Abstract
T lymphocyte activation requires signal 1 from the T cell receptor (TCR) and signal 2 from co-stimulatory receptors. For long-lasting immunity, growth and survival signals imparted through the Akt/PKB pathway in activated or effector T cells are important, and these can be strongly influenced by signaling from OX40 (CD134), a member of the TNFR superfamily. In the absence of OX40, T cells do not expand efficiently to antigen and memory formation is impaired. How most costimulatory receptors integrate their signals with those from antigen through the TCR is not clear, including whether OX40 directly recruits PKB or molecules that regulate PKB. We show that OX40 after ligation by OX40L assembled a signaling complex that contained the adaptor TRAF2 as well as PKB and its upstream activator PI-3-Kinase. Recruitment of PKB and PI3K were dependent on TRAF2 and on translocation of OX40 into detergent insoluble membrane lipid microdomains, but independent of TCR engagement. However, OX40 only resulted in strong phosphorylation and functional activation of the PI3K/PKB pathway when antigen was recognized. Therefore OX40 primarily functions to augment PKB signaling in T cells by enhancing the amount of PI3K and PKB available to the TCR. This highlights a quantitative role of this TNFR family second signal to supplement signal 1.
Introduction
T cell activation is initiated by recognition of peptide-major histocompatibility (MHC) complexes and the interaction of costimulatory receptors with their ligands present on the surface of antigen-presenting cells (APCs) (1). T cell antigen recognition is followed by activation of phosphoinositide 3-kinases (PI3K) and a sustained rise in the lipid second messenger phophatidylinositol(3,4,5)triphosphate (PIP3). The localization of PIP3 at the inner leaflet of the plasma membrane of T cells recruits pleckstrin homology (PH) domain containing signaling molecules, such as Akt, known also as protein kinase B (PKB) (2–4). PKB is phosphorylated at threonine 308 and serine 473, resulting in its fully active form. This can promote several cellular responses, including controlling growth, cell cycle entry, survival, and glucose metabolism. Thus, the PI3K-PKB axis is essential for clonal expansion, differentiation, and longevity of T cells (5–8).
Several costimulatory receptors in the Ig superfamily have been described to directly target PI3K and result in PKB activation. The first- and best-characterized costimulatory molecule, CD28, which is constitutively expressed on T cells, directly recruits the p85α regulatory subunit of PI3K through a pYMNM motif located in the cytoplasmic tail (9, 10). Mutational analyses of CD28 by replacing the tyrosine of the YMNM motif with phenylalanine revealed that the CD28-PI3K axis transmitted survival signals, but was not necessary for the production of IL-2 or proliferation (11–13). Inducible costimulator (ICOS, CD278), which is not constitutive on T cells but is expressed on activated effector T cells, also recruits p85α and p50α of PI3K through a pYMFM motif (14, 15). A mutational analysis in the cytoplasmic tail by replacing the tyrosine with phenylalanine showed that the ICOS-PI3K axis controlled IL-4 and IL-21 production, which is critical for follicular helper T cell differentiation and humoral immunity (16, 17).
The TNFR family molecule OX40 (CD134), which is induced on a T cell after recognition of antigen, can also regulate the overall level of PI3K-PKB activity in a T cell. CD4 T cells that lacked OX40 did not sustain PI3K activity and PKB signaling over time after antigen encounter, which correlated with impaired expansion and survival of effector cells, and poor generation of T cell memory (18, 19). Moreover, the phenotype exhibited by OX40-deficient T cells included defective expression of Bcl-xL, Bcl-2, Bfl-1, and survivin, and this was rescued by introducing a constitutively active form of PKB into the antigen-responding OX40−/− T cells (18, 19). However, in contrast to CD28 and ICOS, OX40 does not have the consensus YXXM motif in its cytoplasmic tail that might recruit PI3K. It is therefore not clear how OX40 regulates the PI3K-PKB pathway and whether the activity exhibited by OX40 is direct.
Here, we show that engagement of OX40L expressed on an APC resulted in OX40 moving into cholesterol- and sphingolipid-rich detergent-insoluble membrane lipid microdomains (DIM), and the assembly of a signaling complex that contained TRAF2, PKB, and the p85 subunit of PI3K. The association of PKB and p85/PI3K with OX40 was preceded by recruitment of TRAF2, and was dependent on TRAF2 and movement into DIM. However, the complex containing p85/PI3K and PKB was not dependent on the TCR or antigen recognition. In contrast, the OX40 signalosome only augmented total cellular PKB phosphorylation and PKB activity when antigen was presented. Thus, OX40 can recruit PI3K and PKB, but it regulates activation through a conventional role as a true co-signal, quantitatively enhancing the TCR-initiated signal 1.
Materials and methods
Cells and constructs
CD4 T cells from AND (Tg(TcrAND)53Hed) x ox40−/− (tnfsf4−/−) TCR-transgenic mice (Vβ3/Vα11) on a B10.BR-H2k H2-T18a/SgSnJ were activated with moth cytochrome c (MCC) peptide (MCC88-103), and primary effector T cells fused with a thymoma cell line, BW5147, using polyethylene glycol. A representative IL-2 producing clone was selected. N-terminal cMyc-tagged mouse OX40 was subcloned into the pEF1 vector (Invitrogen). T hybridoma cells were transfected with control or cMyc-OX40 vector and selected with G418 (Invitrogen). MSCV-LTRmiR30-PIG (LMP) vector (Open Biosystems) containing the micro-RNA–adapted short hairpin RNA (shRNAmir) against mouse TRAF2 was provided by Dr. Hideki Sanjo (20). Retrovirus production was as described (19). A fibroblast DCEK cell line expressing endogenous CD80 was transfected with I-Ek and OX40L (21). T cells (5 × 105 cells per ml) were stimulated with DCEK cells (1.5 to 10 × 105 cells per ml) and various concentrations of T102S, a superagonist MCC peptide. In general, 10 μM peptide was used. For DIM depletion, T cells were pre-incubated in varying concentrations of the cholesterol-depleting drug methyl-β-cyclodextrin (MβCD) for 1 hr. Inhibition of sphingolipid and cholesterol biosynthesis was performed (22) culturing in serum-containing complete medium for 48 h in the presence of 25 μM myriocin and then serum-starved for 14 h in the presence of 5 μM zaragozic acid and 25 μM myriocin.
Peptides, Chemicals, and Antibodies
T102S (amino acids 88-103; ANERADLIAYLKQASK) was synthesized by Abgent (San Diego). LY294002 (#440202) was from Calbiochem. Sucrose (#84097), Methyl-β-cyclodextrin (MβCD, C4555), peroxidase-conjugated cholera toxin B (CT-HRP, C3741), Myriocin (M1177), and Zaragozic acid (Z2626) were from Sigma. Anti-cMyc (R950-25) and Dynabeads® Protein G (100.04D) were from Invitrogen. Brij-58 (#28336), Nonidet P-40 (#28324), and n-Dodecyl-β-maltoside (#89903) were from Thermo scientific. Protein G sepharose (17-0618-01) was from GE Healthcare. Anti-CD80 (16-10A1, #104710) and -OX40 (OX86, #119408) were from Biolegend. Anti-cMyc (9B11, #2233 and #2276), -p-Erk (#9101), -p-PI3 kinase p85 (Tyr458, #4228), -PI3 kinase p85 (#4257), -p-PKB (Ser473, #9271), -p-PKB (Thr308, #4056), and -PKB (#9272) were from Cell Signaling Technology. Anti-actin (C4, MAB1501), -phosphotyrosine (4G10®, 05-321), and -LAT (06-807) were from Millipore. Anti-TRAF2 (592) and -TRAF2 (M112-3) were from MBL. Anti-PDK1 (ab31406) was from abcam. Anti-OX40 (AF1256) was from R&D systems. Anti-Erk (K-23, sc-94) was from Santa Cruz Biotech.
IL-2 production
IL-2 was measured by ELISA with antibodies JES6-1A12 (#554424) and biotin-JES6-5H4 (#554426), from BD PharMingen.
Western blotting
For total lysates, cells were lysed in ice-cold RIPA buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 1% Na deoxycholate, 0.1% SDS, 50 mM NaF, 1 mM Na3VO4) containing protease inhibitor cocktail (Sigma, P8340) for 15 min. After vortexing at 4 °C for 60 min, insoluble material was removed by centrifugation at 13,000 rpm for 10 min. Protein content was determined by bicinchoninic acid (BCA) assay (Thermo scientific). Equal amounts (5–20 μg) were dissolved in LDS sample buffer (NP0007, Invitrogen) containing DTT (NP0004, Invitrogen) and reduced at 70°C for 10 min. Samples were loaded onto 4–12% NuPage Bis-Tris precasting gels (SDS-PAGE), transferred onto PVDF membrane (Invitrogen), and immunoblotted. All blots were developed with Immobilon western HRP substrate (Millipore).
PIP3 measurement
The amount of PIP3 was measured by the PIP3 mass ELISA kit (Echelon, K-2500s) according to the manufacturers instructions. Briefly, cMyc-OX40 T cells (1 × 107 cells) were stimulated with DCEK cells (3 × 106 cells) in the presence (50 μM) or absence of MCC peptide for 15 min. Cells were washed with 0.5 M Trichloroacetic acid (TCA) and then 5% TCA containing with 1 mM EDTA. Neutral lipids were firstly excluded from the cell pellets with MeOH : CHCl3 (2:1). Acidic lipids were then extracted with 2.25 ml of MeOH : CHCl3 : 12M HCl (80:40:1) and partitioned by centrifugation after addition of 0.75 ml CHCl3 and 1.35 ml 0.1 M HCl. The lower phase was vacuum dried and dissolved in PIP3 buffer. Controls, standards and samples were incubated with PIP3 detector, secondary detection reagent and TMB solution sequentially. The reaction was terminated by adding stop solution (0.5M H2SO4) and the absorbance was measured at 450nm.
DIM isolation
For discontinuous sucrose gradient ultracentrifugation, cells were lysed in 1 ml of 1% Brij-58 lysis buffer (50 mM Tris-HCl (pH 7.4), 25 mM KCl, 5 mM MgCl2, 1 mM EDTA, 50 mM NaF, 1 mM Na3VO4) containing protease inhibitor cocktail for 30 min. Cells were disrupted by passages (10 ×) through a 22-gauge needle on a 1-ml syringe. Lysates were centrifuged at 1,000g for 10 min at 4 °C and supernatants mixed with 1 ml of 80% wt/vol sucrose in the same lysis buffer, and overlaid with 2 ml of 30% sucrose followed by 1 ml 5% sucrose. Samples were ultracentrifuged in a Beckman SW50Ti rotor at 200,000g for 16 hr at 4 °C. Twelve fractions (0.4 ml/fraction) were collected from the top of the gradient. For visualization of GM1, fractions were dot blotted on a nitrocellulose membrane and detected using CT-HRP. Proteins from each fraction were precipitated with trichloroacetic acid (TCA) before immunoblotting.
Alternatively, the DIM fractions were prepared by a 2-step separation method. Postnuclear lysates in 1% Brij-58 lysis buffer were prepared and ultracentrifuged at 100,000g for 50 min at 4 °C. Cell pellets were collected and dissolved in 1% SDS containing ice-cold RIPA buffer. After vortex for 60 min at 4 °C, the supernatants containing extracted DIM proteins were recovered by centrifugation at 13,000 rpm for 10 min at 4 °C.
Immunoprecipitation
Cells were lysed in ice-cold RIPA or 1% n-Dodecyl-β-maltoside for 30 min. Cells were disrupted by passage (30 ×) through a 22-gauge needle on a 1-ml syringe. Lysates were centrifuged at 1,000g for 10 min at 4 °C to remove insoluble material, followed by preclearing with Protein G sepharose beads for 30 min at 4 °C. Lysates were immunoprecipitated with primary Abs, cMyc (9B11) and TRAF2 (M112-3), and protein G beads at 4 °C for overnight. After washing with RIPA or 1% n-Dodecyl-β-maltoside, beads were incubated at 70 °C in 4 × LDS sample buffer for 10 min. After collection of IP lysates from beads and dilution with ultrapure water, those samples in 2 × LDS buffer were further reduced with DTT at 70 °C for 10 min for immunoblotting.
Results
OX40 co-signal supports TCR-dependent PKB activation
Using OX40-deficient TCR-transgenic T cell systems, we previously demonstrated in antigen-responding effector T cells that OX40 was required for optimal activation of the PI3K/PKB pathway, and this coordinately regulated T cell proliferation, survival, and cytokine secretion (18). To understand how OX40 regulates PI3K/PKB, we established an MCC peptide-specific T cell hybridoma, derived from effector T cells obtained from OX40-deficient AND Vα11/Vβ3 TCR transgenic mice. These T cells were then transfected with a cMyc-OX40 construct to allow OX40 to be efficiently immunoprecipitated via the Myc tag.
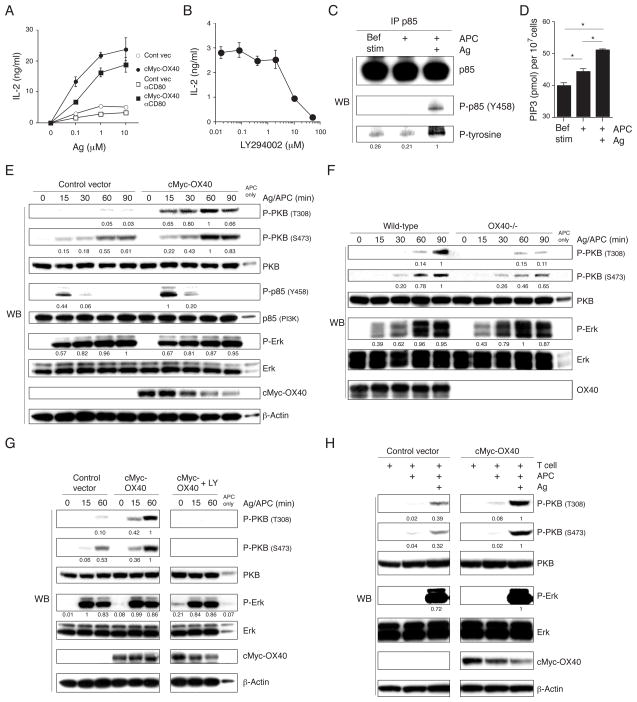
T cells were stimulated with a fibroblast APC that expresses B7 (CD80) and OX40L, and that can present MCC peptide on I-Ek (21). Without antigen, the T cells did not produce any IL-2 even in the presence of OX40-OX40L and CD28-CD80 interactions (Fig. 1A). OX40 ligation strongly promoted IL-2 when antigen was presented. Some of this was independent, and a proportion dependent on CD28, shown by blocking CD80. In contrast, antigen and CD28 signals resulted in only modest IL-2 production (Fig. 1A). In Jurkat human T cell lines, IL-2 production induced by the TCR and CD28 is insensitive to the PI3K inhibitors wortmannin and LY294002, in part due to loss of expression of PTEN (phosphatase and tensin homologue) (23–25). In our murine T cell hybridoma, LY294002 suppressed antigen and OX40L dependent IL-2 production in a dose dependent manner (Fig. 1B), indicating PI3K activity. In accordance with this, when the p85 subunit of PI3K was immunoprecipitated after antigen and OX40L stimulation from APCs, we found that it was strongly tyrosine phosphorylated (Fig. 1C). Furthermore, PIP3 was elevated in antigen/OX40L stimulated T cells (Fig. 1D).
Figure 1. OX40 promotes antigen-dependent PKB activation.
(A) IL-2 production from control vector OX40−/− (open symbol) and cMyc-OX40 (closed symbol) transduced AND T hybridoma cells, evaluated in the presence or absence of indicated concentrations of MCC peptide and B7+OX40L+ DCEK APC, and anti-CD80 blocking antibody (10 μg/ml, square), after 24 h. (B) IL-2 from cMyc-OX40 expressing T cells stimulated with antigen and APC in the presence of indicated concentrations of LY294002 for 4 h. Data in A and B are average values with SD from triplicate cultures. (C and D) cMyc-OX40 T cells were stimulated with APC in the presence or absence of antigen for 1 hr (C) or 15 min (D). (C) p85 was immunoprecipitated and samples analyzed for the indicated proteins. (D) PIP3 was extracted from the cells and quantified as described in the materials and methods. Statistical significance was evaluated by Student t-test (*, p < 0.01). (E and F) Protein expression levels were evaluated by immunoblotting of total lysates from T cell hybridoma cells (E) and primary wt or OX40−/− AND effector CD4 T cells (F) cultured with peptide and APC for 0–90 mins. (G) T hybridoma cells were stimulated with antigen and APC in the presence or absence of 10 μM LY294002, and total lysates analyzed for the indicated proteins. (H) T hybridoma cells were stimulated with APC in the presence or absence of antigen for 1 hr, and cytosolic protein levels were evaluated. Densitometry was performed using the software YabGelImageX1.2 using 1 as a reference of the highest level. All results are representative of at least two experiments.
During initial T cell activation in the presence of antigen, phosphorylation of PKB (T308 and S473) and p85 (Y458) were induced in OX40-deficient cells (control vector), but these activities were strongly upregulated and maintained at later times by engagement of OX40 with OX40L (cMyc-OX40 T cells) (Fig. 1E). Similar to our previous results (18), when primary effector CD4 T cells were stimulated with antigen, phosphorylation of T308 and S473 on PKB was severely impaired over time in the absence of OX40 (Fig. 1F), showing endogenous OX40 provides the same signals as transfected OX40 in the hybridoma cells. Consistent with data in Fig. 1B, LY294002 completely inhibited PKB phosphorylation mediated by antigen and OX40L in the hybridoma (Fig. 1G). Thus, PI3K controls PKB activation, and OX40 contributes strongly to activation of this PI3K/PKB pathway when antigen is recognized.
To address the role of the TCR, cells were stimulated without antigen. OX40/OX40L interactions alone could not induce efficient phosphorylation of PI3K and PKB (Fig. 1C and H, respectively). This indicates that OX40 facilitates TCR-dependent PI3K activation and resulting PKB phosphorylation. Phosphorylation of Erk was markedly induced by antigen, but unaffected by OX40 signals, demonstrating some selectivity (Fig. 1E–H). Therefore, OX40 represents a dominant stimulus for promoting the PI3K-PKB axis in effector-like T cells, but does so only as a co-signal to augment antigen/TCR-induced activity.
OX40 recruits TRAF2, PI3K, and PKB
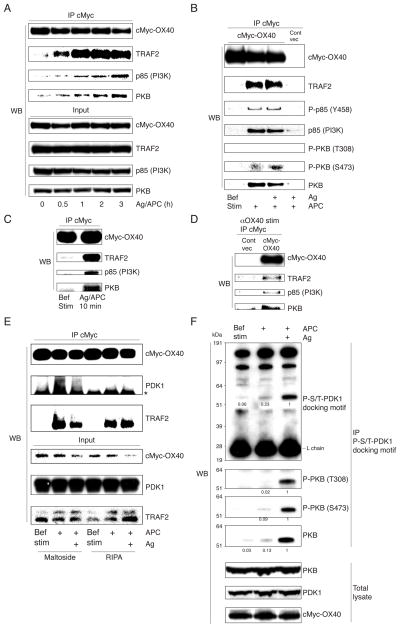
To understand how OX40 augments TCR-dependent PKB activation, OX40 was immunoprecipitated via the cMyc tag and associated proteins were examined by immunoblotting. The adaptor protein TRAF2 has been shown to associate with OX40 (26, 27). Before stimulation with OX40L expressed on APC, OX40 did not appreciably interact with TRAF2 or other signaling molecules. After stimulation with OX40L, OX40 was strongly associated with endogenously expressed TRAF2 (Fig. 2A–C). Moreover, p85 and PKB were found within this immunoprecipitate (Fig. 2A–C). In cultures where T-APC contact was not forced and likely increased over time, prolonged complex formation was observed (Fig. 2A), however, 10 min stimulation was sufficient to detect the complex (Fig. 2C). Furthermore, it was not dependent on TCR engagement, occurring regardless of peptide presentation (Fig. 2B). The complex was also induced with an agonist antibody to OX40 in the absence of APC (Fig. 2D). We evaluated any possible recruitment of the TCR, CD3, or CD28, but failed to detect these molecules and to detect the proximal kinases, Zap70, Lck, or SLP-76 in the immunoprecipitates (data not shown), suggesting that OX40 forms a unique signalosome separate from the TCR and CD28 signalosomes.
Figure 2. OX40 recruits PI3K, PKB, and PDK1, independent of antigen.
(A–E) cMyc-OX40 or control vector T cells were stimulated with B7+OX40L+ APC, with or without antigen, as in Fig. 1 (A–C, and E) or were stimulated with an anti-OX40 agonistic antibody (D). Cells were lysed in RIPA buffer containing NP-40 (A–E), or in dodecyl-maltoside (E), and OX40 was immunoprecipitated. Samples were analyzed for the indicated proteins at 0–3 hr (A), 3 hr (B, D, and E), or 10 min (C). Asterisk in E shows H chain bands. (F) cMyc-OX40 T cells were stimulated with APC with or without antigen for 1 hr and lysates immunoprecipitated with anti-phospho-serine/threonine-PDK1 docking motif mAb. Bef. Stim. represents OX40 immunoprecipitated from unstimulated T cells. All results are representative of at least two experiments.
Interestingly, a low level of phosphorylated p85, and serine-, but not threonine-, phosphorylated PKB was found in the OX40 signalosome and again this was also antigen-independent (Fig. 2B). Activation of PI3K induces relocalization of phosphoinositide-dependent kinase 1 (PDK1) to the phospholipid-enriched plasma membrane. PDK1 is activated on the membrane and phosphorylates substrate kinases such as PKB. We then investigated whether PDK1 was recruited to OX40. We did not find PDK1 in the standard IP conditions using RIPA, but when n-dodecyl-β-maltoside was used, a detergent that preserves membrane protein structure, we found a low level of PDK1 (bands of 58–68 kD) precipitated after OX40 was ligated by OX40L (Fig. 2E). Again, this was independent of TCR engagement (Fig. 2E). This implied that the small amount of phosphorylated PKB present in the antigen-independent OX40 signaling complex was likely due to this minor amount of recruited PDK1. We also immunoprecipitated PDK1 but failed to detect OX40 (data not shown), also suggesting that the quantity of total cellular PDK1 that complexes with OX40 is small. As shown before, the majority of total cellular phosphorylated p85 and both serine- and threonine-phosphorylated PKB were dependent on antigen recognition (Fig. 1C and 1H). This further implied that PDK1 that might promote this activity was primarily engaged by TCR signaling, suggesting that the role of OX40 is to recruit both PI3K and PKB and make them available for further or continued phosphorylation when the TCR is engaged. To correlate this with the activity of PDK1, T cell lysates were prepared before and after stimulation with antigen/OX40L and proteins were immunoprecipitated with mAb specific for phosphorylated substrate peptide sequences of PDK1 (PDK1 docking motifs). As demonstrated in Fig. 2F, upper panel, antigen/OX40L stimulation induced strong phosphorylation in a ~60 kDa cytoplasmic protein, but without an antigen signal, this was marginal. The protein was found to be PKB (Fig. 2F, middle panel). Thus, OX40 ligation by OX40L in the context of antigen presentation likely provides increased amounts of PKB that can then be targeted by PDK1 activity that primarily results from engagement of the TCR.
TRAF2 plays a critical role for PKB activation by OX40
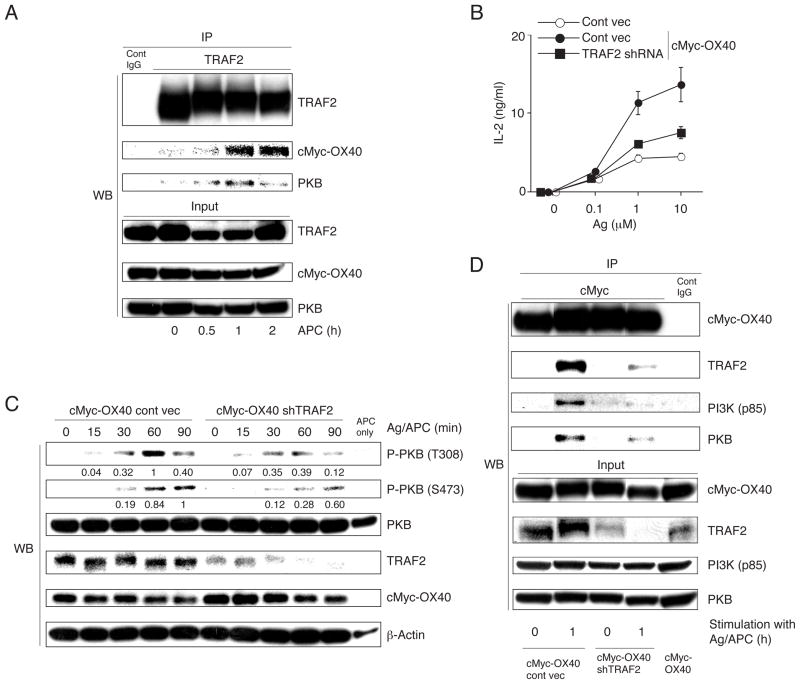
An important question is how OX40 recruits PKB. By immunoprecipitating TRAF2, we found that both OX40 and PKB were pulled down, but only after OX40-OX40L interaction (Fig. 3A). This correlates with some studies that have suggested that certain members of the TRAF family adaptor proteins could directly or indirectly interact with PKB (1, 29). To investigate the functional importance of TRAF2 to the signalosome, expression was reduced by treatment with shRNA in cMyc-OX40 T cells. ~70% loss of TRAF2 was observed, but OX40 and cMyc expression were unaltered (Fig. 3C and D). Inhibiting TRAF2 expression blocked IL-2 production in response to antigen presented on B7/OX40L APC, almost to the level secreted by OX40 negative T cells (Fig. 3B). In parallel, activation of total cellular PKB was impaired following antigen and OX40L stimulation (Fig. 3C). Correspondingly, the association of OX40 with PKB was severely reduced when TRAF2 was knocked down (Fig. 3D). Unlike CD28 and ICOS, OX40 does not contain consensus phospho-tyrosine motifs in the cytoplasmic tail to interact with PI3K (28). Interestingly, the association of OX40 with p85 was also strongly reduced when TRAF2 was knocked down (Fig. 3D), suggesting that TRAF2 directly or indirectly plays a pivotal role for recruitment of PI3K as well as PKB.
Figure 3. TRAF2 is critical for PKB activation mediated by OX40.
(A) cMyc-OX40 T cells were stimulated with APC without antigen for 0–2 hr and TRAF2 was immunoprecipitated for analysis of associated proteins. (B) cMyc-OX40 or control (OX40−/−) T cells were transduced with shTRAF2 or control shRNA, and IL-2 levels were determined at 24 hr after stimulation with varying doses of antigen/APC. (C) Phosphorylation of PKB was evaluated over 0–90 mins after antigen stimulation of T cells transduced as in B. (D) Proteins co-immunoprecipitated with cMyc-OX40 were visualized by immunoblotting in shTRAF2 or control shRNA transduced T cells, before stimulation (0 hr) or 1 hr after stimulation with antigen/APC. Results are representative of at least two experiments.
Detergent insoluble lipid microdomains are essential for PKB activation
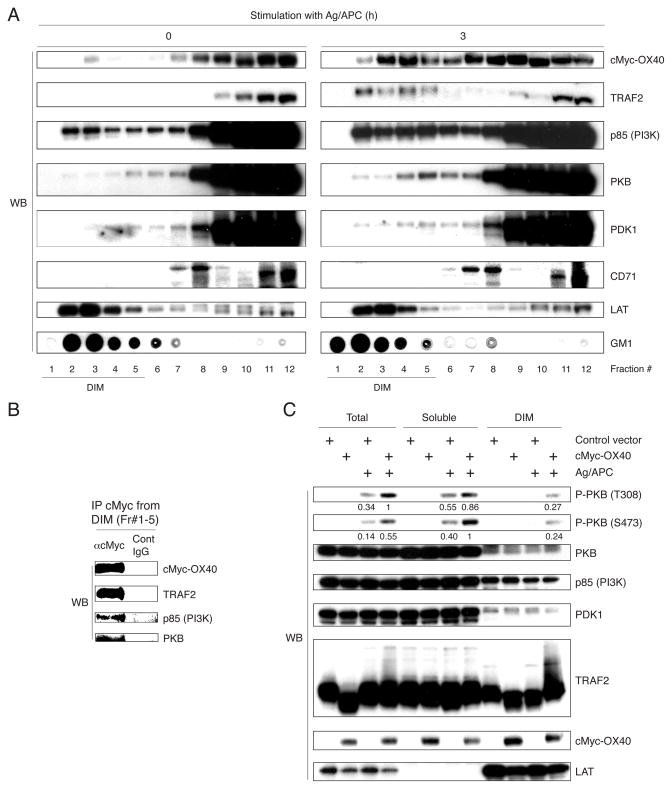
An important step in PKB activation is its translocation from the cytosol to the plasma membrane. We then focused on detergent insoluble lipid microdomains (DIM) that might be necessary for efficient intracellular signaling (22, 30). Antigen/APC stimulated cMyc-OX40 T cells were lysed in buffer containing Brij-58, a weak nonionic detergent that preserves DIM, and analyzed after sucrose gradient ultracentrifugation. Activation with antigen/OX40L induced strong translocation of OX40 into the DIM (Fig. 4A). The levels initially decreased to ~60% of the starting level within 1 hr then further increased up to ~300% at 3–4 hr (data not shown). OX40 moving into DIM was accompanied by TRAF2 (Fig. 4A). A proportion of p85, PKB, and PDK1 was expressed in the DIM before and after T cell stimulation suggesting these molecules were available to OX40 that moved into this compartment. Importantly, OX40 that translocated into the DIM associated with TRAF2, p85, and PKB (Fig. 4B), implying that the DIM environment is important to initiate the PI3K/PKB pathway. After stimulation, the DIM fractions recovered from Brij-58-insoluble pellets of cMyc-OX40 T cells contained phosphorylated PKB, but this was dependent on antigen recognition and not seen after OX40L interaction in the absence of antigen (Fig. 4C and data not shown). These results suggested that OX40 might recruit PKB into its signaling complex in the DIM, which then allows PKB to be available for phosphorylation triggered by TCR-related signaling events.
Figure 4. OX40 translocation into DIM is associated with activation of PKB.
(A and B) cMyc-OX40 T cells were stimulated with APC in the presence of antigen for 3 hr. (A) Fractions from sucrose gradient of lysates of unstimulated (0 hr) and stimulated T cells were immunoblotted. LAT and GM1 are markers of the DIM. CD71 (transferrin receptor) represents a non-DIM marker. (B) OX40 was immunoprecipitated from pooled fractions #1–5 in A. (C) Control vector and cMyc-OX40 T cells were stimulated with APC in the presence of antigen for 30 min as in A. The DIM and non-DIM (soluble) fractions were obtained by a 2-step separation method, and protein levels were evaluated by immunoblotting. Results are representative of at least two experiments.
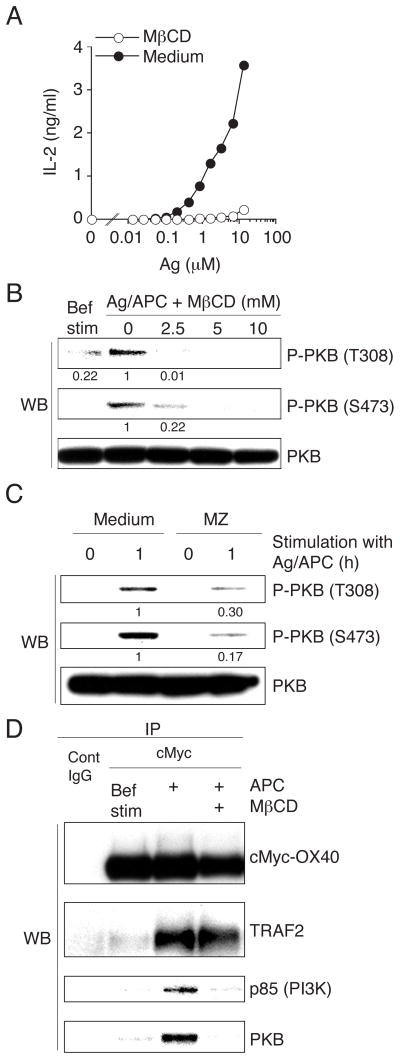
To investigate the importance of the membrane microdomain environment, the cholesterol-depleting drug methyl-β-cyclodextrin (MβCD) was used. Cholesterol depletion completely blocked antigen and OX40L-dependent IL-2 production (Fig. 5A), and correlating with this, it strongly inhibited phosphorylation of PKB (Fig. 5B). Suppression of sphingolipid and cholesterol synthesis, by the inhibitors myriocin (M) and zaragozic acid (Z), respectively, which also cause DIM disruption, similarly resulted in impaired PKB phosphorylation (Fig. 5C). Importantly, OX40 association with TRAF2 was not altered after DIM disruption, whereas that with p85 and PKB was essentially abolished (Fig. 5D). Thus, OX40 recruits TRAF2 outside the DIM and then p85 and PKB are recruited into the DIM-resident OX40-TRAF2 complex. The DIM therefore serves as an important platform for OX40 to integrate with the TCR to enhance downstream PKB activation.
Figure 5. Disruption of DIM blocks PI3K and PKB recruitment to OX40 and inhibits PKB activation.
(A) cMyc-OX40 T cells were pretreated with 10 mM MβCD. IL-2 levels were measured at 4 hr from T cells stimulated with APC and varying concentrations of antigen. (B) cMyc-OX40 T cells, treated with various concentrations of MβCD, were cultured with antigen and APC for 1 hr and PKB protein levels were evaluated by immunoblotting. Bef. Stim. represents unstimulated T cells. (C) cMyc-OX40 T cells, treated with MZ, were cultured with antigen and APC for 0 and 1 hr and protein levels were evaluated. (D) cMyc-OX40 T cells, pretreated in the presence or absence of 10 mM MβCD, were cultured with APC in the absence of antigen for 1hr. cMyc-OX40 was immunoprecipitated from total cell lysates and associated proteins analyzed. Bef. Stim represents OX40 precipitated from unstimulated T cells. Results are representative of at least two experiments.
Discussion
In this study, we show that the TNFR family member OX40 forms a signaling complex that contains PI3K and PKB. Recruitment of these molecules is dependent on TRAF2, and occurs in detergent insoluble membrane lipid microdomains after OX40 and TRAF2 translocate into this cellular compartment driven by ligation of OX40 by OX40L. We show this is a key event that strongly augments antigen/TCR-dependent activation of the PI3K-PKB pathway.
Physiological interactions between T cells and antigen bearing APC promote increased PIP3 in the T cell inner membrane over many hours (2, 3). It is known that after the TCR is cross-linked, PI3K generates PIP3 through phosphorylation of PI4,5-biphosphate (PIP2). The PH domain of PKB recognizes PIP3, triggering PKB recruitment from the cytosol to the plasma membrane. PKB then can undergo phosphorylation on Thr308 and Ser473, in a process that is not completely understood but has been suggested to involve PDK-1 and a second kinase called PDK-2. PDK-2 has been proposed to be the mammalian target of rapamycin complex 2 (mTORC2) (22, 31). The two phosphorylation events on PKB promote its release into the cytosol, thereby allowing phosphorylation of downstream targets, of which there are many possible including GSK3, FoxO1, TSC, IKKα, and Bad.
Although signal 1 from the TCR is critical for production of PIP3 and activation of the kinases at early time points, it has not been clear how kinase activation is maximized or maintained for long periods. CD28 has been known for many years to be distributed in the DIM (32–34), and recent studies showed that ligation of several receptors including CD28 resulted in accumulation of PIP3 in the plasma membrane and formation of functional hot spots in what were termed “raft nanodomains” that concentrated PKB in regions of the DIM and allowed sustained activation of the PI3K-PKB pathway (22). Furthermore, results from Saito and Davis demonstrated that TCR-initiated signaling occurred in ‘microclusters’ or ‘islands’ in the plasma membrane, and such spatially and temporally regulated protein structures were critical for T cell activation (35, 36). These microclusters or islands were either found to be preformed or to be induced by TCR ligation, and contained the TCR and several kinases and adaptors, namely Zap70, SLP-76, and LAT. After antigen recognition they were also shown to transiently link or aggregate together. Collectively, these results promote the hypothesis that signal 2 from costimulatory receptors might support signal 1 by recruiting or maintaining a high concentration of PI3K and PKB in the vicinity of the TCR that is located in these membrane microclusters or nanodomains after peptide recognition. Our previous results (18), combined with our current results here, also could be interpreted into this model. We demonstrated that OX40 signals in the presence of antigen maximized and prolonged activation of PKB in a DIM-dependent fashion, and that OX40 associates in a complex with both PI3K and PKB. However, OX40 did not appreciably or strongly affect the total amount of PI3K or PKB in the DIM. Therefore it is likely that OX40 acts in very specific regions of the DIM and the primary role of OX40 is to promote highly concentrated depots of these molecules either in, or close to, the TCR microclusters/nanodomains. Although this needs to be investigated in the future, OX40 may therefore increase the amount of PI3K and PKB available to these units.
Several other TNFR family members can activate the PI3K-PKB pathway. However, the proximal steps that are utilized by TNFRs to recruit or lead to activation of PI3K or PKB might vary and are not fully understood. RANK (CD265), after binding RANKL (CD254), has been described to activate PKB through recruiting TRAF6, Cbl-b, and PI3K into a complex, in a mechanism dependent on the kinase activity of c-Src (37, 38). CD40 induces recruitment of PI3K and c-Cbl (38), and deficiencies in Cbl-b, c-Cbl, and TRAF6 abrogated CD40L-dependent PKB phosphorylation (38, 39). TNF can also activate PI3K and PKB (40, 41). TNFR1 constitutively forms a complex with PI3K, c-Src, and Jak2, and the tyrosine kinase activities of both c-Src and Jak2 were found to be important for activation of the PI3K-PKB pathway (41). Furthermore, TRAILR has been shown to interact with c-Src, c-Cbl, and PI3K, with again c-Src activity being critical for TRAILR-dependent PI3K and PKB activation (42, 43). Our study shows that OX40 recruits PI3K and PKB in a manner dependent on TRAF2, but whether TRAF2 directly interacts with these molecules is not clear. c-Src appears to be common to the aforementioned receptors, but we failed to detect c-Src in the OX40 signalosome. Critical regulators for PI3K-PKB activation after TNFR family engagement in T cells might be ubiquitin ligases in the TRAF family as well as Cbl. It has recently been reported that PKB undergoes lysine (K) 63-linked ubiquitination at K8 and K14 within its PH domain by TRAF6, and this can regulate PKB membrane recruitment and phosphorylation (29). We have not found TRAF6 in the OX40 signaling complex, but OX40L interaction with OX40 resulted in ubiquitination of OX40, which was also sensitive to MβCD treatment (unpublished results), suggesting that this DIM-dependent ubiquitination event might also play a functional role for enhancing the molecular association of OX40 with p85 and PKB. Further experiments are then needed to understand the role of ubiquitination, and if other molecules might associate with the OX40 signalosome within the DIM to allow recruitment and activation of PI3K and PKB.
In summary, our results shed light on the mechanism by which the T cell costimulatory receptor OX40 promotes PKB activation. The data add to our knowledge of the molecular mechanisms by which ligation of OX40 amplifies TCR-driven T cell activation, and provides further information that in part explains why OX40 is an important regulator of effector T cells.
Acknowledgments
We thank Dr. Jianxun Song and Dr. Hideki Sanjo for the DNA constructs. This is manuscript #1302 from the La Jolla Institute for Allergy and Immunology.
The work was supported by NIH grants AI49453 and CA91837 to M.C.
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- 3.Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- 4.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 5.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 6.Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantrell D. Protein kinase B (Akt) regulation and function in T lymphocytes. Semin Immunol. 2002;14:19–26. doi: 10.1006/smim.2001.0338. [DOI] [PubMed] [Google Scholar]
- 8.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 10.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 11.Okkenhaug K, Wu L, Garza KM, La Rose J, Khoo W, Odermatt B, Mak TW, Ohashi PS, Rottapel R. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 12.Harada Y, Tokushima M, Matsumoto Y, Ogawa S, Otsuka M, Hayashi K, Weiss BD, June CH, Abe R. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–3803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- 13.Burr JS, Savage ND, Messah GE, Kimzey SL, Shaw AS, Arch RH, Green JM. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 14.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 15.Fos C, Salles A, Lang V, Carrette F, Audebert S, Pastor S, Ghiotto M, Olive D, Bismuth G, Nunes JA. ICOS ligation recruits the p50alpha PI3K regulatory subunit to the immunological synapse. J Immunol. 2008;181:1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- 16.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 17.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 19.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Sanjo H, Zajonc DM, Braden R, Norris PS, Ware CF. Allosteric regulation of the ubiquitin:NIK and TRAF3 E3 ligases by the lymphotoxin-{beta} receptor. J Biol Chem. 2010;285:17148–17155. doi: 10.1074/jbc.M110.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 22.Lasserre R, Guo XJ, Conchonaud F, Hamon Y, Hawchar O, Bernard AM, Soudja SM, Lenne PF, Rigneault H, Olive D, Bismuth G, Nunes JA, Payrastre B, Marguet D, He HT. Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nat Chem Biol. 2008;4:538–547. doi: 10.1038/nchembio.103. [DOI] [PubMed] [Google Scholar]
- 23.Ueda Y, Levine BL, Huang ML, Freeman GJ, Nadler LM, June CH, Ward SG. Both CD28 ligands CD80 (B7-1) and CD86 (B7-2) activate phosphatidylinositol 3-kinase, and wortmannin reveals heterogeneity in the regulation of T cell IL-2 secretion. Int Immunol. 1995;7:957–966. doi: 10.1093/intimm/7.6.957. [DOI] [PubMed] [Google Scholar]
- 24.Crooks ME, Littman DR, Carter RH, Fearon DT, Weiss A, Stein PH. CD28-mediated costimulation in the absence of phosphatidylinositol 3-kinase association and activation. Mol Cell Biol. 1995;15:6820–6828. doi: 10.1128/mcb.15.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 26.Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J Biol Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 28.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG, Lin HK. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–1138. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 31.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 32.Sadra A, Cinek T, Imboden JB. Translocation of CD28 to lipid rafts and costimulation of IL-2. Proc Natl Acad Sci U S A. 2004;101:11422–11427. doi: 10.1073/pnas.0403792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 34.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 35.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 36.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 38.Arron JR, Vologodskaia M, Wong BR, Naramura M, Kim N, Gu H, Choi Y. A positive regulatory role for Cbl family proteins in tumor necrosis factor-related activation-induced cytokine (trance) and CD40L-mediated Akt activation. J Biol Chem. 2001;276:30011–30017. doi: 10.1074/jbc.M100414200. [DOI] [PubMed] [Google Scholar]
- 39.Benson RJ, Hostager BS, Bishop GA. Rapid CD40-mediated rescue from CD95-induced apoptosis requires TNFR-associated factor-6 and PI3K. Eur J Immunol. 2006;36:2535–2543. doi: 10.1002/eji.200535483. [DOI] [PubMed] [Google Scholar]
- 40.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 41.Pincheira R, Castro AF, Ozes ON, Idumalla PS, Donner DB. Type 1 TNF receptor forms a complex with and uses Jak2 and c-Src to selectively engage signaling pathways that regulate transcription factor activity. J Immunol. 2008;181:1288–1298. doi: 10.4049/jimmunol.181.2.1288. [DOI] [PubMed] [Google Scholar]
- 42.Secchiero P, Gonelli A, Carnevale E, Milani D, Pandolfi A, Zella D, Zauli G. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107:2250–2256. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- 43.Song JJ, Kim JH, Sun BK, Alcala MA, Jr, Bartlett DL, Lee YJ. c-Cbl acts as a mediator of Src-induced activation of the PI3K-Akt signal transduction pathway during TRAIL treatment. Cell Signal. 2010;22:377–385. doi: 10.1016/j.cellsig.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]