Abstract
We present a biobehavioral model that explains the neurobiological mechanisms through which measures of vagal regulation of the heart (e.g., respiratory sinus arrhythmia) are related to infant self-regulatory and social engagement skills. The model describes the sequential development of the neural structures that provide a newborn infant with the ability to regulate physiological state in response to a dynamically changing postpartum environment. Initially, the newborn uses primitive brainstem-visceral circuits via ingestive behaviors as the primary mechanism to regulate physiological state. However, as cortical regulation of the brainstem improves during the first year of life, reciprocal social behavior displaces feeding as the primary regulator of physiological state. The model emphasizes two sequential phases in neurophysiological development as the fetus transitions to postpartum biological and social challenges: 1) the development of the myelinated vagal system during the last trimester, and 2) the development of cortical regulation of the brainstem areas regulating the vagus during the first year postpartum.
Keywords: social behavior, infancy, polyvagal theory, heart rate variability, respiratory sinus arrhythmia
During the last trimester and continuing through the first year postpartum, the autonomic nervous system is rapidly changing. These changes insure that the infant can breathe, obtain food, and maintain body temperature. Coupled with the development of these abilities to obtain basic biological needs is a progressive change in the infant’s ability to regulate physiological and behavioral state through interactions with another person (e.g., mother). We propose that the developmental changes in the neural pathways that regulate autonomic state provide a neural platform to support the expanding abilities of the infant to engage objects and people in a dynamically changing environment Thus, the emerging behavioral repertoire and social-interactive needs of the rapidly developing young infant should be studied within the context of the maturational changes in the autonomic nervous system.
Contrary to the hypothesized dependence of social behavior on the autonomic nervous system, the autonomic nervous system has played a limited role within predominant theories in developmental psychology. Similarly, given the critical role that the autonomic nervous system plays in the infant’s survival during the transition from prenatal to postnatal environments, it is surprising that an understanding of the central mechanisms mediating the autonomic nervous system has been tangential to pediatric medicine. In general, measures of autonomic activity have been conceptualized as a correlate of motivated or adaptive behaviors within psychology and as indices of health risk in medicine.
We propose, from a biobehavioral perspective, that the maturational shifts in neural regulation of the autonomic nervous system provide a developing resource, which, in part, mediate much of the dynamic interaction that the infant has with both objects and others. Within this perspective, we identify sequential, developmental, neurophysiological processes, which provide the biological basis to explain how and why individual and developmental differences in vagal control of the heart are related to social behavior and, when relevant, clinical outcome.
Dependence on others: Parallels with maturation of the autonomic nervous system
At birth, mammalian infants are incapable of caring for themselves. The newborn is vulnerable and dependent on the mother, or a concerned caregiver, to survive. Support from others is required to obtain basic biological needs such as food, warmth, and protection. This dependence on other decreases as the infant develops. This decrease in dependence is paralleled by changes in neural regulation of the autonomic nervous system. During development, as higher brain circuits begin to regulate the brainstem nuclei, which control the autonomic nervous system, the infant becomes more independent and is increasingly capable of initiating social interactions with others to regulate physiological state. As these self-regulatory skills develop, the dependence on the caregiver to elicit ingestive-vagal reflexes (i.e., feeding) as a primary strategy of regulation, decreases. Behaviorally, the infant appears to be more socially skilled and better able to spend time alone. This is observed as the infant’s ability to both rapidly calm after disruptive challenges and to remain calm for longer periods, even in the absence of others.
Developmentally, as skills of state regulation improve, the central nervous system expands to promote enhanced cognition and greater control over peripheral motor systems. These global systems (autonomic, cognitive, and motor) mature in combination and enable the maturing infant to become more independent and explorative in a complex environment.
Similar to most mammals and distinct from our phylogenetically related reptilian ancestors, humans maintain a need for social interactions throughout their lifespan. Social separation and isolation for humans, regardless of age, leads to profound disruption in the ability to regulate physiological state and compromises both physical and mental health. This disruption may impact all aspects of development and may be expressed as delays in motor development, growth, and cognition, as well as global health vulnerabilities and atypical social and emotional behaviors. Studies reliably report that various degrees of social abandonment can have disastrous effects on child development. Studies of the Romanian orphans illustrate that caregiving, defined solely by the physical features of food, warmth, and protection without consistent and predictable social engagement opportunities, is insufficient for typical development. For example, in a three-year follow-up study of Romanian orphans, who had been adopted after living at least eight months in orphanages, Chrisholm (1998) found that the orphans had significantly more behavior problems, insecure attachment, and lower IQ scores than non-adopted or early-adopted children. Adoptive parents of these orphans also reported significantly more parenting stress. More recent anatomical studies have revealed that early social and emotional deprivation in many Romanian orphans may lead to functional and structural changes in brain regions, including orbital frontal gyrus, infralimbic prefrontal cortex, medial temporal structures (i.e., amygdale and head of hippocampus), and left uncinate fasciculus (Chugani et al., 2001; Eluviathingal et al., 2006). Impairment in functions of these brain regions could certainly contribute to lower verbal skills, diminished attention and concentration abilities, impaired impulsivity, conduct problems, and depression frequently reported in these children.
Unlike reptiles and other phylogenetically more ancient vertebrates, birth for mammals is not a transition into independence, but an extension of the period of dependence that begins in utero. For humans, maturation does not lead to a total independence from others, but leads to ability to function independent of other people for short periods. Moreover, humans, as they become more independent of their caregivers, search for appropriate others (e.g., friends, partners, etc.) with whom they may form dyads capable of symbiotic regulation. In fact, individuals, who prefer to use interactions with objects instead of people to regulate physiological state, often receive psychiatric diagnoses (e.g., autism spectrum disorders, borderline personality disorder).
Evolution of the autonomic nervous system prepares the human infant for social behavior
It is necessary to understand the phylogenetic origins of mammalian autonomic nervous systems to understand how autonomic state is linked to social behavior. This conceptualization has been described as the Polyvagal Theory (Porges, 1995, 1998, 2001, 2003, 2007). The theory was derived from the identification of the two motor branches of the vagus nerve, the Xth cranial nerve, that provides both motor and sensory pathways between brainstem structures and visceral organs. The theory emphasizes not only the phylogenetic shifts in neuroanatomy of the vertebrate autonomic nervous system, but also proposes specific, adaptive behaviors that co-occur with these transitions.
As evolutionary forces molded the human nervous system, new structures were added and older structures were modified to allow greater dynamic range and more fine control of physiological state and to facilitate emergence of new, adaptive social behaviors. The Polyvagal Theory, by incorporating an evolutionary approach, provides a strategy to investigate developmental shifts in social behavior from a phylogenetic perspective. The theory provides insights into how developmental shifts in neural regulation of the autonomic nervous system are related to the changing repertoire of adaptive behaviors that, depending on context, either limit or expand expression of social behavior. The theory emphasizes unique features in neural regulation of the autonomic nervous system that distinguish mammals from reptiles and explains how these features serve as a biobehavioral platform for the emergence of face-to-face social behaviors. In addition, the theory provides insights into the biobehavioral mechanisms that are rapidly developing in utero and in the young infant. As the neural mechanisms facilitating self-regulation improve during normal development, the infant’s dependence on others to regulate physiological state decreases. This allows social communication to expand beyond the cueing of basic physical survival needs (i.e., warmth, safety, food) and into the realm of prosocial engagements.
The Polyvagal Theory articulates how each of three phylogenetic stages in the development of the vertebrate autonomic nervous system is associated with a distinct autonomic subsystem that is retained and expressed in mammals. These autonomic subsystems are phylogenetically ordered and behaviorally linked to social communication (e.g., facial expression, vocalization, listening), mobilization (e.g., fight-flight behaviors, tantrums or behavioral meltdowns), and immobilization (e.g., feigning death, vasovagal syncope, and behavioral and physiological shutdown). The product of this phylogeny is a mammalian nervous system with three identifiable circuits that regulate adaptive behaviors and physiological reactions to challenges.
In this phylogenetically organized hierarchy, the newest circuit associated with social communication is used first. If that circuit fails to provide safety, then the older survival-oriented circuits are recruited sequentially. From a developmental perspective, the oldest circuits develop first, and the newest circuit develops last, leaving it the most vulnerable to neural insult and the most sensitive to postpartum experience. The newest circuit becomes only partially available during the last trimester and is expressed at term as the brainstem reflexes that enable the coordination of sucking, swallowing, and breathing. By six months postpartum, these brainstem reflexes, which become coordinated with cortical processes, provide a biobehavioral pathway through which reciprocal social engagement behavior can calm and soothe physiological state in both participants of a social dyad (e.g., mother-infant interactions).
Development of the “vagal brake:” Consequences for postpartum adaptation and social behavior
The mammalian nervous system did not develop solely to survive in dangerous and life-threatening contexts, but also to promote social interactions and social bonds in safe environments. To accomplish this adaptive flexibility, a new neural strategy requiring safe environments emerged, while the more primitive neural circuits to regulate defensive strategies were retained. To accommodate both fight-flight and social engagement behaviors, the new mammalian vagus evolved to enable rapid, adaptive shifts in autonomic state. The mammalian myelinated vagus functions as an active vagal brake (Porges, Doussard-Roosevelt, Portales & Greenspan, 1996) in which inhibition and recovery of the vagal tone to the heart can rapidly mobilize or calm an individual. Tonic vagal influences to the sinoatrial node (i.e., the primary cardiac pacemaker) produce a resting heart rate that is substantially lower than the intrinsic rate of the pacemaker alone. When vagal tone, through myelinated vagal pathways, to the pacemaker is high, the vagus acts as a restraint, or brake, limiting the rate at which the heart can beat and functionally calming the individual. When vagal tone to the pacemaker is low, there is little or no inhibition of the pacemaker, and the heart rate increases. The vagal brake construct may be used to describe functional modulation of heart rate by myelinated vagal efferent pathways.
The state of the vagal brake can be quantified as the amplitude of a periodic component in the beat-to-beat heart rate pattern known as respiratory sinus arrhythmia (RSA). RSA is a naturally occurring rhythm in the heart rate pattern that oscillates at approximately the frequency of spontaneous breathing. RSA represents only a portion of beat-to-beat heart rate variability. By quantifying RSA and the relation between RSA and heart rate during various challenges, it is possible to measure the dynamic regulation of the myelinated vagal brake to study the responses of infants and young children to people and to objects (Bazhenova, Plonskaia, & Porges, 2001).
The human infant is not born with a completely functioning myelinated vagal system. The mammalian vagus is only partially myelinated at birth and continues to develop during the first few months postpartum. Morphological studies demonstrate a rapid developmental increase in total number of myelinated vagal fibers from 24 weeks through adolescence with the greatest increases observed from approximately 30-32 weeks of gestational age to approximately 6 months postpartum (see Sachis, Armstrong, Becker, & Bryan, 1982). More recent neuroanatomical research suggests that the increase in myelinated fibers may be occurring in the absence of an increase in unmyelinated vagal fibers, since the increase in the number of myelinated vagal fibers is paralleled by a decrease in the ratio of unmyelinated to myelinated vagal fibers (Pereyra, Zhang, Schmidt, & Becker, 1992). A relative increase in myelinated vagal fibers would functionally improve visceral regulation and enable the infant to express better behavioral regulation, which would support spontaneous social engagement behaviors. Based on these studies, preterm infants born before approximately 30 weeks gestational age are likely compromised due to lack of an appropriately functioning mammalian vagus, or vagal brake. Without a functioning mammalian vagus, the preterm has a limited ability to regulate visceral state and is dependent solely on the sympathetic nervous system and on the phylogenetically older unmyelinated vagus to meet physiological needs. This compromised profile of autonomic regulation obligates the preterm infant to rely on the sympathetic nervous system to increase heart rate in response to distress, in order to support tantrums and mobilization behaviors. Preterm infants are also more vulnerable to clinically dangerous hypotensive states and lowered oxygen saturation caused by episodes of bradycardia and apnea (i.e., massive slowing of the heart and cessation of breathing), which may be triggered by ingestive behaviors (e.g., sucking and swallowing) through activity in the more primitive unmyelinated vagus that has already developed.
RSA in the preterm infant follows a maturational trajectory that parallels the reported changes in both number and ratio of myelinated vagal fibers. During the preterm period there is a monotonic increase in RSA from 32-37 weeks gestational age (Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, 1997). Opportunities for skin-to-skin contact (i.e., Kangeroo care) between mother and preterm (Feldman & Eidelman, 2003) enhance the development of RSA. Paralleling the enhanced vagal regulation, these authors also reported more rapid improvement in state organization and a more mature neurodevelopmental profile. However, the enhanced development of RSA was only relative to preterm controls not receiving skin-to-skin contact and was still substantially lower than reports of RSA in typically delivered fullterms (Porges, 1992).
In longitudinal studies evaluating heart rate and RSA in term infants during the first year postpartum, heart rate reliably slows with age, although RSA changes are less obvious (i.e., Fracasso, Porges, Lamb, & Rosenberg, 1994; Izard et al., 1991). In these studies the effects of development on RSA appear to be maximized during the first 6 months postpartum and taper during the 6 – 12-month period. Although these developmental trends have been reported and replicated, closer inspection of the data indicates that individual differences in RSA during the first year postpartum are even greater than the developmental shifts.
Based on parallel literatures that describe developmental features in both RSA and the neuroanatomy of the vagus nerve, we may make two inferences: 1) RSA during early infancy reflects the functional outflow of the myelinated vagus; and 2) efficient RSA reactivity and recovery are dependent on both the number of myelinated vagal fibers and the ratio of myelinated to unmyelinated vagal fibers. The literature on young infants supports these assumptions by demonstrating increases in RSA during the last trimester through the first few months postpartum. The second assumption has primarily been tested during feeding when ingestive-vagal reflexes are recruited, although additional research will need to examine reactivity during social challenges during the first few months postpartum (e.g., Bazhenova et al., 2001;Weinberg & Tronick, 1996; Moore & Calkins, 2004).
Feeding: Challenging and exercising the vagal brake through an ingestive-vagal reflex
To ingest food properly and efficiently, the newborn must have the neural resources to implement the complex sequence of sucking, swallowing, and breathing. This sequence requires the coordination of the striated muscles of the face, head, and neck with the myelinated vagal regulation of the bronchi and the heart. Unlike the striated muscles of the limbs, striated muscles of the face,head, and neck are regulated by pathways traveling from the brainstem through several cranial nerves. These pathways are known as special visceral efferent pathways, although anatomically, their targets are not considered visceral. These pathways are often called branchiomeric, since they regulate the structures that embryologically emerged from the pharyngeal (branchial or ancient gill) arches. As the infant matures, the special visceral efferent pathways are recruited by corticobulbar pathways and expressed through social engagement behaviors. Autonomic support for these muscles is provided by the myelinated vagus, which can be dynamically monitored by quantifying RSA. This face-heart connection provides the necessary elements for an integrated social engagement system (see below).
Since the structures involved in the neural regulation and coordination of the striated muscles involved in sucking, swallowing, vocalizing, and breathing are all linked to the myelinated vagus, the functioning of these behaviors, and the link between these behaviors and RSA, may provide an early indicator of the functional status of a system that will later be involved in social engagement behaviors.
The status of the face-heart connection can be evaluated by measuring RSA changes during the ingestive behavior of sucking, which requires coordination of the striated muscles of the face with visceral changes in heart rate and breathing. Porges and Lipsitt (1993) monitored the integration of sucking behaviors with heart rate and RSA as infants sucked to obtain sucrose. In response to sucrose, reductions in RSA and corresponding increases in heart rate paralleled increased sucking frequency. When the availability of sucrose was terminated, RSA and heart rate returned to pre-sucrose levels. Moreover, individual differences in baseline RSA were correlated with the magnitude of heart rate reactivity to the gustatory stimulation. These findings illustrate that in the healthy neonate, there is a coordinated ingestive response in which the vagal brake is systematically removed to support the increased metabolic demands of sucking.
Because ingestive-vagal responses can be systematically elicited, a feeding challenge paradigm provides an opportunity in newborns and preterm infants to evaluate the status of coordinated physiological-behavioral sequences that require vagal regulation and control of the striated muscles of the face, head, and neck. Similar to the full term infants described in the Porges and Lipsitt study, clinically stable low-birthweight preterm infants (near the time of discharge) also decreased RSA and increased heart rate during feeding (Portales et al., 1997). When feeding was terminated, heart rate and RSA returned to pre-feeding levels. In a second study, with more clinically compromised infants (Suess et al., 2000), RSA and heart rate were monitored before, during, and after oral or gastric-tube bolus feedings in preterm infants at approximately 33 – 34 weeks postmenstrual age. The preterm neonates were categorized into two groups based on gestational age at birth. The earlier-born group had gestational ages less than 30 weeks and the later-born group had gestational ages greater than 30 weeks. Consistent with the above studies, RSA decreased in both groups during feeding. However, post-feeding RSA increased toward pre-feed levels (i.e., recovered) only in later-born infants. The results confirmed the assumption that the higher risk group, independent of corrected gestational age, experienced compromised vagal regulation during the feeding paradigm.
The primary characteristics of the feeding response is a withdrawal of vagal control of the heart to increase metabolic activity necessary to ingest food and a post-feeding recovery of vagal tone to foster a calm state and support digestion. Thus, during metabolically demanding ingestive behaviors such as sucking, there is a reduction of myelinated cardiac vagal tone to allow greater mobilization of energy resources. Following these ingestive behaviors, there is a recovery of vagal function to support digestion and calm the infant.
The studies described above indicate that term infants and stable older preterm infants modulate the “vagal brake” during feeding. This ingestive-vagal reflex might be mediated solely at the brainstem level and might not be sensitive to the increasing number and efficiency of corticobulbar pathways connecting the cortex with the source nucleus of the myelinated vagus (the nucleus ambiguus) that arise during maturation. From an evolutionary perspective, the adaptive consequence of these higher-order neuroanatomical changes would enable the older infant to use social cues to regulate the vagal brake. It is possible that through neuroception (see Porges, 2003), the loving caregiver’s facial features and vocal prosody trigger temporal corticolimbic pathways that dampen defensive reactions and recruit the vagal brake to calm. In contrast, the facial and vocal features of a stranger might inhibit the vagal brake to allow mobilizing, protesting, and defensive behaviors.
The Social Engagement System
A face-heart connection evolved in mammals as source nuclei of vagal pathways shifted ventrally from the phylogenetically older dorsal motor nucleus (e.g., unmyelinated vagal pathways) to the nucleus ambiguus (e.g., myelinated vagal pathways). This resulted in an anatomical and neurophysiological linkage between neural regulation of the heart via the myelinated vagus and the special visceral efferent pathways that regulate the striated muscles of the face, head, and neck. Together this linkage between brainstem motor systems responsible for cardiovascular functions and those necessary for regulating the face, head, and neck form an integrated “Social Engagement System.”
With increased cortical development, the cortex exhibits greater control over the brainstem via direct (e.g., corticobulbar) and indirect (e.g., corticoreticular) neural pathways originating in the motor cortex and terminating in the source nuclei of the myelinated motor fibers emerging from the brainstem (e.g., specific neural pathways embedded within cranial nerves V, VII, IX, X, and XI). These cranial nerves then extend from their source nuclei to control visceromotor structures (i.e., heart, bronchi) as well as branchiomotor structures (muscles of the face, head, and neck).
Specifically, the Social Engagement System includes the regulation of the eyelids through the orbicularis oculi (e.g., social gaze and gesture), muscles of facial expression (e.g., emotional expression), middle ear muscles (e.g., extracting human voice from background sounds), muscles of mastication (e.g., ingestion, sucking), laryngeal and pharyngeal muscles (e.g., vocalizing, swallowing, breathing), and muscles of head turning and tilting (e.g., social gesture and orientation). Collectively, these muscles act as filters for social stimuli (i.e., observing others’ facial expressions and the detection of prosody in human voice), and they allow the expression of the motor behaviors necessary for engagement with the social environment.
Based on the Polyvagal Theory, the development of the mammalian myelinated vagus is critical in the development of the face-heart connection, which links social behavior and autonomic regulation. Thus, with more optimal vagal regulation, features of more adaptive social behavior emerge. Without a functioning myelinated vagus, social behavior would be compromised and more primitive defensive strategies, such as fight-flight mobilization and tantrums (mediated by the sympathetic nervous system) and shutdown behaviors (mediated by the unmyelinated vagal system), would be more frequently expressed. Clinically, the status of vagal myelination becomes critical for the newborn and the young infant as they attempt to engage and disengage the caregiver and to explore social reciprocity as a mechanism to regulate physiology and behavior.
Model: Autonomic state provides a neural platform for social behavior
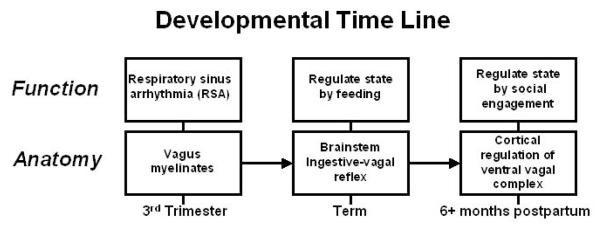
The unique features of the autonomic nervous system that support mammalian social behavior start to develop during the last trimester of fetal life. Specifically, myelination of vagal efferent fibers from the nucleus ambiguus to the sinoatrial node (i.e., cardiac pacemaker) begins during the last trimester. This process continues during the first few months postpartum and can be monitored by quantifying the amplitude of RSA. Regulation of the myelinated vagus (i.e., vagal brake) provides a mechanism to rapidly increase metabolic output by releasing the “brake” on the heart’s pacemaker (i.e., sinoatrial node). The release of the vagal brake results in an instantaneous increase in heart rate. Subsequently, when the metabolic demands decrease, inhibitory vagal influence on the heart is reinstated, and heart rate instantaneously decreases. The regulation of the vagal brake impacts important developmental processes related to survival, including 1) improved reactivity and recovery of the ingestive-vagal reflexes involved during feeding, 2) an expanded capacity to self-regulate and calm, and 3) improved abilities to both spontaneously engage others and to be soothed by others. This developmental time line is illustrated in Figure 1.
Figure 1.
Developmental timeline relating myelination of the vagus to social behavior.
Prematurity, illness, or negelect may dampen the developmental trajectory of the vagal circuit. Atypical maturation of this circuit may be reflected in myelination of the vagus, interneuronal connections in the brainstem that form the face-heart connection, and/or corticobulbar regulation of the brainstem circuits regulating both vagal activity and the striated muscles of the face, head, and neck. The consequences of these delays or disruptions in typical neural maturation would be expressed as lower levels of RSA, less efficient reactivity and recovery of the “vagal brake,” difficulties in behavioral state regulation, poor affective tone, and diminished abilities for reciprocal social engagement behaviors.
The myelinated vagus is not the sole mediator of autonomic state, in general, or heart rate, specifically. Heart rate is influenced by intrinsic cardiac mechanisms, surrounding thoracic anatomy, the sympathetic nervous system, and the unmyelinated vagus originating in the dorsal nucleus of the vagus. The development of the sympathetic nervous system and the unmyelinated vagal circuit has not been extensively studied in the human fetus and has been assumed to be functioning at term. Within our phylogenetic perspective, we assume that these circuits are functioning at the start of the last trimester. Below, we support this assumption with a brief review of the limited literature focusing on studies of the embryological development of the central regulators and peripheral pathways directly influencing heart rate or the contractility of the heart. Although only the development of efferent (motor) fibers are discussed, afferent (sensory) pathways play a vital role in both autonomic function and in providing sufficient input to trigger normal neural development.
The development of the autonomic nervous system in the human fetus mirrors the broader phylogenetic progression described above. The oldest existing autonomic system, comprised of unmyelinated vagal fibers originating from the dorsal motor nucleus of the vagus (DMNX), is also, embryologically, the earliest system to develop in utero. An immature, undifferentiated DMNX first appears in the brainstem at 9 weeks gestation (Cheng, Zhou, Qu, Ashwell, & Paxinos, 2004; Nara, Goto, & Hamano, 1991). Magnocellular subdivisions become visible by 13 weeks, and clear demarcation of DMNX subnuclei, including the lateral cardiomotor subnucleus, occurs by 23 weeks. At 28 weeks, all magnocellular subnuclei are considered essentially mature (Cheng et al, 2004). Some, however, including Nara et al, 1991, believe there may be some postnatal changes in the DMNX, such as increased nuclear columnar length and volume. Even if present, however, these postnatal changes are not considered to have much functional significance or physiological consequence in the neonate.
The other major component of the parasympathetic, cardioinhibitory ANS is the newest, myelinated vagal system, which originates in the nucleus ambiguus (NA). This system, as predicted by the Polyvagal Theory, develops last in the fetus, and continues functional development well into the first postnatal year. Mature neurons appear in the rostral NA by 8 or 9 weeks gestation and fill the nucleus by 12.5 weeks (Brown, 1990). Unlike mature neurons in the lateral subnucleus of the DMNX, however, axons of these mature neurons have not yet reached cardiac tissue to exert cardioinhibitory effects. The functional significance of vagal fibers from the NA depends heavily on myelination, which does not begin until 23 weeks gestation, when near mature axon diameter is achieved (Woźniak and O’Rahilly, 1981). Myelination of NA vagal fibers increases linearly from 24 to 40 weeks gestation and, again, continues actively during the first year postpartum (Sachis et al., 1982; Pereyra et al., 1992).
Development of the sympathetic, cardioexcitatory ANS is less well described in the literature. Phylogenetically, this largely catecholaminergic system appears before the mammalian NA vagal system and after the older DMNX vagus. According to the Polyvagal Theory, then, this system should begin development in the human fetus sometime between the two parasympathetic systems. Anatomically, sympathetic innervation of cardiac tissue is complex and difficult to isolate. Postganglionic cardiomotor nuclei lie mostly within the cardiothoracic and middle cervical ganglia, which lie caudal to the sympathetic superior cervical ganglion. Functionally, sympathetic influence on the heart is also varied. Unlike the two vagal circuits, which exert mostly chronotropic effects (slowing heart rate), sympathetic activity leads to both chronotropic (increasing heart rate) by innervating pacemaker tissue and inotropic (increasing cardiac contractility) by innervating ventricular myocardium. Investigations using fetal heart rate monitoring to infer sympathetic activity provide potential insight into development of this system. Using continuous 24-hour fetal heart rate monitoring in 28 healthy women, who were 16 to 28 weeks pregnant, Kinbraia, Zarnadze, Kintraia, & Kashakashvili (2005) reported that fetal locomotor activity increased between 16 and 20 weeks gestation. At this stage, increases in activity are accompanied by corresponding increases in heart rate, which returns to normal during “quiet” fetal periods. Since the vagal brake is not functional during this period of fetal development, these increases in heart rate are most likely due to activity in the sympathetic nervous system. Furthermore, the authors interpret an absence of such coordinated heart rate increase with increased locomotion by 24 weeks gestation as “developmental retardation.”
Measurement of RSA can map the development of the myelinated vagus and also enable dynamic monitoring of vagal reactivity and recovery elicited during feeding to quantify the status of ingestive-vagal reflexes. The literature documents that both amplitude of RSA and pattern of RSA responses during feeding are sensitive indices of risk in preterm and term infants. Ingestive-vagal reflexes elicited during feeding provide an opportunity early in development to evaluate a neural circuit that will later be involved in social engagement behaviors. Eliciting these reflexes provides an opportunity to exercise the neural circuits coordinating both the striated muscles of the face and neck and the vagal brake. As the infant develops, the brainstem structures involved in ingestive-vagal reflexes are increasingly recruited by higher brain structures, which regulate the facial and vocal features involved in reciprocal social interactions. Thus, when brainstem ingestive-vagal reflexes are functional, the first year postpartum is characterized by an increase in the efficiency with which corticobulbar pathways recruit and regulate these same brainstem nuclei for the purpose of social engagement. If, during early infancy, ingestive-vagal reflexes are not efficiently working, then there will be difficulties coordinating sucking, swallowing, and breathing. Problems in regulating these survival-related processes may provide a sensitive prognostic index leading to difficulties not only in social behavior, but also in the development of cognitive and language skills that are dependent on appropriate behavioral and physiological state regulation.
Changes in RSA represent a dynamic adjustment of the inhibitory action of the vagus (“vagal brake”) on the heart. Functionally, the removal of the vagal brake provides a physiological state that promotes vigilance as an intermediary and precautionary psychological process to monitor risk in the environment. The outcome of this assessment includes the induction of different physiological states either in which social behaviors can proceed, or in which defensive fight-flight strategies associated with increased sympathetic excitation are necessary. If defensive behaviors are not necessary to maintain or to negotiate safety, then the rapid vagal regulatory mechanisms that dampen autonomic state are reinstated, allowing the individual to calm and self-soothe. Further support for this interpretation can be seen in the infant data in which suppression of RSA is correlated with maternal reports of longer attention spans and being more easily soothed (Huffman et al., 1998). For example, Huffman et al. (1998) found that 12-week-old infants with higher baseline RSA expressed fewer negative behaviors and were less disrupted by experimental procedures than age-matched infants with lower baseline RSA. Moreover, consistent with the vagal brake concept, the infants who decreased RSA during the laboratory assessment were rated on maternal report temperament scales as having longer attention spans and being more easily soothed.
The regulation of behavioral state is a critical determinant of the range of social behaviors an individual can express. The underlying mechanisms mediating behavioral state are tightly linked to the autonomic nervous system. Investigation of the early maturational changes in vagal regulation of autonomic state unmasks several of the behavioral features that infants exhibit. For example, greater suppression of RSA during challenging situations is related to better state regulation, greater self-soothing, more attentional control, and greater capacity for social engagement (Calkins, 1997; DeGangi, DiPietro, Porges, & Greenspan, 1991, Porges et al., 1996; Huffman et al, 1998; Stifter & Corey, 2001).
The ability to regulate state follows a developmental trajectory during the early part of life. As the neural circuits involved in state regulation become more available to the developing child, there are parallel opportunities for social engagement behaviors and the development of strong social bonds. Without the dynamic, efficient myelinated vagus, it is difficult to regulate behavioral state and to use the features of the Social Engagement System (i.e., facial expression, vocal prosody), which at birth are involved in feeding behaviors (i.e., ingestive-vagal reflexes). Developmental limitations in the vagal system, expressed as low level of RSA and difficulties in regulating RSA, may lower thresholds to negative or ambiguous environmental cues with resultant hyper-reactivity and severe limitations in the ability to self-sooth and calm.
Social behavior and the capacity to manage challenge are dependent on the neural regulation of physiological state. The neural circuits involved in the regulation of physiological state are modified during gestation and continue during postnatal life. When these circuits are easily available and efficiently functioning, then the laws of learning and the impact of experience can shape behavior. However, when these circuits are not available, either as a function of phase of development or during periods of increased environmental risk, then state regulation is compromised, social skills are not easily learned, and social bonds become difficult to establish. During most of the lifespan, the vagal brake and the other features of the Social Engagement System are readily available and contribute to the numerous opportunities for social learning to occur. Without the efficient vagal brake turning off defensive systems and blunting their disruptive manifestations (e.g., fight-flight behaviors), prosocial behavior is limited and opportunities for social learning and social bonding are minimized.
Acknowledgments
The research described in this manuscript was supported, in part, by NIH Grant R01 HD053570 from the National Institute of Child Health and Human Development and NIH Grant T32 MH18882 from the American Psychological Association Diversity Program in Neuroscience.
References
- Bazhenova OV, Plonskaia O, Porges SW. Vagal Reactivity and Affective Adjustments in Infants during Interaction Challenges. Child Development. 2001;72:1314–1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- Brown JW. Prenatal development of the human nucleus ambiguous during the embryonic and early fetal periods. American Journal of Anatomy. 1990;189:267–283. doi: 10.1002/aja.1001890310. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Zhou X, Qu J, Ashwell KWS, Paxinos G. Central vagal sensory and motor connection: human embryonic and fetal development. Autonomic Neuroscience: Basic and Clinical. 2004;114:83–96. doi: 10.1016/j.autneu.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Chisholm K. A Three Year Follow-Up of Attachment and Indiscriminate Friendliness in Children Adopted from Romanian Orphanages. Child Development. 1998;69:1092–1106. [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. Brain Funcational Activity Following Early Deprivation: A Study of Postinstitutionalized Romanian Orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- DeGangi GA, DiPietro JA, Greenspan SI, Porges SW. Psychophysiological Characteristics of the Regulatory Disordered Infant. Infant Behavior and Development. 1991;14:37–50. [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68:173–186. [PubMed] [Google Scholar]
- Eluvanthingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Magbool M, Chugani DC, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Developmental Medicine & Child Neurology. 2003;45:274–281. doi: 10.1017/s0012162203000525. [DOI] [PubMed] [Google Scholar]
- Fracasso MP, Porges SW, Lamb ME, Rosenberg AA. Cardiac Activity in Infancy: Reliability and Stability of Individual Differences. Infant Behavior and Development. 1994;17:277–284. [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Izard CE, Porges SW, Simons FR, Haynes OM, Hyde C, Parisi M, Cohen B. Infant Cardiac Activity: Developmental changes and Relations with Attachment. Developmental Psychology. 1991;27:432–439. [Google Scholar]
- Kintraia PI, Zarnadze MG, Kintraia NP, Kashakashvili IG. Development of daily rhythmicity in heart rate and locomotor activity in the human fetus. Journal of Circadian Rhythms. 2005;3:1–12. doi: 10.1186/1740-3391-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ Vagal Regulation in the Still-Face Paradigm is Related to Dyadic Coordination of Mother-Infant Interaction. Developmental Psychology. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Nara T, Goto N, Hamano S. Development of the human dorsal nucleus of vagus nerve: a morphometric study. Journal of the Autonomic Nervous System. 1991;33:267–276. doi: 10.1016/0165-1838(91)90027-z. [DOI] [PubMed] [Google Scholar]
- Pereyra PM, Zhang W, Schmidt M, Becker LE. Development of myelinated and unmyelinated fibers of human vagus nerve during the first year of life. Journal of Neurological Sciences. 1992;110:107–13. doi: 10.1016/0022-510x(92)90016-e. [DOI] [PubMed] [Google Scholar]
- Porges SW. Vagal Tone: A physiological marker of stress vulnerability. Pediatrics. 1992;90:498–504. [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32:301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: An Emergent Property of the Mammalian Autonomic Nervous System. Psychoneuroendocrinology. 1998;23:8, 837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–46. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Annals of New York Academy of Sciences. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Lipsitt LP. Neonatal Responsivity to gustatory Stimulation: The Gustatory-Vagal Hypothesis. Infant Behavior and Development. 1993;16:487–494. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant Regulation of the Vagal “Brake” Predicts Child Behavior Problems: A Psychobiological Model of Social Behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Portales AL, Porges SW, Doussard-Roosevelt JA, Abedin M, Lopez R, Young MA, Beeram MR, Baker M. Vagal regulation during bottle feeding in low-birthweight neonates: support for the gustatory-vagal hypothesis. Developmental Psychobiology. 1997;30:225–233. [PubMed] [Google Scholar]
- Sachis PN, Armstrong DL, Becker LE, Bryan AC. Myelination of the human vagus nerve from 24 weeks postconceptional age to adolescence. Journal of Neuropathology and Experimental Neurology. 1982;41:466–72. doi: 10.1097/00005072-198207000-00009. [DOI] [PubMed] [Google Scholar]
- Stifter C, Corey J. Vagal regulation and observed social behavior in infancy. Social Development. 2001;10:189–201. [Google Scholar]
- Suess PE, Alpan G, Dulkerian SJ, Doussard-Roosevelt J, Porges SW, Gewolb IH. Respiratory sinus arrhythmia during feeding: a measure of vagal regulation of metabolism, ingestion, and digestion in preterm infants. Developmental Medicine and Child Neurology. 2000;42:353. doi: 10.1017/s001216220000030x. [DOI] [PubMed] [Google Scholar]
- Weinberg MK, Tronick EZ. Infant affective reactions to the resumption of maternal interaction after the still-face. Child Development. 1996;67:905–14. [PubMed] [Google Scholar]
- Woźniak W, O’Rahilly R. Fine structure and myelination of the human vagus nerve. Acta Anatomica. 1981;109:118–130. [PubMed] [Google Scholar]