Abstract
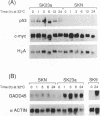
Utilizing a temperature sensitive p53 mutant (pLTRp53cGval135) which expresses mutant p53 at 37 degrees C and a wild-type like p53 at 32 degrees C, we transfected a human ovarian cancer cell line (SKOV3) which does not express endogenous p53. Among the different clones obtained, we selected three clones. Two were obtained from simultaneous transfection of p53 and neomycin resistance expression plasmids (SK23a and SK9), the other was obtained from transfection experiments utilizing the neomycin resistance gene only (SKN). Introduction of mutant p53 did not alter the morphology or growth characteristics of this ovarian cancer cell line. Upon shifting to the permissive temperature, a dramatic change in morphology and growth rate was observed in SK23a and SK9 cells that is associated with the presence of a wild-type like p53. SKN and SKOV3 cells maintained at 32 degrees C did not change morphology and only slightly reduced proliferation. Both SK23a and SK9 cells did not show evidence of apoptosis when measured up to 72 hours of maintenance at 32 degrees C. In contrast to what observed in other cell lines, SK23a and SK9 cells maintained at 32 degrees C were not blocked in G1, but they were accumulated in G2-M. This accumulation was transient and could be due either to a blockade or to a delay in the G2 progression. No down-regulation of c-myc was observed in p53 expressing clones when shifted to the permissive temperature. In these conditions gadd45 mRNA expression was highly stimulated in SK9 and SK23a cells but not in SKN cells. In both clones Gas1 mRNA was not detected either at 37 degrees C or 32 degrees C. This system represents a new and useful model for studying the effect of the absence of p53 (SKOV3 or SKN), presence of mutated p53 (SK23a and SK9 kept at 37 degrees C) or wild type p53 (SK23a and SK9 kept at 32 degrees C) on the mechanism of response of cancer cells to DNA damaging agents.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baisch H., Göhde W., Linden W. A. Analysis of PCP-data to determine the fraction of cells in the various phases of cell cycle. Radiat Environ Biophys. 1975 Jun 13;12(1):31–39. doi: 10.1007/BF02339807. [DOI] [PubMed] [Google Scholar]
- Broggini M., Erba E., Ponti M., Ballinari D., Geroni C., Spreafico F., D'Incalci M. Selective DNA interaction of the novel distamycin derivative FCE 24517. Cancer Res. 1991 Jan 1;51(1):199–204. [PubMed] [Google Scholar]
- Busse P. M., Bose S. K., Jones R. W., Tolmach L. J. The action of caffeine on X-irradiated HeLa cells. III. Enhancement of X-ray-induced killing during G2 arrest. Radiat Res. 1978 Nov;76(2):292–307. [PubMed] [Google Scholar]
- Casey G., Lo-Hsueh M., Lopez M. E., Vogelstein B., Stanbridge E. J. Growth suppression of human breast cancer cells by the introduction of a wild-type p53 gene. Oncogene. 1991 Oct;6(10):1791–1797. [PubMed] [Google Scholar]
- Cheng J., Haas M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol Cell Biol. 1990 Oct;10(10):5502–5509. doi: 10.1128/mcb.10.10.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- D'Incalci M., Torti L., Damia G., Erba E., Morasca L., Garattini S. Ovarian reticular cell sarcoma of the mouse (M5076) made resistant to cyclophosphamide. Cancer Res. 1983 Dec;43(12 Pt 1):5674–5680. [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erba E., Sen S., Lorico A., D'Incalci M. Potentiation of etoposide cytotoxicity against a human ovarian cancer cell line by pretreatment with non-toxic concentrations of methotrexate or aphidicolin. Eur J Cancer. 1992;28(1):66–71. doi: 10.1016/0959-8049(92)90387-h. [DOI] [PubMed] [Google Scholar]
- Foord O., Navot N., Rotter V. Isolation and characterization of DNA sequences that are specifically bound by wild-type p53 protein. Mol Cell Biol. 1993 Mar;13(3):1378–1384. doi: 10.1128/mcb.13.3.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Mechta F., Yaniv M., Oren M. Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Michael-Michalovitz D., Ginsberg D., Oren M. Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decreased stability of the protein. Mol Cell Biol. 1991 Jan;11(1):582–585. doi: 10.1128/mcb.11.1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut P., Milner J. Interaction of heat-shock protein 70 with p53 translated in vitro: evidence for interaction with dimeric p53 and for a role in the regulation of p53 conformation. EMBO J. 1992 Oct;11(10):3513–3520. doi: 10.1002/j.1460-2075.1992.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Isobe M., Emanuel B. S., Givol D., Oren M., Croce C. M. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986 Mar 6;320(6057):84–85. doi: 10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- Karantza V., Maroo A., Fay D., Sedivy J. M. Overproduction of Rb protein after the G1/S boundary causes G2 arrest. Mol Cell Biol. 1993 Nov;13(11):6640–6652. doi: 10.1128/mcb.13.11.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Krishan A., Paika K., Frei E., 3rd Cytofluorometric studies on the action of podophyllotoxin and epipodophyllotoxins (VM-26, VP-16-213) on the cell cycle traverse of human lymphoblasts. J Cell Biol. 1975 Sep;66(3):521–530. doi: 10.1083/jcb.66.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Kavanagh J. J., Wildrick D. M., Wharton J. T., Blick M. Frequent loss of heterozygosity on chromosomes 6q, 11, and 17 in human ovarian carcinomas. Cancer Res. 1990 May 1;50(9):2724–2728. [PubMed] [Google Scholar]
- Levine A. J. The p53 tumor suppressor gene and gene product. Princess Takamatsu Symp. 1989;20:221–230. [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Merry D., Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci U S A. 1986 Jan;83(1):130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Shields M. T., Amin M., Sauve G. J., Appella E., Romano J. W., Ullrich S. J. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Miller C., Mohandas T., Wolf D., Prokocimer M., Rotter V., Koeffler H. P. Human p53 gene localized to short arm of chromosome 17. 1986 Feb 27-Mar 5Nature. 319(6056):783–784. doi: 10.1038/319783a0. [DOI] [PubMed] [Google Scholar]
- Milner B. J., Allan L. A., Eccles D. M., Kitchener H. C., Leonard R. C., Kelly K. F., Parkin D. E., Haites N. E. p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res. 1993 May 1;53(9):2128–2132. [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Oren M. p53: the ultimate tumor suppressor gene? FASEB J. 1992 Oct;6(13):3169–3176. doi: 10.1096/fasebj.6.13.1397838. [DOI] [PubMed] [Google Scholar]
- Osley M. A. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- Papathanasiou M. A., Kerr N. C., Robbins J. H., McBride O. W., Alamo I., Jr, Barrett S. F., Hickson I. D., Fornace A. J., Jr Induction by ionizing radiation of the gadd45 gene in cultured human cells: lack of mediation by protein kinase C. Mol Cell Biol. 1991 Feb;11(2):1009–1016. doi: 10.1128/mcb.11.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramqvist T., Magnusson K. P., Wang Y., Szekely L., Klein G., Wiman K. G. Wild-type p53 induces apoptosis in a Burkitt lymphoma (BL) line that carries mutant p53. Oncogene. 1993 Jun;8(6):1495–1500. [PubMed] [Google Scholar]
- Ryan J. J., Danish R., Gottlieb C. A., Clarke M. F. Cell cycle analysis of p53-induced cell death in murine erythroleukemia cells. Mol Cell Biol. 1993 Jan;13(1):711–719. doi: 10.1128/mcb.13.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam U., Ray A., Sehgal P. B. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- Velu T. Involvement of the p53 tumor suppressor gene in Ph1-positive and Ph1-negative myeloid leukemia. Blood. 1990 Nov 1;76(9):1893–1894. [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Yaginuma Y., Westphal H. Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res. 1992 Aug 1;52(15):4196–4199. [PubMed] [Google Scholar]
- Yonish-Rouach E., Grunwald D., Wilder S., Kimchi A., May E., Lawrence J. J., May P., Oren M. p53-mediated cell death: relationship to cell cycle control. Mol Cell Biol. 1993 Mar;13(3):1415–1423. doi: 10.1128/mcb.13.3.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]