Abstract
1,4,7-Tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane is widely used as an intermediate in the preparation of medically important DO3A and DOTA metal chelators. Despite its commercial availability and importance, the literature describing the preparation and properties of the free base is limited and sometimes unclear. We present herein an efficient synthesis of the hydrobromide salt of 1,4,7-tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane, characterize this compound spectroscopically and by X-ray crystallographic analysis, describe its simple conversion to the corresponding free base, characterize this compound spectroscopically and by X-ray crystallographic analysis, and make observations on the reactivity of this interesting and useful compound.
Keywords: Synthesis, DOTA, DO3A, Contrast Agent, MRI, X-ray crystallography
Introduction
Many Magnetic Resonance Imaging (MRI) examinations employ gadolinium(III)-based contrast agents (CAs).1–3 Clinically-approved MRI CAs are primarily suited for highlighting anatomical features after intravenous administration and vascular distribution throughout the body. The efficacy of an MRI CA is dependent on its relaxivity. Much work in the last two decades has been devoted to understanding the parameters that influence the relaxation properties of MRI CAs.4–6 Recent years have seen the development of targeted MRI CAs that are sensitive to physiochemical and biochemical changes, such as pH, pO2, metal ion concentration, enzymatic activity, and redox state.7 This work has been largely driven by structural modifications of the chelators 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid (1, DO3A) and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (2, DOTA). Derivatives of 1 and 2 are often synthesized from 1,4,7-tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (3) or the corresponding hydrobromide or hydrochloride salts 4 and 5.8 Despite its commercial availability and importance, the literature describing the preparation and properties of the free base 3 is limited and often lacks clarity. We present herein an efficient, scalable synthesis of the hydrobromide salt 4, characterize this salt spectroscopically and by X-ray crystallographic analysis, describe the conversion of 4 to the corresponding free base 3, characterize the free base spectroscopically and by X-ray crystallographic analysis, and make observations on the reactivity of this interesting and useful compound.

Background
The synthesis of the hydrobromide salt 4 in pure form and in high yield once presented a challenge owing to contamination of the desired product with di- and tetra-alkylated biproducts. A literature search revealed that there are two generally applied methods for the synthesis of 4. The first method involves tris-alkylation of cyclen with tert-butyl bromoacetate in dimethylacetamide in the presence of sodium acetate. The procedure of Himmelsbach, et al.,9 employed a reaction time of nineteen days to produce a 56% yield of 4. The related protocol of Berg, et al.,10 required six days and a chromatographic purification to afford 4 in 60% yield. The procedure of Axelsson, et al.,11 took five days and employed modified work up procedures to produce 4 in 73% yield. Recently, Moore12 prepared 4 in 65–80% yield by a related procedure that required 60 hours.
The second method for the synthesis of 4, originated by Dadabhouy, et al.,13 involves reacting tert-butyl bromoacetate with cyclen in acetonitrile using sodium bicarbonate as the base. Although the procedure is simple, the reported yields range from 42–54% and the method has thus far been limited to relatively small scale syntheses.13–15 Li, et al.,16 reported preparation of the hydrochloride salt 5 in 77% yield by tris-alkylation of cyclen with tert-butyl bromoacetate in chloroform using triethylamine as the base, followed by chromatographic purification.
Kohl, et al.,17 published a synthesis and characterization of 1,4,7-tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclo-dodecane in the free base form (3) by a multistep procedure unrelated to the syntheses described above. Moore,18 Barge, et al.,8 and Liu, et al.,19 describe methods for obtaining 3 from 4, but in each case the identity and purity of 3 were not established. Other articles claim syntheses of 3, but due to incomplete characterization it is unclear if the compounds isolated were the free base or hydrohalide salts.20–24
What is clear is that most of the existing synthetic routes to the free base 3 are overly long, time consuming, involve complex work-up procedures and/or a chromatographic purification step, and are thus unattractive for use in large scale syntheses. Most fail to establish the identity and purity of the product. Our interest in the development of redox sensitive MRI CAs25 motivated the development of a simple, concise, scalable synthesis of 3.
Synthesis of 1,4,7-Tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane Hydrobromide (4) and Generation of the Corresponding Free Base (3)
Our synthesis of the trialkylated hydrobromide salt 4 is an adaptation of the first method described above. This method offers the advantage of selectivity in that the trialkylated salt precipitates from the reaction mixture, thus making it attractive for large-scale preparations. We reasoned that if the addition of tert-butyl bromoacetate to cyclen were done at a lower temperature, tetraalkylation would be further prevented, thereby increasing the yield of 4.
In a typical procedure, 3.3 equivalents of tert-butyl bromoacetate in dimethylacetamide were added to a stirred suspension of 1 equivalent of cyclen and 3.3 equivalents of sodium acetate in dimethylacetamide at −20 °C. After stirring for 24 h at room temperature, the reaction mixture was poured into water to give a clear solution. Solid potassium bicarbonate was then added until 4 precipitated as a white solid. The precipitate was collected by filtration and dissolved in chloroform. The solution was washed with water, dried (MgSO4), filtered and concentrated. Upon the addition of ether, 4 crystallized as a white fluffy solid in ~80% yield. Repetition of the procedure on scales from 1–5 g of cyclen gave similar results.
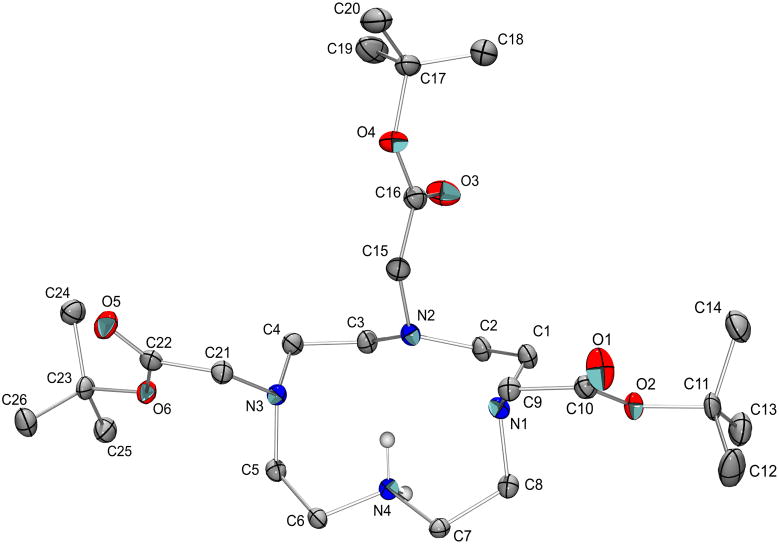
Recrystallization of the hydrobromide salt 4 was attempted from ethyl acetate and toluene without much success. Both solvents failed to yield a crystalline material, and instead shiny amorphous solids were obtained. However, crystallization from water produced crystals that were suitable for X-ray crystallographic analysis. The hydrobromide salt 4 crystallized in space group P2/c as a hemihydrate (Figure 1) with half of a molecule of water per asymmetric unit.26 In the crystal, compound 4 forms an intramolecular N—H···N hydrogen bond between N4 and N2 and exhibits an intermolecular N—H···Br interaction. Two O—H···Br interactions connect adjacent formula units.
Figure 1.
The structure of the cation in 4. Displacement ellipsoids are at the 50% probability level and C-bound H atoms are omitted.
The hydrobromide salt 4 was dissolved in water at 70 °C. The solution was allowed to cool to 40 °C, at which point 2 equivalents of 10% aqueous potassium hydroxide were added. After stirring for 15 min, 3 had separated as viscous oil. This oil was extracted into hexanes, the organic layer was dried (MgSO4), filtered, and concentrated to give 2 as a colorless oil in 95% yield. When stored at −20 °C, the oil solidified to give a white solid, mp 44–47 °C, lit17 mp 47–50 °C, that was characterized by 1H NMR, 13C NMR, HRMS, and elemental analysis. The free base 3 (1 g) was partitioned between water and ether (1:1, 100 mL). Slow evaporation of the ether produced crystals of 3 suitable for X-ray analysis. The free base 3 crystallized in space group C2/c as a hydrate (Figure 2) with 1.3 disordered water molecules per asymmetric unit.27 The structure exhibited substantial positional disorder of the part of the ring which contains the secondary amine. This was modeled using a two-part disorder model with a refined major:minor occupancy ratio of 56:44%.
Figure 2.
The structure of the free base 3. Displacement ellipsoids are at the 30% probability level. The minor disorder component, and C-bound H atoms, and water molecules are omitted.
When dichloromethane was used instead of hexanes for extraction in the above procedure, a crystalline, high melting product (mp 204–205 °C) was isolated. Upon X-ray crystallographic analysis, this product was discovered to be the known hydrochloride salt 5 which crystallized as the hemihydrate16 and was isomorphous with the hydrobromide salt 4 (Figure 3).28,29 Secondary amines are known to react rapidly with dichloromethane to give mixtures of amine hydrochlorides and aminals.30 In the case of 3, the secondary amine appears to have decomposed dichloromethane to generate the hydrochloride salt, which was obtained in quantitative yield. This result is consistent with the observation of Li, et al., who obtained 5 from the reaction of cyclen with with tert-butyl bromoacetate in chloroform.16
Figure 3.
An overlay of the cationic parts of 4 (solid lines) with 5 (dashed lines) formed by a least squares fit of all non-H atoms of the tetraazacyclododecane rings (rms deviation = 0.016 Å).
In summary, a simple and concise synthesis of the free base 3 was demonstrated via the hydrobromide salt 4. The procedure was scalable to 5 grams of cyclen and seems amenable to further scale-up. Water, a green solvent, was used for crystallization of 4. The first X-ray crystal structures of the hydrobromide salt 4 and the free base 3 were obtained, along with an X-ray crystal structure of the hydrochloride salt 5.
Experimental Section
1,4,7-Tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane Hydrobromide (4)
To a suspension of cyclen (5.00 g, 29 mmol) and sodium acetate (7.86 g, 96 mmol) in N,N-dimethylacetamide (DMA, 60 mL) at −20 °C was added a solution of t-butyl bromoacetate (18.7 g, 14.1 mL, 96 mmol) in DMA (20 mL) dropwise over a period of 0.5 h. The temperature was maintained at −20 °C during the addition, after which the reaction mixture was allowed to come to room temperature. After 24 h of vigorous stirring, the reaction mixture was poured into water (300 mL) to give a clear solution. Solid KHCO3 (15 g, 150 mmol) was added portionwise, and 4 precipitated as a white solid. The precipitate was collected by filtration and dissolved in CHCl3 (250 mL). The solution was washed with water (100 mL), dried (MgSO4), filtered, and concentrated to about 20–30 mL. Ether (250 mL) was added, after which 4 crystallized as a white fluffy solid, mp 190–191 °C (lit12 mp 179–181 °C, lit13 mp 178–180 °C), Rf 0.28 (10:1 DCM/MeOH on silica gel 60 F254). Yield: 13.6 g (23 mmol, 79%). 1H NMR (500 MHz, CDCl3) δ 1.45 (s, 9H), 1.46 (s, 18H), 2.87 (m, 4H), 2.93 (m, 8H), 3.10 (m, 4H), 3.29 (s, 2H), 3.38 (s, 4H), 10.03 (br s, 1H); 13C NMR (125 MHz, CDCl3) δ 28.1, 28.2, 47.4, 48.7, 49.1, 51.2, 51.3, 58.1, 81.6, 81.8, 169.5, 170.4.
Anal. Calcd for C26H51BrN4O6: C 52.43, H 8.63, N 9.41. Found: C 52.36, H 8.69, N 9.45.
1,4,7-Tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (3)
Hydrobromide 4 (5.00 g, 8.40 mmol) was dissolved in water (250 mL) at 70 °C. The solution was allowed to cool to 40 °C, after which 10% aqueous KOH solution (9.4 mL, 16.8 mmol) was added. The reaction mixture was stirred for 15 min, then extracted with hexanes (3 × 100 mL). The combined organic layers were washed with water (3 × 100 mL), dried (MgSO4), filtered, and concentrated under reduced pressure to give 3 as a colorless, viscous oil which solidified upon storage at −20 °C; mp 44–47 °C (lit17 mp 47–50 °C). Yield: 4.10 g (8.00 mmol, 95%). 1H NMR (500 MHz, CDCl3) δ 1.45 (s, 9H), 1.46 (s, 18H), 2.56 (m, 4H), 2.72–2.78 (m, 8H), 2.81 (m, 4H), 3.30 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 28.2 (2), 47.7, 50.7, 52.3, 52.4, 53.2, 57.1, 80.7 (2), 171.2, 171.4; HRMS-ESI Calcd for C26H51N4O6 (M + H)+ 515.3803, Obsd 515.3806.
Anal. Calcd for C26H50N4O6: C 60.67, H 9.79, N 10.89. Found: C 60.26, H 9.28, N 10.80.
Acknowledgments
This work was supported by grants R01-CA118359 and P30-CA023074 from the National Institutes of Health. GLB-A thanks Research Corporation for an Arizona Partners in Science Fellowship. The single crystal X-ray diffractometer was purchased with funds from grant CHE-0741837 from the National Science Foundation.
Footnotes
Dedicated to Professor Harry Wasserman on the occasion of his 90th birthday.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Lauffer RB. Chem Rev. 1987;87:901–927. [Google Scholar]
- 2.Tóth É, Helm L, Merbach AE. Top Curr Chem. 2002;221:61–101. [Google Scholar]
- 3.Westbrook C, Roth CK, Talbot J. MRI in Practice. 3. Blackwell; Malden, MA: 2005. [Google Scholar]
- 4.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 5.Caravan P. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 6.Hermann P, Kotek J, Kubìcek V, Lukes I. Dalton Trans. 2008:3027–3047. doi: 10.1039/b719704g. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet CS, Tóth É. Am J Neuroradiol. 2010;31:401–409. doi: 10.3174/ajnr.A1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barge A, Tei L, Upadhyaya D, Fedeli F, Beltrami L, Stefanìa R, Aime S, Cravotto G. Org Biomol Chem. 2008;6:1176–1184. doi: 10.1039/b715844k. [DOI] [PubMed] [Google Scholar]
- 9.Himmelsbach RJ, Rongved P, Klaveness J, Strande P, Dugstad H. 93/02045 WO.
- 10.Berg A, Almen T, Klaveness J, Rongved P, Thomassen T. 5,198,208 US.
- 11.Axelsson O, Olsson A. 2006/112723 A1 WO.
- 12.Moore DA. Org Synth. 2008;85:10–14. [Google Scholar]
- 13.Dadabhoy A, Faulkner S, Sammes PG. J Chem Soc, Perkin Trans 2. 2002:348–357. [Google Scholar]
- 14.Prasuhn DE, Yeh RM, Obenaus A, Manchester M, Finn MG. Chem Commun. 2007:1269–1271. doi: 10.1039/b615084e. [DOI] [PubMed] [Google Scholar]
- 15.Mizukami S, Tonai K, Kaneko M, Kikuchi K. J Am Chem Soc. 2008;130:14376–14377. doi: 10.1021/ja800322b. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Wong W-T. Tetrahedron. 2004;60:5595–5601. [Google Scholar]
- 17.Kohl SW, Kuse K, Hummert M, Schumann H, Mügge C, Janek K, Weißhoff H. Z Naturforsch. 2007;62b:397–406. [Google Scholar]
- 18.Moore DA. 2007/106546 A2 WO.
- 19.Liu W, Hajibeigi A, Lin M, Rostollan CL, Kovacs Z, Öz OK, Sun X. Bioorg Med Chem Lett. 2008;18:4789–4793. doi: 10.1016/j.bmcl.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke T, Feng Y, Guo J, Parker DL, Lu ZR. Magn Reson Imaging. 2006;24:931–940. doi: 10.1016/j.mri.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Rami M, Cecchi A, Montero JL, Innocenti A, Vullo D, Scozzafava A, Winum JY, Supuran CT. ChemMedChem. 2008;3:1780–1788. doi: 10.1002/cmdc.200800267. [DOI] [PubMed] [Google Scholar]
- 22.Machitani K, Sakamoto H, Nakahara Y, Kimura K. Anal Sci. 2008;24:463–469. doi: 10.2116/analsci.24.463. [DOI] [PubMed] [Google Scholar]
- 23.Chong H-S, Lim S, Baidoo KE, Milenic DE, Ma X, Jia F, Song HA, Brechbiel MW, Lewis MR. Bioorg Med Chem Lett. 2008;18:5792–5795. doi: 10.1016/j.bmcl.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo B, Sheth VR, Pagel MD. Tetrahedron Lett. 2009;50:4459–4462. doi: 10.1016/j.tetlet.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghunand N, Guntle GP, Gokhale V, Nichol GS, Mash EA, Jagadish B. J Med Chem. 2010;53:6747–6757. doi: 10.1021/jm100592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crystal data for 4: C52H104Br2N8O13, Mr = 1209.25 g.mol−1; colorless rod 0.35 × 0.08 × 0.07 mm3; T = 100K, MoKα radiation (λ = 0.71073 Å); space group P2/c, unit cell parameters a = 21.631(2) Å, b = 6.8054(7) Å, c = 23.564(3) Å, V = 3213.0(6) Å3; 22521 measured reflections, 6804 unique reflections, 5002 reflections with F2>2σ; final R1 = 0.0344 (F2>2σ), wR2 = 0.0768 (all data).
- 27.Crystal data for 3: C26H52.60N4O7.30, Mr = 538.12 g.mol−1; colorless rod 0.37 × 0.07 × 0.06 mm3; T = 100K, MoKα radiation (λ = 0.71073 Å); space group C2/c, unit cell parameters a = 25.515(5) Å, b = 5.9055(10) Å, c = 41.474(8) Å, V = 6196.7(19) Å3; 25268 measured reflections, 3222 unique reflections, 2546 reflections with F2>2σ; final R1 = 0.0531 (F2>2σ), wR2 = 0.1559 (all data).
- 28.Crystal data for 5: C52H104Cl2N8O13, Mr = 1120.33 g.mol−1; colorless rod 0.55 × 0.08 × 0.07 mm3; T = 100K, MoKα radiation (λ = 0.71073 Å); space group P2/c, unit cell parameters a = 21.8420(12) Å, b = 6.6031(3) Å, c = 23.3715(13) Å, V = 3126.9(3) Å3; 42356 measured reflections, 8394 unique reflections, 6035 reflections with F2>2σ; final R1 = 0.0383 (F2>2σ), wR2 = 0.0971 (all data).
- 29.Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC-795054, CCDC-793547, and CCDC-793548 for compounds 3, 4, and 5, respectively. Copies of the data can be obtained, free of charge, on application to CCDC by e-mail: deposit@ccdc.cam.ac.uk.
- 30.Mills JE, Maryanoff CA, McComsey DF, Stanzione RC, Scott L. J Org Chem. 1987;52:1857–1859. [Google Scholar]