the brain. Neuroscientist. 2004;10:501–512. doi: 10.1177/1073858404269955. [DOI] [PubMed] [Google Scholar]
- 11.Gorbacheva LR, Storozhevykh TP, Pinelis VG, Ishiwata S, Strukova SM. Modulation of hippocampal neuron survival by thrombin and factor Xa. Biochemistry (Mosc) 2006;71:1082–1089. doi: 10.1134/s000629790610004x. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Luo W, Reiser G. Activation of protease-activated receptors in astrocytes evokes a novel neuroprotective pathway through release of chemokines of the growth-regulated oncogene/cytokine-induced neutrophil chemoattractant family. Eur J Neurosci. 2007;26:3159–3168. doi: 10.1111/j.1460-9568.2007.05938.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee da Y, Park KW, Jin BK. Thrombin induces neurodegeneration and microglial activation in the cortex in vivo and in vitro: proteolytic and non-proteolytic actions. Biochemical and biophysical research communications. 2006;346:727–738. doi: 10.1016/j.bbrc.2006.05.174. [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Lee DY, Ryu JK, Kim J, Joe EH, Jin BK. Thrombin induces nigral dopaminergic neurodegeneration in vivo by altering expression of death-related proteins. Neurobiology of disease. 2003;14:181–193. doi: 10.1016/s0969-9961(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 15.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- 16.Hermann GE, Tovar CA, Rogers RC. LPS-induced suppression of gastric motility relieved by TNFR:Fc construct in dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2002;283:G634–639. doi: 10.1152/ajpgi.00412.2001. [DOI] [PubMed] [Google Scholar]
- 17.Hermann GE, Tovar CA, Rogers RC. TNFalpha-stimulation of cFos-activation of neurons in the solitary nucleus is suppressed by TNFR:Fc adsorbant construct in the dorsal vagal complex. Brain Res. 2003;976:69–74. doi: 10.1016/s0006-8993(03)02687-8. [DOI] [PubMed] [Google Scholar]
- 18.Hathaway CA, Appleyard CB, Percy WH, Williams JL. Experimental colitis increases blood-brain barrier permeability in rabbits. Am J Physiol. 1999;276:G1174–1180. doi: 10.1152/ajpgi.1999.276.5.G1174. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen C, Coelho AM, Grady E, et al. Colitis induced by proteinase-activated receptor-2 agonists is mediated by a neurogenic mechanism. Can J Physiol Pharmacol. 2003;81:920–927. doi: 10.1139/y03-080. [DOI] [PubMed] [Google Scholar]
- 20.O’Lynnger T, He Y, Hu H, Hua Y, Muraszko KM, Xi G. Concomitant intracerebral infusion of tissue plasminogen activator and thrombin leads to brain injury. Acta Neurochir Suppl. 2008;105:55–58. doi: 10.1007/978-3-211-09469-3_12. [DOI] [PubMed] [Google Scholar]
- 21.Smirnova IV, Salazar A, Arnold PM, Glatt S, Handler M, Festoff BW. Thrombin and its precursor in human cerebrospinal fluid. Thromb Haemost. 1997;78:1473–1479. [PubMed] [Google Scholar]
- 22.Gingrich MB, Traynelis SF. Serine proteases and brain damage - is there a link? Trends Neurosci. 2000;23:399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- 23.Souto JC, Martinez E, Roca M, et al. Prothrombotic state and signs of endothelial lesion in plasma of patients with inflammatory bowel disease. Dig Dis Sci. 1995;40:1883–1889. doi: 10.1007/BF02208650. [DOI] [PubMed] [Google Scholar]
- 24.Bustos D, Negri G, De Paula JA, et al. Colonic proteinases: increased activity in patients with ulcerative colitis. Medicina (B Aires) 1998;58:262–264. [PubMed] [Google Scholar]
- 25.Hayat M, Ariens RA, Moayyedi P, Grant PJ, O’Mahony S. Coagulation factor XIII and markers of thrombin generation and fibrinolysis in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14:249–256. doi: 10.1097/00042737-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Saibeni S, Saladino V, Chantarangkul V, et al. Increased thrombin generation in inflammatory bowel diseases. Thromb Res. 2009 doi: 10.1016/j.thromres.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Zezos P, Papaioannou G, Nikolaidis N, et al. Elevated markers of thrombin generation and fibrinolysis in patients with active and quiescent ulcerative colitis. Med Sci Monit. 2009;15:CR563–572. [PubMed] [Google Scholar]
- 28.van Bodegraven AA. Haemostasis in inflammatory bowel diseases: clinical relevance. Scand J Gastroenterol Suppl. 2003:51–62. doi: 10.1080/00855920310002708. [DOI] [PubMed] [Google Scholar]
- 29.Thevenet J, Angelillo-Scherrer A, Price M, Hirt L. Coagulation factor Xa activates thrombin in ischemic neural tissue. J Neurochem. 2009;111:828–836. doi: 10.1111/j.1471-4159.2009.06369.x. [DOI] [PubMed] [Google Scholar]