Abstract
Treatment of substituted 4-methoxyanilines with ceric ammonium nitrate in a 1:1 mixture of water and acetonitrile resulted in formation of 1,4-benzoquinones in acceptable yields.
Keywords: oxidation, ceric ammonium nitrate, aniline, benzoquinone, quinone
1. Introduction
In the course of our work on the synthesis of various antitubercular terpenes1 and related compounds,2 we were challenged by the need to oxidize a relatively complex aniline to the corresponding benzoquinone.3 This letter summarizes our successful development of ceric ammonium nitriate for that purpose.
The oxidation of aromatic amines to quinones is a potentially convenient way to generate the latter, since anilines are readily available via a number of processes, including the reduction of nitroarenes. While a number of methods have been reported for this oxidation,4–12 they can be tedious due to long reaction times, complicated work-up procedures or low yields and often work best when using anilines soluble in water.
Ceric ammonium nitrate (CAN) is a powerful oxidant that has many uses in organic synthesis.13 This reagent is well known to oxidize electron rich aromatic groups. The removal p-methoxyphenyl and related electron rich aromatic groups from amines has been established.14 For example, electron rich aromatics can be removed from secondary amines and secondary amides by oxidation with CAN.15 It is likely that such deprotections proceed via the formation of a quinone, but generally in these processes, the quinone is not the desired organic material. Other electron rich systems are readily oxidized by CAN to quinones,16 but it appears that the direct oxidation of substituted, unprotected anilines to quinones has not previously been reported.
2. Results and Discussion
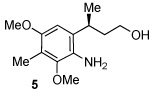
We specifically wanted to accomplish the conversion of 1 to 2 (eq. 1). To this end, 1 was treated with a variety of oxidants. After several attempts with known procedures17 that proved ineffective, we turned to ceric ammonium nitrate, and found that a good yield of the quinone 2 could be obtained simply by stirring
 |
(1) |
the aniline 1 in the presence of the reagent in a 1:1 mixture of water and acetonitrile. Thus, an acetonitrile solution (4 mL) of 1 (0.6 mmol) was added dropwise into a 16 mL solution (acetonitrile : water = 1:1) of ceric ammonium nitrate (4.0 equiv) at room temperature with vigorous stirring. After 20 min, the reaction was quenched. Chromatographic work-up afforded a 73% yield of 2. Compound 2 was obtained without any epimerization at the stereocenter adjacent to the quinone ring according to 1H NMR analysis.
This procedure turned out to be general and was applied to a small selection of other anilines that were available in our laboratories. The results are presented in Table 1. The results suggest that highly substituted anilines are best for this process, presumably as that minimizes side reactions that might arise as a result of reactions between the starting materials and the products. Indeed, it should be noted that in general this reaction not only gave quinone, but also resulted in the formation of a number of colored by products, none of which have been isolated and characterized.
Table 1.
Conversion of anilines to quinones with CAN
3. Conclusion
We found that treatment of p-methoxyanilines with ceric ammonium nitrate afforded the corresponinding 1,4-quinones in good yield. The efficacy of CAN as an oxidant suggests its potential applicability to many oxidation problems, some of which we plan to test. Results will be reported in due course.
Supplementary Material
Acknowledgments
This work was supported by the NIH (1R01-A159000-01A1) to whom we are very grateful.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data (experimental procedures, charaterization data; 1H and 13C spectra for new compounds) associated with this letter can be found in the online versions at doi:XXXXXXXX
References and notes
- 1.(a) Harmata M, Zheng P. Heterocycles. 2009;77:279. [Google Scholar]; (b) Harmata M, Cai Z, Chen Y. J. Org. Chem. 2009;74:5559. doi: 10.1021/jo9009112. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Harmata M, Hong X, Schreiner PR. J. Org. Chem. 2008;73:1290. doi: 10.1021/jo701935s. [DOI] [PubMed] [Google Scholar]; (d) Harmata M, Hong X. Tetrahedron Lett. 2005;46:3847. [Google Scholar]; Harmata M, Hong X. Org. Lett. 2005;7:3581–3583. doi: 10.1021/ol0515412. [DOI] [PubMed] [Google Scholar]
- 2.Harmata M, Hong X, Barnes CL. Tetrahedron Lett. 2003;44:7261–7264. [Google Scholar]
- 3.The work described herein was initiated during a formal total synthesis of floresolide B, the details of which shall be communicated shortly.
- 4.Rao AV, Chavan SP, Sivadasan L. Tetrahedron. 1986;42:5065. [Google Scholar]
- 5.Teuber HJ, Hasselbach M. Chem. Ber. 1959;92:674. [Google Scholar]
- 6.Haslam E. Shikimic Acid Metabolism and Metabolites. New York: John Wiley & Sons; 1993. [Google Scholar]
- 7.Martin EI, Fieser LF. Org. Synth. 1941;21:91. [Google Scholar]
- 8.Fieser LF. Org. Synth. 1937;17:68. [Google Scholar]
- 9.Sakave S, Tsubakino T, Nishiyama Y, Ishi Y. J. Org. Chem. 1993;58:3633. [Google Scholar]
- 10.Adams R, Nagarkatti AS. J. Am. Chem. Soc. 1950;72:4601. [Google Scholar]
- 11.Willstätter R, Benz M. Chem. Ber. 1906;39:3482. [Google Scholar]
- 12.Hashemi MM, Beni YA. J. Chem. Research. 1999:672. [Google Scholar]
- 13.Nair V, Deepthi A. Chem. Rev. 2007;107:1862. doi: 10.1021/cr068408n. [DOI] [PubMed] [Google Scholar]
- 14.For examples see: Wuts PGM, Greene TW. Greene’s Protective Groups in Organic Synthesis. 4th ed. New York: Wiley; 2007. pp. 813–814. Uraguchi D, Nakashima D, Ooi T. J. Am. Chem. Soc. 2009;131:7242. doi: 10.1021/ja903271t. Cheemala MN, Knochel P. Org. Lett. 2007;9:3089. doi: 10.1021/ol071168t.
- 15.(a) Verniest G, Wang X, Kimpe ND, Padwa A. J. Org. Chem. 2009;75:424. doi: 10.1021/jo902287t. [DOI] [PubMed] [Google Scholar]; (b) McErlean CSP, Sperry J, Blake AJ, Moody CJ. Tetrahedron. 2007;63:10963. [Google Scholar]
- 16. For example, see: Radeke HS, Digits CA, Bruner SD, Snapper ML. J. Org. Chem. 1997;62:2823. doi: 10.1021/jo962292l.
- 17.We focused on using FeCl3 under a variety of conditions giving results ranging from no reaction to complicated mixtures. Attempts to generated diazonium ions from nitrite and acid or isoamyl nitrite were also not successsful. The use of sodium hypochlorite did not afford the desired quinone and it was after these initial attempts that we pursued CAN oxidation.
- 18.Higuchi T, Satake C, Hirobe M. J. Am Chem. Soc. 1995;117:8879. [Google Scholar]
- 19.Ying W, Barnes CL, Harmata M. Tetrahedron Lett. doi: 10.1016/j.tetlet.2010.10.109. 20xx, XX, 0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.