Abstract
Context: The level and duration of exposure to circulating low-density lipoprotein-cholesterol (LDL-C) are major contributors to coronary atherosclerosis. Therefore, optimal prevention will require long-term LDL-C reduction, making it important to select the most effective agent for each individual.
Objective: We tested the hypothesis that individuals with high fractional absorption of cholesterol respond better to the cholesterol absorption inhibitor ezetimibe than to simvastatin, whereas low absorbers, who have elevated rates of cholesterol synthesis, respond better to simvastatin.
Design, Setting, and Participants: A randomized, double-blind, placebo-controlled, crossover trial was performed in 215 African- and European-American men.
Intervention: Participants were randomized to placebo, ezetimibe (10 mg/d), simvastatin (10 mg/d), and both drugs for 6 wk each.
Main Outcome: Plasma levels of LDL-C, surrogate markers for cholesterol absorption (campesterol) and synthesis (lathosterol), and proprotein convertase subtilisin-like kexin type 9 were measured at baseline and after treatment.
Results: LDL-C levels were reduced by 19% (ezetimibe), 25% (simvastatin), and 41% (ezetimibe+simvastatin) from a baseline of 146 ± 20 mg/dl; results were similar between ethnic groups. Reduction in LDL-C correlated poorly with baseline levels of noncholesterol sterols and proprotein convertase subtilisin-like kexin type 9. Although individual responses varied widely, change in LDL-C on ezetimibe correlated with response to simvastatin (r = 0.46, P < 0.001). Combination therapy lowered LDL-C by 15% or greater in more than 95% of participants.
Conclusions: Baseline cholesterol absorption and synthesis did not predict responsiveness to LDL-lowering drugs. Responsiveness to simvastatin and ezetimibe were highly correlated, suggesting that factors downstream of the primary sites of action of these drugs are a major determinant of response.
Responsiveness to simvastatin and ezetimibe are correlated, suggesting that factors downstream of the primary sites of action of these drugs are major determinants of response.
Sustained exposure of coronary arteries to elevated levels of circulating low-density lipoprotein cholesterol (LDL-C) is both necessary and sufficient for the development of coronary heart disease (CHD). Genetic forms of primary hypercholesterolemia, irrespective of the underlying mechanism, all result in severe, premature CHD (1). In contrast, individuals who maintain low-plasma LDL-C levels over a lifetime, whether due to prudent diets or genetic differences, are remarkably protected from CHD (2,3). The therapeutic utility of lowering LDL-C has been demonstrated by clinical trials with statin drugs, which have consistently produced significant reductions in CHD (4). Despite the proven efficacy of statins, the reduction in CHD achieved in clinical trials is invariably smaller than reductions associated with LDL-lowering mutations (3,5). This finding indicates that duration of LDL-C exposure, not just level of LDL-C, is an important determinant of whether an individual develops coronary atherosclerosis. Accordingly, we and others have proposed that the most effective strategy to reduce CHD is to initiate LDL-C-lowering therapy earlier in life (3,5,6).
If cholesterol-lowering agents are to be started at earlier ages and continued for decades, it is important that medication selected for each individual be effective and well tolerated. Responses to cholesterol-lowering drugs vary over a wide range, yet the reasons underlying the variability in response are largely unknown. Here we tested the hypothesis that optimal cholesterol-lowering regimens can be personalized for each individual based on indicators of cholesterol metabolism.
Two of the most widely used cholesterol-lowering agents are statins and ezetimibe. Ezetimibe blocks absorption of dietary and biliary cholesterol by inhibiting the sterol transporter Niemann-Pick C1-like1 (NPC1L1) (7). Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, an enzyme required for cholesterol synthesis (8). Decreased delivery of sterols from the gut or reduced endogenous synthesis of cholesterol triggers the cholesterol homeostatic machinery to up-regulate hepatic LDL receptors (LDLRs) (9). The increase in LDLR activity accelerates LDL clearance and reduces blood levels of LDL. Thus, although working by different mechanisms, the cholesterol-lowering effect of both ezetimibe and statins is largely a result of increased expression of hepatic LDLRs.
Relative rates of cholesterol absorption and synthesis can be inferred from circulating levels of noncholesterol sterols (10). Plasma levels of plant sterols, such as sitosterol and campesterol, are proportional to the fractional absorption of cholesterol (10,11), which varies from 20 to 80% among individuals (12). Plasma levels of cholesterol precursor sterols, such as desmosterol or lathosterol, provide an index of cholesterol synthesis (13). Because noncholesterol sterols are transported in lipoproteins, levels are influenced by lipoprotein turnover rates. Accordingly, plant sterols and precursor sterols are indexed to cholesterol or expressed as a ratio (campesterol to lathosterol) to provide indices of sterol absorption and synthesis (14).
In a post hoc analysis of the Scandinavian Simvastatin Survival Study, individuals with high plasma levels of plant sterols at baseline were more likely to require higher doses of statins to achieve adequate cholesterol lowering (15). This finding was interpreted as indicating that individuals who are high cholesterol absorbers are less responsive to statins than are low absorbers because the dietary sterols suppress endogenous cholesterol synthesis. Conversely low absorbers should have elevated HMG-CoA reductase activity and thus be more sensitive to statins.
If indices of intestinal and hepatic cholesterol metabolism predict responsiveness to cholesterol-lowering drugs, then circulating levels of noncholesterol sterols could potentially be used as biomarkers to individualize cholesterol-lowering therapy and allow the most effective therapeutic regimen to be selected for each person. To test this hypothesis, we compared the LDL-C lowering effects of a low dose of a statin (simvastatin, 10 mg/d), a cholesterol absorption inhibitor (ezetimibe, 10 mg/d), and a combination of the two drugs in the same individuals. We also tested whether relative responsiveness to either hypocholesterolemic agent is related to circulating plasma levels of proprotein convertase subtilisin-like kexin type 9 (PCSK9), a sterol-regulated protein that degrades the LDLR.
Subjects and Methods
Study design
This study was a randomized, double-blind, placebo-controlled, crossover trial comprising a 3-d screening period followed by a 2-wk, placebo run-in period and then four 6-wk treatment periods (Fig. 1). The institutional review board reviewed and approved the study protocol and all participants provided written informed consent. This study was initiated by the investigators and funded by the Donald W. Reynolds Foundation and Merck/Schering-Plough. Subjects were recruited between October 2005 and October 2006 through workplace screenings in the Dallas-Fort Worth area.
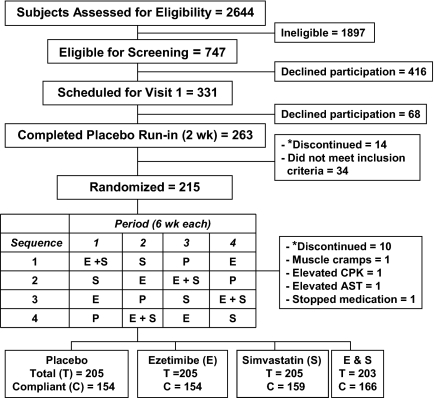
Figure 1.
Eligibility, enrollment, and compliance of randomized, placebo-controlled, crossover study. *, Refused continued participation or moved out of state. CPK, Creatine phosphokinase.
African-American and European-American men, aged 20 to 70 yr, were eligible for the study. Inclusion criteria were body mass index between 20 and 35 kg/m2, fasting plasma LDL-C levels between 130 and 175 mg/dl and triglyceride <250 mg/dl. Exclusion criteria included a major illness, hospitalization within 6 months, adverse reaction to HMG-CoA reductase inhibitors, substance abuse (including alcohol), or treatment with cholesterol-lowering medications or investigational drugs within 30 d. Individuals with a history of CHD, peripheral vascular disease, cerebrovascular disease, congestive heart failure, clinically significant arrhythmia, diabetes, a serum creatinine level >1.5 mg/dl, an abnormal thyroid-stimulating hormone level, uncontrolled hypertension [systolic blood pressure (BP) >160 mm Hg or diastolic BP >100 mm Hg], or elevated serum transaminases [alanine aminotransferase (ALT) and aspartate aminotransferase >1.5 times the upper limit of normal] were excluded.
European- and African-Americans were randomized separately. Participants were stratified by race before randomization and allocated in equal numbers to four treatment sequences by the sponsor. Investigation site, sponsor personnel, and patients were blinded to treatment assignments. Eligible subjects were scheduled for a second visit to begin a 2-wk, single-blind, placebo run-in phase before randomization. A total of 34 individuals were excluded after the placebo run-in: four had a chronic disease,15 had plasma LDL-C outside the required range, seven had side effects with cholesterol-lowering agents, and eight had abnormal levels of glucose, TSH, creatinine, AST, or creatine phosphokinase.
The subjects were called in the middle of each treatment period to review dietary and medication compliance. Fasting blood samples were obtained on 2 d during the last week of each treatment period. Pills were counted at the end of each treatment period to determine the percent of pills that were taken. Noncompliance was defined for each treatment period as taking 80% or less of the study medication.
Blood sampling and measurements
Venous blood was collected after an overnight fast and plasma was isolated within 2 h. Serum chemistries and plasma lipid levels were measured using enzymatic assays. Plasma lipoprotein-cholesterol levels were measured by β-quantification and noncholesterol sterols (lathosterol, desmosterol, sitosterol, campesterol) by gas chromatography (16). Plasma levels of PCSK9 were measured using a sandwich ELISA (17). Investigators were blinded to lipid/lipoprotein values after visit 1.
Statistical methods
Statistical analyses were performed using SAS software 9.1 (SAS Institute, Cary, NC). The primary analysis was performed on an intention-to-treat basis. The primary end point of the study was percent change in plasma LDL-C concentrations in response to each agent in African-Americans and European-Americans. The study was designed to provide 80% power to detect a difference in treatment effect of greater than 6% between the two ethnic groups. For the primary analysis, two-sided t tests were used to assess differences in plasma LDL-C levels by treatment arm. Pearson correlation coefficients were used to assess correlations between responses to each treatment. Additional analyses were performed after stratification by race. A Bonferroni-corrected significance threshold of 0.01 was used to account for multiple testing. A secondary analysis considered only individuals who consumed 80% of the study drug (compliant group). In a post hoc analysis, we assessed differences in LDL-C response and plasma levels of noncholesterol sterols and PCSK9 for both African- and European-Americans. Generalized linear modeling was used to examine the relationship between race and LDL response for each drug regimen and included body mass index (BMI) and medication compliance in the adjusted model; these two covariates were included to account for ethnic differences in compliance and baseline BMI (P < 0.001 for both). Spearman correlations were calculated to determine the relationship between LDL-C response and baseline noncholesterol sterols and PCSK9 levels. Kruskal-Wallis tests were used to assess differences in noncholesterol sterol levels by treatment arm between African- and European-American subjects, and compare plasma campesterol to lathosterol ratios in individuals who showed the best (>90th percentile) and poorest (<10th percentile) LDL-C lowering with each regimen.
Results
A total of 215 men were randomized into one of four treatment sequences consisting of 6 wk each of treatment with 10 mg each of ezetimibe, simvastatin, ezetimibe plus simvastatin, and placebo. After randomization, 14 men dropped out of the study (10 elected not to continue or moved to another location, one had muscle cramps, one each had elevated serum levels of creatine phosphokinase and AST, and one stopped taking the medications) (Fig. 1). No serious adverse events were reported and the enzyme elevations resolved with discontinuation of study drug.
The trial was based on intention-to-treat analysis. Of the 215 subjects who were randomized, 43% were African-Americans and the others were European-Americans. On average the participants were middle aged (44 yr), overweight (BMI 29 ± 4 kg/m2) and moderately hypercholesterolemic (mean LDL-C 146 ± 20 mg/dl) with normal levels of HDL-C and triglycerides (Table 1). The baseline characteristics were similar in the compliant and noncompliant (defined as taking <80% of pills during the treatment period) groups, except that a greater proportion of noncompliant subjects were African-American than European-Americans.
Table 1.
Baseline characteristics of all subjects, compliant subjects, and noncompliant subjects by study medication
| Total (n = 215) | Placebo
|
Ezetimibe
|
Simvastatin
|
Ezetimibe+simvastatin
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| C (n = 154) | NC (n = 51) | C (n = 154) | NC (n = 51) | C (n = 159) | NC (n = 46) | C (n = 166) | NC (n = 37) | ||
| Age (yr) | 44 (9) | 45 (9) | 43.6 (8.4) | 45 (8) | 43 (9) | 45 (8) | 43 (9) | 44 (8) | 44 (10) |
| African-American (n) | 93 | 54 | 32b | 50 | 34b | 52 | 32b | 64 | 20 |
| European-American (n) | 122 | 100 | 19 | 104 | 17 | 107 | 14 | 102 | 17 |
| BMI (kg/m2) | 29 (4) | 29 (4) | 30 (4) | 29 (3) | 30 (4) | 29 (4) | 30 (4) | 29 (4) | 29 (4) |
| Systolic BP (mm Hg) | 128 (11) | 128 (11) | 130 (10.0) | 128 (11) | 130 (10) | 128 (11) | 129 (11) | 128 (11) | 129 (11) |
| Fasting glucose (mg/dl) | 90 (8) | 89 (8) | 91 (7) | 90 (8) | 90 (7) | 90 (8) | 90 (7) | 90 (7) | 90 (9) |
| LDL-C (mg/dl) | 146 (20) | 146 (20) | 146 (20) | 146 (21) | 145 (18) | 146 (20) | 146 (21) | 146 (20) | 144 (20) |
| HDL-C (mg/dl) | 46 (9) | 46 (9) | 46 (10) | 46 (9) | 46 (9) | 46 (9) | 47 (8) | 46 (9) | 46 (8) |
| Triglycerides (mg/dl)a | 106 (52) | 106 (58) | 108 (508) | 106 (59) | 106 (49) | 106 (62) | 103 (51) | 106 (57) | 125 (72) |
Mean (standard deviation) unless otherwise indicated.
Median (interquartile range). C, Compliant (took >80% of the medications during the treatment period, as determined by pill count); NC, noncompliant; HDL, high-density lipoprotein.
Proportion of noncompliant subjects among African-Americans was significantly greater than European-Americans (P < 0.001).
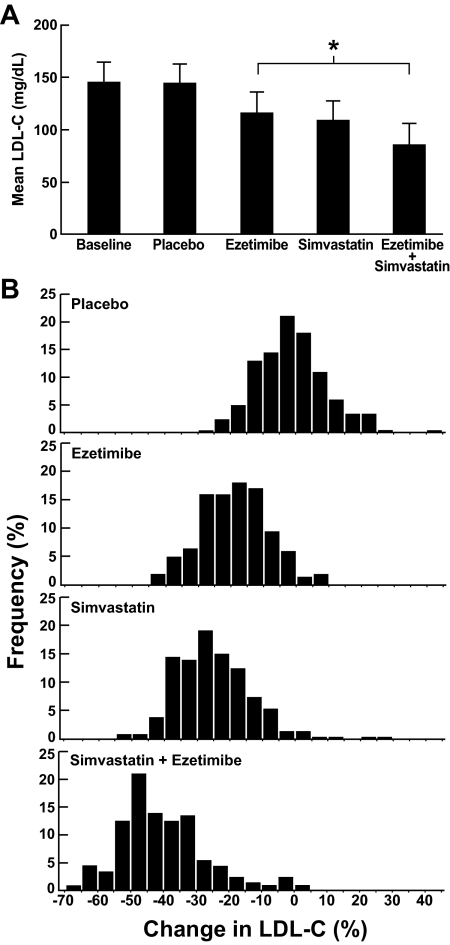
All three drug regimens had a significant impact on plasma LDL-C levels with mean reductions in LDL-C of 19, 25, and 41% on ezetimibe, simvastatin, and both drugs, respectively (Fig. 2A). The response to each drug was normally distributed and showed substantial individual variation (Fig. 2B). No significant differences in percent change in LDL-C levels were seen between European- and African-Americans with ezetimibe therapy, irrespective of compliance (Table 2). As reported previously (18,19,20), the reduction in LDL-C levels in response to simvastatin was lower in African- than European-Americans (−21.8 vs. −26.8%, P = 0.006), although no differences were apparent in the subset of compliant subjects. Similarly, the percent change in plasma LDL-C levels on combination therapy (ezetimibe+simvastatin) was lower in African- than European-Americans (−36.5 vs. −42.6%, P = 0.002), but this difference was abolished after adjustment for BMI and compliance (Table 2). Lastly, exclusion of noncompliant subjects did not significantly affect the mean LDL-lowering responses of the entire group to the three treatments (data not shown); therefore all participants were included in the subsequent analyses.
Figure 2.
A, Mean plasma levels of LDL-C at baseline and after 6 wk of placebo, ezetimibe (10 mg/d), simvastatin (10 mg/d), and ezetimibe (10 mg/d) plus simvastatin (10 mg/d). B, The frequency distribution of percent change in LDL-C level for each treatment arm. *, P < 0.001.
Table 2.
Percent change in plasma levels of LDL-C by treatment arm in African- and European-Americans
| Total
|
P value | Complianta
|
P value | P valueb | |||
|---|---|---|---|---|---|---|---|
| African-Americans | European-Americans | African-Americans | European-Americans | ||||
| Placebo | −0.4 (10.7) | −2.2 (10.8) | 0.24 | 1.2 (10.7) | −2.7 (9.5) | 0.02 | 0.16 |
| Ezetimibe | −18.3 (9.8) | −19.8 (10.7) | 0.31 | −19.1 (9.7) | −19.8 (10.9) | 0.63 | 0.36 |
| Simvastatin | −21.8 (13.7) | −26.8 (10.9) | 0.006 | −24.4 (12.5) | −26.9 (10.7) | 0.2 | 0.46 |
| Ezetimibe+simvastatin | −36.5 (14.6) | −42.6 (11.3) | 0.002 | −37.5 (14.5) | −43.8 (10.9) | 0.002 | 0.10 |
Values in the table are mean percent change compared with baseline (sd).
Participants who took 80% of the medication based on pill counts performed at the end of each treatment regimen.
Adjusted for medication compliance and BMI.
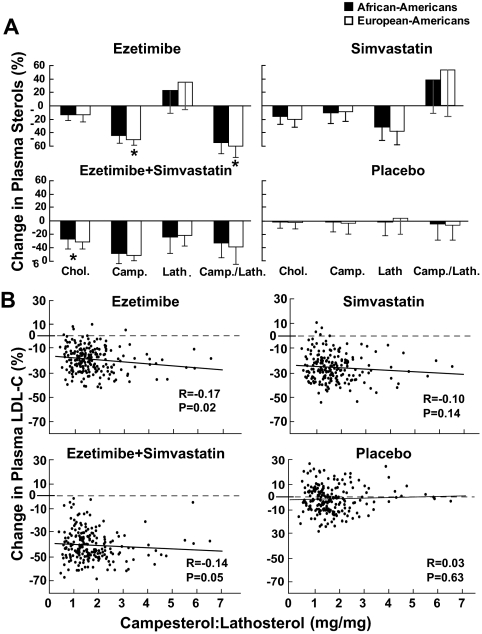
Changes in median plant sterol levels in response to the various treatments were consistent with the mode of action of each drug (Fig. 3A). Inhibition of sterol absorption by ezetimibe reduced campesterol levels and increased lathosterol levels, thus reducing the campesterol to lathosterol ratio. Simvastatin treatment reduced lathosterol levels, produced a modest reduction in campesterol levels, and resulted in an increase in the campesterol to lathosterol ratio. Combination therapy reduced all three parameters: campesterol, lathosterol, and the campesterol to lathosterol ratio. These results are consistent with those of prior studies (14,21,22,23).
Figure 3.
A, Median percent change (interquartile range) in plasma sterol levels by treatment regimen in African- and European-Americans. Median values were compared between African- and European-Americans using the Kruskal-Wallis test. *, P < 0.01. B, Relationship between baseline plasma levels of the campesterol to lathosterol ratio and percent change in plasma LDL-C levels after 6 wk of treatment with ezetimibe, simvastatin, and ezetimibe+simvastatin. Spearman correlations were used to determine the relationship between LDL-C response and baseline plasma levels of noncholesterol sterols. Chol., cholesterol; Camp., campesterol; Lath., lathosterol; Camp./Lath., campesterol to lathosterol ratio.
Baseline campesterol to lathosterol ratios were weakly (r = −0.17), although statistically significantly (P = 0.02), related to response to ezetimibe but did not correlate with responses to simvastatin or simvastatin+ezetimibe (Fig. 3B). Changes in campesterol to lathosterol ratios with treatment were correlated with LDL-C response in the three groups, but the correlation coefficients were modest (Supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). The change in LDL-C levels was directly related to the change in the campesterol to lathosterol ratio in the ezetimibe (r = 0.25, P < 0.001) and ezetimibe+simvastatin groups (r = 0.19, P = 0.008) and inversely related to the change in the campesterol to lathosterol ratio in the simvastatin treatment group (r = 0.24, P < 0.001).
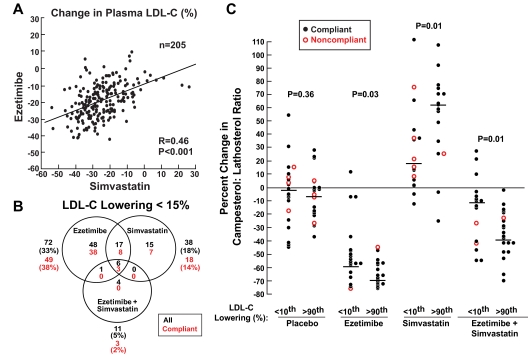
If high absorbers respond better to ezetimibe and low absorbers respond better to statins, then responses to the two drugs should be inversely correlated. Our data reveal no evidence for an inverse relationship between response to simvastatin and response to ezetimibe. Rather, individual changes in LDL-C levels on ezetimibe monotherapy were strongly positively correlated with the responses to simvastatin monotherapy (r = 0.46, P < 0.001) (Fig. 4A). Similar high correlation coefficients were seen when the responses to ezetimibe and simvastatin were compared with both drugs together (r = 0.50 and 0.59, respectively) (Supplemental Fig. 2). The results were similar when African- and European-Americans were analyzed separately or when the analysis was limited to only participants who were compliant (data not shown).
Figure 4.
A, Correlation between changes in plasma LDL-C levels on ezetimibe and simvastatin. Pearson correlation coefficients were used to assess correlations between responses to each treatment regimen. B, Number (percent) of individuals (including both the compliant and noncompliant subjects) that had a reduction in plasma LDL-C that was less than 15% for each drug regimen. C, Changes in campesterol to lathosterol ratios (percent) in subjects in the top and bottom tenth percentile of LDL-C reduction (percent) in response to each drug regimen. Kruskal-Wallis tests were used to compare plasma campesterol to lathosterol ratios in individuals who showed the best (>90th percentile) and poorest (<10th percentile) LDL-C lowering with each regimen. Horizontal line designates median change for each high- and low-response group. Noncompliant subjects were defined as those taking 80% of the medication, as determined by pill counts at the end of each treatment period.
The observation that responses to ezetimibe and simvastatin were highly correlated suggests that the major factors determining the magnitude of LDL-C lowering on these agents are downstream of their primary sites of action. Both drugs increase the activity of the cholesterol-responsive transcription factor sterol regulatory element binding protein-2 in the liver, resulting in up-regulation of the LDLR (24), and of PCSK9, a proprotein convertase that promotes LDLR degradation (25). To test the hypothesis that individuals with elevated baseline levels of PCSK9 respond less well to simvastatin (26,27), we examined the relationship between PCSK9 levels and responses to ezetimibe, simvastatin, or ezetimibe+simvastatin (Supplemental Fig. 1B). No significant relationships were found between baseline PCSK9 levels and response to LDL-lowering therapy. Median PCSK9 levels were not significantly increased by any of the treatment modalities, as is observed when statins are administered at higher doses (26), and changes in plasma PCSK9 levels did not correlate with the magnitude of LDL-lowering response for any treatment regimens.
Despite the correlation in LDL-C-lowering response to ezetimibe and simvastatin (Fig. 4A), combination therapy proved to be more efficacious than either drug individually. On average, combination therapy provided incremental reductions in LDL-C of 22 and 16% when compared with ezetimibe or simvastatin alone, respectively. We also examined the proportion of the sample that had a suboptimal response to each drug regimen, defined by an LDL-C reduction of less than 15% (Fig. 4B). This cutoff was chosen because this level of long-term reduction in LDL-C is associated with a 40 to 50% reduction in CHD (3,28,29). A greater proportion of subjects taking simvastatin alone (81%) than taking ezetimibe alone (65%) achieved a 15% or greater reduction in plasma levels of LDL-C, which is consistent with the known LDL-C-lowering potencies of these regimens (30,31). When both drugs were taken together, LDL-C levels were reduced by 15% or greater in 95% of the entire sample. Only 2% of compliant subjects failed to achieve an LDL-C reduction of at least 15% on combined therapy.
Finally, we probed the possible reasons for the variation in magnitude of response to all three treatments (Fig. 2B) by comparing plasma campesterol to lathosterol ratios in individuals with the most exuberant (>90th percentile) and poorest (<10th percentile) LDL lowering with each regimen (referred to as high and low responders, respectively). No significant differences were found in the change in campesterol to lathosterol levels between the high and low responders on placebo treatment (Fig. 4C). In all three treatment groups the high responders had a greater change in campesterol to lathosterol than the low responders, but there was significant overlap between the extremes in each group. The greatest overlap in distribution was in the ezetimibe group in which all of the high responders and all but three (of 20) of the low responders had a reduction in campesterol to lathosterol of greater than 35%; these three individuals were compliant with the medication. In the simvastatin and combination therapy groups, the increase in the campesterol to lathosterol ratio was greater in the high responders, and a greater proportion of individuals in the low responders were noncompliant. Nonetheless, there was still significant overlap in the distributions. Thus, the wide variation in responsiveness to these drugs cannot be ascribed simply to poor compliance or the drug failing to inhibit cholesterol absorption or synthesis in some individuals.
Discussion
A major finding of this study was that responsiveness to the two major classes of cholesterol-lowering drugs, statins and ezetimibe, was highly correlated among individuals. Although all three drug regimens, ezetimibe alone, simvastatin alone, and both drugs together, achieved the expected mean reductions in LDL-C levels, there was wide variation in responsiveness. Differences in responses among individuals were not fully explained by differences in compliance or indices of cholesterol absorption or synthesis. Plasma levels of noncholesterol sterols and PCSK9 failed to predict the most effective cholesterol-lowering agent for a given individual, at least at the doses of the drugs given in this trial. Thus, we found no evidence to support the use of a personalized medicine approach to select the optimal cholesterol-lowering medication for a patient based on indices of cholesterol metabolism.
Because rates of cholesterol synthesis and cholesterol absorption are inversely correlated, it has been proposed that responsiveness to statins and ezetimibe would depend on the balance between these processes in each individual (32). In this study we found little indication that individuals could be classified as either preferential statin responders or ezetimibe responders based on baseline indicators of cholesterol absorption and synthesis. Individuals tended to be more or less responsive to these cholesterol-lowering therapies, irrespective of whether the intervention targeted cholesterol absorption or cholesterol synthesis. Responses to ezetimibe, simvastatin, and combination therapy were strongly positively correlated (Fig. 4A and Supplemental Fig. 2). Few individuals who responded poorly to one drug showed exuberant responsiveness to the other.
The large variation in individual responsiveness to LDL-C-lowering therapy could potentially be due to differences in compliance, variations in drug metabolism, variations in the pathways targeted by the drugs, or compensatory pathways elicited in response to the drug. Compliance determined from the number of pills remaining after each regimen correlated poorly with LDL-C lowering (Table 2). Most individuals who did not respond to ezetimibe had reductions in plasma campesterol to lathosterol ratios that were greater than those on placebo and were comparable with the most exuberant responders (Fig. 4C). Several nonresponders to simvastatin had changes in campesterol to lathosterol ratios that were comparable to those of the most exuberant responders. Thus, the wide range of responsiveness to lipid-lowering regimens in this study cannot be attributed to systematic differences in compliance.
Genetic variation in drug-metabolizing enzymes can contribute to individual variations in drug responsiveness. Sequence variations in more than 30 genes, including drug-metabolizing enzymes, have been proposed to contribute to variability in responsiveness to statins, but most of the observed associations have been inconsistent and the effect sizes small (33,34). A nonresponder to ezetimibe has been described who has sequence variations in the drug target, NPC1L1 (35). The drugs used in this study are metabolized by distinct mechanisms: ezetimibe is eliminated primarily by glucuronidation (36), whereas simvastatin is metabolized primarily by cytochrome P450 3A (37). Therefore, variation in drug metabolism is unlikely to explain the finding that responses to the three drug regimens were highly correlated. The finding that plasma campesterol to lathosterol ratios fell almost as much in nonresponders to ezetimibe as they did in the most exuberant responders indicates that the primary action of the drug is comparable in the two groups. Thus, it is unlikely that more rapid metabolism of ezetimibe is responsible for the varying levels of LDL lowering observed among individuals taking this drug.
Previous studies suggested that African-Americans have poorer LDL-lowering responses to statins than do European-Americans (18,19,38). African-Americans also have a higher prevalence of mutations in NPC1L1, the target of ezetimibe (39). In the present study, we found no differences between African- and European-Americans in the magnitude of LDL-C lowering with ezetimibe (Table 2). Consistent with previous reports, we did find that African-Americans had a slightly lower response to simvastatin, but this decrease was abolished after adjusting for BMI and compliance. Thus, we found no evidence supporting major racial differences in the response to low doses of ezetimibe, simvastatin, or combined therapy.
Statin treatment increases plasma levels of PCSK9, a proprotein convertase that promotes degradation of the LDLR (26). PCSK9 is a major determinant of plasma LDL-C levels, and changes in PCSK9 activity may influence the efficacy of cholesterol-lowering interventions (27). In the present study, plasma levels of PCSK9 at baseline did not predict responsiveness to any of the three drug regimens, and changes in PCSK9 levels during treatment were not correlated with LDL-C lowering. Whereas these results do not support a major role for PCSK9 in determining individual differences in response to statins or ezetimibe, plasma levels of PCSK9 may provide only a limited indication of functional PCSK9 activity (17). Therefore, it remains possible that PCSK9 limits the efficacy of cholesterol-lowering drugs, perhaps when higher doses of these agents are used.
Guidelines for cholesterol-lowering therapy have been based largely on estimates of CHD risk over 10 yr, emphasizing progressively lower target LDL-C levels for high-risk individuals (40). Data from genetic studies have indicated that modest lifelong reductions in LDL-C confer a disproportionate degree of protection against CHD (3). A mutation in PCSK9 that lowers LDL-C by about 15% was associated with about 50% reduction in CHD in three independent studies (3,28,29), whereas more severe mutations that lowered LDL-C by 28% reduced CHD by 88% (3). In the present study, combination therapy with low-dose ezetimibe+simvastatin lowered LDL-C levels by at least 15% in more than 95% of study participants and by greater than 30% in more than 80% of subjects. Thus, highly clinically significant cholesterol lowering can be achieved in a substantial proportion of individuals using modest doses of currently available drugs.
Finally, this study had certain limitations. The study was restricted to the subset of African- and European-American men with mild to moderate hypercholesterolemia. The results of this study may not be generalizable to women, other ethnic groups, or individuals with more severe hypercholesterolemia. The relatively small sample size combined with the low-dose regimens used in this study limited the ability to detect small differences between groups in the post hoc analysis. A major strength of the study is the crossover design, which permitted comparison within individuals to responses to cholesterol-lowering agents that work by different mechanisms. To our knowledge, this is the first study using this design to assess direct indices of drug action and compliance in both African- and European-Americans.
Note added to proof.
Whereas this paper was in review, a post hoc analysis of the ENHANCE study provided reported that baseline plasma plant sterol levels did not predict response to ezetimibe+statin (Jakulj L, et al. J Lipid Res Oct 14; PMID: 19828909).
Supplementary Material
Acknowledgments
We thank Teresa Eversole, Maria Bailey, and Sharon Ayacannoo for their contributions to this study. We also thank Tuyet Dang and Norma Anderson for performing the PCSK9 ELISAs and Jay Horton for helpful discussions.
Footnotes
This work was supported by the Donald W. Reynolds Foundation and Merck and Co., Inc.
Disclosure Summary: H.H.H. reported serving as a consultant for Pfizer, receiving research support from Donald W. Reynolds Foundation, Howard Hughes Medical Institute, National Institutes of Health, and for this study from Merck and Co., Inc. J.C.C. reported serving as a consultant for Merck, GlaxoSmithKline, and Amgen; receiving research support from the National Institutes of Health, and Pfizer. G.L.V. and S.M.G. reported serving as a consultant for Daiichi Sankyo and receiving research support from Donald W. Reynolds Foundation and Merck. M.C. reported serving on the speaker’s bureau for Merck/Schering-Plough, Daiichi Sankyo, Pfizer, and Takeda. C.L., R.S.L., M.E.S., and T.A.M. are employed by Merck and Co., Inc. The other authors have nothing to declare.
First Published Online December 4, 2009
Abbreviations: ALT, Alanine aminotransferase; BMI, blood mass index; BP, blood pressure; C, cholesterol; CHD, coronary heart disease; HMG CoA, 3-hydroxy-3-methylglutaryl coenzyme A; LDL, low-density lipoprotein; LDLR, LDL receptor; NPC1L1, Niemann-Pick C1-like1; PCSK9, proprotein convertase subtilisin-like kexin type 9.
References
- Rader DJ, Cohen J, Hobbs HH 2003 Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest 111:1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD 2000 Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 284:311–318 [DOI] [PubMed] [Google Scholar]
- Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH 2006 Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 354:34–42 [DOI] [PubMed] [Google Scholar]
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R 2005 Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366:1267–1278 [DOI] [PubMed] [Google Scholar]
- Steinberg D, Glass CK, Witztum JL 2008 Evidence mandating earlier and more aggressive treatment of hypercholesterolemia. Circulation 118:672–677 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 2006 Biomedicine. Lowering LDL—not only how low, but how long? Science 311:1721–1723 [DOI] [PubMed] [Google Scholar]
- Davis HR, Jr., Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW 2004 Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem 279:33586–33592 [DOI] [PubMed] [Google Scholar]
- Endo A 1981 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors. Methods Enzymol 72:684–689 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 1997 The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340 [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Tilvis RS, Kesaniemi YA 1989 Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism 38:136–140 [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Miettinen TA 1986 Serum plant sterols and their relation to cholesterol absorption. Am J Clin Nutr 43:92–97 [DOI] [PubMed] [Google Scholar]
- Bosner MS, Lange LG, Stenson WF, Ostlund Jr RE 1999 Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res 40:302–308 [PubMed] [Google Scholar]
- Bjorkhem I, Miettinen T, Reihner E, Ewerth S, Angelin B, Einarsson K 1987 Correlation between serum levels of some cholesterol precursors and activity of HMG-CoA reductase in human liver. J Lipid Res 28:1137–1143 [PubMed] [Google Scholar]
- Miettinen TA, Strandberg TE, Gylling H 2000 CHECK DASH Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients—related to basal serum cholestanol. Arterioscler Thromb Vasc Biol 20:1340–1346 [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Gylling H 2002 Ineffective decrease of serum cholesterol by simvastatin in a subgroup of hypercholesterolemic coronary patients. Atherosclerosis 164:147–152 [DOI] [PubMed] [Google Scholar]
- Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH 2004 No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol 24:2326–2332 [DOI] [PubMed] [Google Scholar]
- Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH 2009 Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab 94:2537–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, Rotter JI, Nickerson DA, Yang H, Saad M, Krauss RM 2006 Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol 97:843–850 [DOI] [PubMed] [Google Scholar]
- Shear CL, Franklin FA, Stinnett S, Hurley DP, Bradford RH, Chremos AN, Nash DT, Langendorfer A 1992 Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. Effect of patient characteristics on lovastatin-induced changes in plasma concentrations of lipids and lipoproteins. Circulation 85:1293–1303 [DOI] [PubMed] [Google Scholar]
- Rodney RA, Sugimoto D, Wagman B, Zieve F, Kerzner B, Strony J, Yang B, Suresh R, Veltri E 2006 Efficacy and safety of coadministration of ezetimibe and simvastatin in African-American patients with primary hypercholesterolemia. J Natl Med Assoc 98:772–778 [PMC free article] [PubMed] [Google Scholar]
- Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K 2002 Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106:1943–1948 [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Gylling H, Lindbohm N, Miettinen TE, Rajaratnam RA, Relas H 2003 Serum noncholesterol sterols during inhibition of cholesterol synthesis by statins. J Lab Clin Med 141:131–137 [DOI] [PubMed] [Google Scholar]
- Assmann G, Kannenberg F, Ramey DR, Musliner TA, Gutkin SW, Veltri EP 2008 Effects of ezetimibe, simvastatin, atorvastatin, and ezetimibe-statin therapies on non-cholesterol sterols in patients with primary hypercholesterolemia. Curr Med Res Opin 24:249–259 [DOI] [PubMed] [Google Scholar]
- Horton JD, Bashmakov Y, Shimomura I, Shimano H 1998 Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA 95:5987–5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH 2009 PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 50(Suppl):S172–S177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, Prat A 2004 Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 24:1454–1459 [DOI] [PubMed] [Google Scholar]
- Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD 2005 Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA 102:5374–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S 2008 A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med 358:2299–2300 [DOI] [PubMed] [Google Scholar]
- McPherson R, Kavaslar N 2007 Statins for primary prevention of coronary artery disease. Lancet 369:1078; author reply 1079 [DOI] [PubMed] [Google Scholar]
- Dujovne CA, Ettinger MP, McNeer JF, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP 2002 Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol 90:1092–1097 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Merz CN, Brewer Jr HB, Clark LT, Hunninghake DB, Pasternak RC, Smith Jr SC, Stone NJ 2004 Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 44:720–732 [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Gylling H, Strandberg T, Sarna S 1998 Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Finnish 4S Investigators. BMJ 316:1127–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrefors V, Orho-Melander M, Krauss RM, Hedblad B, Almgren P, Berglund G, Melander O 2009 A gene score of nine LDL and HDL regulating genes is associated with fluvastatin induced cholesterol changes in women. J Lipid Res doi: 10.1194/jlr.P001792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajinami K, Akao H, Polisecki E, Schaefer EJ 2005 Pharmacogenomics of statin responsiveness. Am J Cardiol 96:65K–70K; discussion 34K–35K [DOI] [PubMed] [Google Scholar]
- Wang J, Williams CM, Hegele RA 2005 Compound heterozygosity for two non-synonymous polymorphisms in NPC1L1 in a non-responder to ezetimibe. Clin Genet 67:175–177 [DOI] [PubMed] [Google Scholar]
- Jeu L, Cheng JW 2003 Pharmacology and therapeutics of ezetimibe (SCH 58235), a cholesterol-absorption inhibitor. Clin Ther 25:2352–2387 [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T, Gorham LM, Ma B, Liu L, Yu X, Zhao JJ, Slaughter DE, Arison BH, Vyas KP 1997 In vitro metabolism of simvastatin in humans [SBT]identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos 25:1191–1199 [PubMed] [Google Scholar]
- Williams ML, Morris 2nd MT, Ahmad U, Yousseff M, Li W, Ertel N 2002 Racial differences in compliance with NCEP-II recommendations for secondary prevention at a Veterans Affairs medical center. Ethn Dis 12:S1–58-62 [PubMed] [Google Scholar]
- Cohen J, Pertsemlidis A, Fahmi S, Esmail S, Vega G, Grundy S, Hobbs H 2006 Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci USA 103:1810–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2002 Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.