Abstract
As the most readily oxidized of DNA’s four natural bases, guanine is a prime target for attack by reactive oxygen species (ROS) and transition metal-mediated oxidants. The oxidation products of a modified guanosine nucleoside and of a single-stranded oligodeoxynucleotide, 5′-d(TTTTTTTGTTTTTTT)-3′ have been studied using oxidants that include CoII, NiII, and IrIV compounds as well as photochemically generated oxidants such as sulphate radical, electron-transfer agents (riboflavin) and singlet oxygen. The oxidized lesions formed include spiroiminodihydantoin (Sp), guanidinohydantoin (Gh), imidazolone (Iz), oxazolone (Z) and 5-carboxamido-5-formamido-2-iminohydantion (2-Ih) nucleosides with a high degree of dependence on the exact oxidation system employed. Interestingly, a nickel(II) macrocyclic complex in conjunction with KHSO5 leads to the recently reported 2-Ih heterocycle as the major product in both the nucleoside and oligonucleotide contexts.
Keywords: Guanine oxidation, DNA damage, Ni(II) complexes, Spiroiminodihydantoin, 2-Iminohydantoin, Singlet Oxygen, Persulfate
1. Introduction
Chemical modification of guanine (G), the most readily oxidized nucleobase in DNA, has been linked to aging, cancer and degenerative diseases [1–4]. The constant attack by endogenous and exogenous oxidants on guanine generates various oxidation products (Figure 1) [5]. Among these products, 8-oxo-7,8-dihydroguanine (8-oxo-G), guanine-derived 2,6-diamino-4-hydroxy-formamidopyrimidine (FaPy-G) [6–8], spiroiminodihydantoin (Sp) [9] and oxazolone (Z) [10] have been found in vivo. The normal level of 8-oxo-G, the most common oxidation product of guanine is 0.3–4 lesions per 106 dGs [11], and it has been used as a biomarker of oxidative stress in cells [12].
Figure 1.
Oxidation products of 2′,3′,5′-tri-O-acetylguanosine (R= 2′,3′,5′-tri-O-acetylribose) and their relative masses
The various oxidation products formed are due to hydroxylation or perhydroxylation at the C5 or C8 positions of guanine. For example, the C5 pathway involves loss of one electron from guanine leading to formation of the guanine radical cation (G+·) that has a pKa of 3.9 [13]; G+· undergoes rapid deprotonation in the nucleoside form, although it may be longer lived in duplex DNA (Scheme 1) [14]. The neutral guanine radical (G-H·) [15–17] is attacked by dioxygen [18–20] or superoxide anion [17, 21, 22] and results in a cascade of decomposition reactions leading to formation of imidazolone (Iz) and eventually oxazolone (Z) products [23–25]. On the other hand, the guanine radical cation (G+·) can be trapped by H2O at C8 [13], followed by deprotonation and formation of an intermediate adduct (G-OH·) [26] that is prone to further oxidation and tautomerization leading to 8-oxo-G (Scheme 2). Under reducing conditions, one-electron reduction of (G-OH·) followed by ring opening leads to FaPy-G. Both 8-oxo-G and FaPy-G are formed during radiation damage to DNA [4, 27]. 8-Oxo-G has a reduction potential of 0.74 V vs NHE [12], and because this is ~0.55V lower than that of G (E = 1.29 V vs NHE at pH 7), further oxidation can readily occur yielding the spirocyclic base Sp as well as guanidinohydantoin (Gh) [28, 29]. Both of the hydantoin lesions are known to be highly mutagenic (Scheme 2) [30].
Scheme 1.
C5 pathway for oxidation of 2′,3′,5′-tri-O-acetylguanosine
Scheme 2.
C8 pathway for oxidation of 2′,3′,5′-tri-O-acetylguanosine
A wide range of aqueous oxidation systems, from reactive oxygen species (ROS) [31] to photochemical oxidants [32] to transition metals [24, 25, 33–35], are known to react with the electron-rich guanine heterocycle (Table 1). Among the sulfoxyl radicals, the sulfate radical anion (SO4·−) is the most potent oxidant with a redox potential of 2.43 V vs. NHE at pH 7, and it acts by a one-electron abstraction mechanism [36]. Another relevant oxidant is singlet oxygen formed by photoexcitation of sensitizers such as Rose Bengal (RB) or porphyrins [32]. Singlet oxygen can potentially act as a four-electron oxidant of G, bypassing 8-oxo-G to yield hydantoin products directly [37].
Table 1.
Aqueous oxidation systems
| Oxidation System | Oxidant | E1/2 (V vs NHE) at pH 7 |
|---|---|---|
| Na2IrCl6 | e− | 0.9 |
| K2S2O8/hv (256 nm) | SO4·− | 2.43 |
| Rose Bengal/hv (360 nm) | 1O2 | NA |
| CoCl2/KHSO5 | SO4·− | >2.0 |
| Ni(II)CR/KHSO5 | L4-Ni-O-SO3· | >2.0 |
| Fe(II)EDTA/H2O2 | OH· | 1.9 |
Furthermore, several transition metal ions act as promoters of nucleic acid oxidation. Mechanistically, the simplest of these oxidants is hexachloroiridate, IrCl62−, a water-soluble, one-electron oxidant with a redox potential of 0.9 V vs NHE [38]. IrCl62− and IrBr62− are capable of facile oxidation of 8-oxo-G and of slow oxidation of G. In the presence of cobalt (II) or nickel (II), KHSO5 (oxone) causes site-specific guanine oxidation [39–41]. The decomposition of KHSO5 catalyzed by these metals generates SO4·− that appears to be responsible for the oxidation of guanine [39]. CoCl2/KHSO5 generates highly diffusible sulfate radicals that induce guanine-specific modification in bulges, loops and single-stranded DNA [33].
It has been reported that simple nickel salts in the presence of H2O2 result in the formation of 8-oxo-G as the oxidation product [42, 43]. Certain four-coordinate, square-planar nickel(II) complexes demonstrate nickel binding to chromatin and have been studied for their toxicity and carcinogenicity [44, 45]. These complexes can facilitate oxidation of DNA causing strand breaks, oxidative base damage and DNA-protein cross-links [46, 47, 48]. Peptide ligands such as glycylglycylhistidine coordinate to nickel (II) and enhance the metal reactivity by stabilizing the +III oxidation state of the metal, thereby promoting guanine oxidation [49–51]. Particularly in the case of Ni(II), the ligand surrounding the metal and the redox properties of the metal play an important role in the determination of the oxidation pathway. Nickel tetraazamacrocycles that possess strong in-plane donor ligands and provide vacant coordination sites mediate oxidative damage of guanine with peracids like KHSO5 [48].
NiCR (Figure 2), unlike nickel peptides, does not react in the minor groove under low salt concentrations [52], but instead incurs base damage at guanines that are solvent accessible. Hence, NiCR/KHSO5 acts as a probe of guanine exposure in determining the three-dimensional folded structure of DNA and RNA [53, 54, 55]. NiCR is thought to prefer oxidation at exposed guanines by binding to the most basic site, N7 in guanine, thus delivering the oxidant to the exposed base via direct metal coordination [34, 56]. The intermediate is proposed to be an octahedral nickel(III)-bound sulphate radical in which the two additional ligands, guanine and sulfate, are cis coordinated (Scheme 3) [46]. Subsequent reductive elimination of these groups results in an oxidized G in DNA that leads to strand scission upon treatment with hot piperidine [34, 48, 57]. The Co(II)/KHSO5 oxidation system has somewhat less specific requirements for guanine exposure suggesting the generation of a free SO4·− species induced by CoCl2 as compared to nickel-coordinated SO4·− in the case of NiCR [33].
Figure 2.

Structure of NiCR
Scheme 3.
Proposed binding of NiCR/KHSO5 to guanine and formation of 2-Ih
Square-planar nickel peptide complexes, NiIIGly-Gly-His and NiIIArg-Gly-His, appear to generate a similar reactive complex with sulfate radical via autoxidation of sulfite. In this mechanism, nickel(II) catalyzes the formation of HSO5− via autoxidation of HSO3− [51,52, 57, 58]. Curiously, the product of G oxidation by the NiIIGly-Gly-His/HSO3−/O2 system appears to be 8-oxo-G [56], which has not been observed from G oxidation by the NiCR/KHSO5 system.
Despite its popularity as a structural tool, the NiCR/KHSO5 system remained elusive in terms of its eventual chemistry with guanine. The final product formed by G oxidation in oligodeoxynucleotides with NiCR/KHSO5 had a mass that was higher than the starting material by 34 amu, although a transient product of mass G+16 could also be detected [59]. The new compound, G+34, is proposed to be the result of two oxygen atoms being incorporated from H2O, based on 18O labeling studies in our laboratory [59]. Three other laboratories have observed this same mass increase: Pratviel, Meunier and coworkers studied oxidation of guanine by the porphyrin complex Mn-TMPyP/KHSO5 and also found a minor product that displayed a mass increase of 34 [25, 60, 61]. It was suggested that the compound arises from hydroxylation at C5 of a (G-H)+ intermediate, leading to an unstable compound with one labeled oxygen atom from H218O added at C5. This intermediate was expected to rearrange at physiological pH into an Sp-like structure (Scheme 3) that would be sensitive to hydrolytic ring opening [62]. The Rokita laboratory also found the mass of G+34 as the major product of DNA oxidation with a dinuclear copper complex, [Cu2II (PD′O)(H2O)2]3+ where PD′OH is a pyridylalkylamine containing binucleating ligand [63]. More recently, epoxidation of guanine by dimethyldioxirane (DMDO) was studied by Ye, et al. This two-electron oxidation yields 5-carboxamido-5-formamido-2-iminohydantoin (2-Ih) as the only characterized product, that also has a mass of G+34 explaining the products observed from Mn-TMPyP/KHSO5, [Cu2II (PD′O)(H2O)2]3+ and NiCR/KHSO5 [64, 65].
In this work, we studied the oxidation chemistry of both a suitably protected nucleoside, namely 2′,3′,5′-tri-O-acetylguanosine, and a 15-mer of single-stranded DNA containing a single 2′-deoxyguanosine site with various oxidants, including NiCR/KHSO5, and the results were compared. Under specific conditions studied, oxidation of guanosine occurred to give 2-Ih as the only isolable oxidation product in both the nucleoside and ssDNA substrates. Hence, 2-Ih may be considered as a primary DNA lesion generated by several transition metal oxidants.
2. Experimental Procedures
2.1 Materials
Guanosine hydrate, KHSO5 (oxone), CoCl2, Na2IrCl6, Rose Bengal, K2S2O8, EDTA, HEPES, sodium chloride and sodium phosphate were purchased from commercially available sources. NiCR was synthesized by the method of Karn and Busch [66]. The oligodeoxynucleotide was obtained from the DNA Core facility of the University of Utah and was 5′-end labeled using T4 polynucleotide kinase and [γ-32P] ATP. Radioactivity was quantified using a Beckman LS6500 scintillation counter.
2.2 Instrumentation
ESI mass spectra were obtained on a Micromass Quattro II spectrometer. HPLC analyses of nucleoside reactions were analyzed on a Beckman System Gold 126NM solvent module attached to a Beckman 168NM diode array detector with a Varian C-18 analytical reversed-phase column (5 μm, 250 mm × 4.6 mm) or Phenomenex Synergi polar reversed-phase column (4 μm, 250 mm × 4.6 mm). All solvents were HPLC-grade and were filtered and sonicated before use. All aqueous solutions were prepared from purified water (Nanopure, Synbron/Barnsted).
The oligodeoxynucleotide reactions were HPLC analyzed using a Beckman System Gold 126NM solvent module attached to a Beckman 166NM diode array detector running a Dionex DNAPac PA-100 analytical ion-exchange column (4 mm × 250 mm).
Synthesis of 2′,3′,5′-tri-O-acetyl-guanosine (OAc)3G was carried out according to the previously published procedure [67, 68].
2.3 Oxidation of (OAc)3G with various oxidants
2.3.1.1 Oxidation with NiCR/KHSO5, Condition A
3 mM (OAc)3G was incubated with 12 μM NiCR and 4 mM KHSO5 in 75 mM sodium phosphate (NaPi) buffer at pH 7.4 and 37 °C. After 30 min the reaction was quenched with 40 mM HEPES. The reaction mixture was analyzed by HPLC using a polar reversed-phase column with a linear gradient of 1% solvent B to 70% solvent B in 65 min at 1mL/min at 245nm. Solvent A was 0.1% aqueous CF3COOH (TFA); solvent B was 0.1% TFA in CH3CN.
2.3.1.2 Oxidation with NiCR/KHSO5, Condition B
3 mM (OAc)3G was incubated with 60 μM NiCR and 4 mM KHSO5 added in intervals every 3 min in 75 mM NaPi at pH 7.4 and 37 °C. The reaction was quenched with 40 mM HEPES after 30 min. HPLC analysis was conducted as described above.
2.3.2 Oxidation with CoCl2/KHSO5
3 mM (OAc)3G was incubated with 12 μM CoCl2 and 4 mM KHSO5 in 75 mM NaPi at pH 7.4 and 37 °C for 30 min and the reaction was quenched with 40 mM HEPES. HPLC analysis was conducted as described above.
2.3.3 Oxidation with Na2IrCl6
3 mM (OAc)3G was treated with 6 mM Na2IrCl6 in 75 mM NaPi at pH 7.4 and 37 °C and was quenched with 60 mM EDTA after 30 min. HPLC analysis was conducted as described above.
2.3.4 Oxidation with singlet oxygen
3 mM (OAc)3G was incubated with 300 μM RB in 75 mM NaPi at pH 7.4 and was irradiated by a sunlamp emitting at 360 nm for 30 min at 37 °C. The RB was removed by passing the reaction mixture through a Nap-25 column following the manufacturer’s protocol. The fractions collected were analyzed by HPLC as described above.
2.3.5 Oxidation with K2S2O8
3 mM (OAc)3G was treated with 70 mM K2S2O8 in pH 7.4 and 75 mM NaPi buffer under 256 nm light for 30 min at a distance of 3 cm. HPLC analysis was conducted as described above.
2.4 Oxidation and piperidine-induced cleavage of an oligodeoxynucleotide
The oligodeoxynucleotide sequence used was a 15-mer with a single guanine at the eighth position, 5′-d(TTT TTT TGT TTT TTT)-3′ (5′-T7GT7-3′). Oxidation reactions were carried out on 5 μM oligodeoxynucleotide, 5 μM NiCR with different concentrations of KHSO5 ranging from 5 – 500 μM in 10 mM NaPi and 100 mM NaCl (pH 7.4). Reactions were quenched with 50 mM HEPES after 30 min. Alkaline cleavage reactions were carried out in 0.4 M piperidine at 90 °C for 30 min. Finally, the smaller DNA fragments were separated by PAGE (20% polyacrylamide/7 M urea) and visualized by storage phosphor autoradiography (Molecular Dynamics Storm 840) and quantified with Image QuaNT version 5.2.
2.4.1 Oxidation with NiCR/KHSO5
5 μM oligodeoxynucleotide was incubated with 5 μM NiCR and then allowed to react with 250 μM KHSO5 in 10 mM NaPi and 100 mM NaCl buffer of pH 7.4 at 37 °C for 30 min.
2.4.2 Oxidation with Na2IrCl6
5 μM oligodeoxynucleotide was allowed to react with 750 μM Na2IrCl6 in 10 mM NaPi and 100 mM NaCl buffer at pH 7.4 at 37 °C for 30 min.
2.4.3 Oxidation with Singlet Oxygen
5 μM oligodeoxynucleotide was incubated with 300 μM RB and irradiated with sunlamp in 10 mM NaPi and 100 mM NaCl buffer of pH 7.4 at 37 °C for 30 min. All the above reactions were analyzed by HPLC using ion-exchange column with gradient of 15 % solvent B to 100 % solvent B in 30 min at a flow rate of 1mL/min. Solvent A was 10% CH3CN; solvent B was 1.5 M sodium acetate at pH 7 and 10% CH3CN. The chromatogram was recorded at 260 nm.
3. Results and Discussion
3.1 Oxidation of guanosine nucleoside
The transition metal complex Na2IrCl6 is a water-soluble, outer-sphere, one-electron oxidant that does not bind to nucleosides or DNA [38], and it provides a convenient reference point for the stepwise one-electron oxidation pathway of G. In this study, the oxidation of (OAc)3G by Ir(IV) resulted in the formation of Sp at pH 7.4. CoCl2 exists as [Co(H2O)6]2+ in aqueous solution, and it is able to effect the decomposition of KHSO5 yielding the potent oxidant sulfate radical. The freely diffusible SO4·−results in guanine oxidation also yielding Sp as the major product at pH 7.4, along with other oxidation products such as Gh and Iz as minor products. In contrast, two roles for the metal ion have been proposed for the square-planar complex NiCR; one is a redox role in which formation of a higher oxidation state is part of the catalytic cycle. The other possible role is that of Ni(II) acting as a Lewis acid for activation of a peracid towards oxidative attack on guanine. It is well documented that Ni(II) ions are able to bind to the N7 of guanine [68]. The intermediate proposed for NiCR is an octahedral Ni(III) species in which the two additional ligands are a guanine and an oxidant monopersulfate and are cis coordinated to nickel.
In the present studies, the oxidation of (OAc)3G with NiCR/KHSO5 yielded a mixture of oxidation products that are well separated on a polar reversed-phase HPLC column (Figure 3). The products 2-Ih, Sp and Gh each exist as two diastereomers. LC-ESI+-MS allowed the identification of the products by comparing their retention times, UV-visible spectra and mass spectra. All products were quantified by integrating the HPLC peak areas that were normalized through each compound’s unique molar extinction coefficient (ε) at 245 nm (Table 2) [69]. The exception was 2-Ih for which the molar extinction coefficient (ε) is assumed to be similar to that of Sp.
Figure 3.
HPLC-chromatogram of (OAc)3G oxidation products with NiCR/KHSO5. The products are identified by LC-ESI+-MS. The oxidation products 2-Ih, Gh and Sp give 2 diastereomers. Apart from these the products Iz, Z and CAC have also been identified
Table 2.
Molar extinction coefficients for guanine oxidized bases
| Oxidized base | ε245 nm (M−1 cm−1) |
|---|---|
| Sp | 2.24 × 103 |
| Gh | 1.17 × 103 |
| Iz | 2.05 × 104 |
| Z | 6.00 × 103 |
| CAC | 2.00 × 103 |
| 2-Ih | 2.24 × 103 * |
( assumed similar to Sp)
Two factors were found to increase the formation of 2-Ih as a major product; the first was to increase the NiCR concentration which, on its own, had little effect. The second was additionally the titration of 500 μM of KHSO5 at regular intervals of every 3.75 min for a total reaction time of 30 min. Both in combination work to yield the 2-Ih product exclusively, and the two diastereomers were well separated on a polar reversed-phase column (Figure 4). The two peaks from the reaction of 3 mM (OAc)3G with 60 μM NiCR and 4 mM KHSO5 added in aliquots were collected to determine their mass. Both peaks in the chromatogram increased with increased NiCR concentration, and titration of KHSO5 at regular intervals exclusively yields the compound with mass of 443.9, which we assign as diastereomers of the 2-Ih structure.
Figure 4.
HPLC-chromatogram of (OAc)3G oxidation by increased NiCR concentration and KHSO5 titration
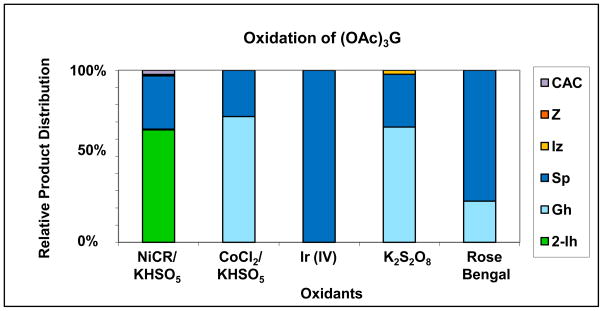
In contrast to the Ni(II) peptide/HSO3−/O2 system [56], no formation of 8-oxo-G was detected using either CoCl2/KHSO5 or NiCR/KHSO5 at pH 7.4. Furthermore, homolytic cleavage of K2S2O8 by photolysis, which generates a clean source of sulphate radical, was also studied, and it resulted in the oxidation of (OAc)3G to yield 70% Sp, 25% Gh and 5% Iz. Similarly, singlet oxygen yielded 85% Sp and 25% Gh as the oxidation products of (OAc)3G. Figure 5 compares the relative product distribution for the oxidation of (OAc)3G with the various oxidants.
Figure 5.
Relative product distribution of (OAc)3G oxidation by various oxidants
3.2 Oxidation of 5′-T7GT7-3′ oligodeoxynucleotide
Oxidation of a 15-mer oligodeoxynucleotide followed by piperidine treatment resulted in exclusive cleavage of the strand at the G site, as analyzed by polyacrylamide gel electrophoresis (Figure 6). Lanes 1–6 show the oxidation of 5 μM 5′-T7GT7-3′ by 5 μM NiCR with increasing concentrations of KHSO5 from 5 μM to 500 μM. Lane 7 shows a control study without addition of the oxidant. Lane 8 is the Maxam-Gilbert G-lane that confirms the location of the G [70].
Figure 6.
Gel analysis for oxidation and piperidine-induced cleavage of 5′-T7GT7-3′ oligodeoxynucleotide. All lanes are piperidine treated. Lanes 1–6: oxidation reactions with 5 μM oligodeoxynucleotide, 5 μM NiCR and increasing concentration of KHSO5 with lane 1 through 6 as 5, 25, 50, 125, 250 and 500 μM, respectively. Lane 7: control lane with 5 μM of 5′-T7GT7-3′oligodeoxynucleotide. Lane 8: Maxam-Gilbert G-lane
The reaction of the 15-mer oligodeoxynucleotide with NiCR/KHSO5 was also analyzed on an ion-exchange HPLC column followed by ESI−-MS analysis. The expected mass for the 15-mer starting material was 4526, however, the observed mass was 4524. The expected mass for the product was 4560 and the observed mass was 4558. The mass analysis indicated a gain in 34 amu on conversion to product, and we therefore assign 2-Ih as the major product of NiCR/KHSO5 oxidation in the oligonucleotide (Figure 7). This result is consistent with the nucleoside study whose mass spectra could be analyzed at higher resolution. (See supplementary information.)
Figure 7.
HPLC trace for oxidation of 15-mer oligodeoxynucleotide with NiCR/KHSO5. The HPLC- chromatogram represents a 15-mer standard (1) and reaction of NiCR/KHSO5 with 15-mer (2)
When the same 15-mer was oxidized by Na2IrCl6 or singlet oxygen at pH 7.4, Sp was the main product according to ESI--MS. The expected mass for the 15-mer starting material was 4526 and observed mass was 4526. The oxidation product expected mass was 4558 for Sp, if the chemistry is the same as the nucleoside, and the observed mass was 4559; this result is most consistent with Sp. The relative product distribution for oxidation of 15-mer with Na2IrCl6, 1O2 and NiCR/KHSO5 is shown in Figure 8. The oxidation of 15-mer with Na2IrCl6 and singlet oxygen yields Sp whereas NiCR/KHSO5 oxidation exclusively yields 2-Ih as the major product.
Figure 8.
Relative product distribution of oxidation of T14G with Na2IrCl6, Rose Bengal and NiCR/KHSO5
4. Conclusions
The oxidation of (OAc)3G yields a wide array of products that follow different oxidation pathways. Some oxidants follow the C8 oxidation pathway and lead to formation of Sp and Gh, with 8-oxo-G, the most common oxidation product, as a possible intermediate. On the other hand, the C5 pathway leads to the formation of Iz/Z when perhydroxylation occurs at C5, and 2-Ih when hydroxylation occurs at C5. Transition metals play an important role in formation of the oxidation products depending on the coordination of the oxidant to the metal. The oxidation of the (OAc)3G with NiCR/KHSO5 can yield a wide range of oxidation products with two diastereomers each of Sp, Gh and 2-Ih. Since no reducing or anaerobic conditions are used, the product FaPy-G is not observed. 2-Ih was formed as the only oxidation product in (OAc)3G under conditions in which the concentration of NiCR was increased and KHSO5 was added in aliquots. The exact mechanism for this is still not clear, but we propose a cis-coordinated, octahedral Ni complex with both sulphate radical and G-N7 bound to the metal ion. Hydroxylation of C5 of G is then followed by an acyl ring shift providing a reduced form of Sp (Spred). Hydrolysis of the unstable amidine ring of Spred then leads to 2-Ih.
The oxidation of a 15-mer single-stranded DNA containing a single G site also results exclusively in the formation of 2-Ih. The NiCR complex is a probe for the structure of DNA and the previously unknown product from the piperidine cleavage site with a mass of M+34 can now be confirmed as the oxidation product 2-Ih. Thus, we conclude that 2-Ih is a two-electron oxidation product, whereas Sp and Gh are four-electron oxidation products of G. The Spred heterocycle has the same mass as 8-oxo-G (G+16), but it is readily hydrolyzed to the final observed G+34 product. Although the hydrolysis could in principle lead to either of two possible structures, (a) and (b) as shown in Figure 9, the structure (a) has been confirmed as the correct structure for 2-Ih by Ye and coworkers [64]. The formation of 2-Ih as the major product in nucleoside and ssDNA in the presence of metals suggests it may be a more widespread lesion from oxidative damage than originally appreciated.
Figure 9.

2-Ih hydrolyses products of Spred
Supplementary Material
Acknowledgments
Various parts of this work were supported by grants from the National Science Foundation (0809483) and the National Institutes of Health (CA090689). MAS was supported by NSF-REU grant no. CHE-0649039. CJB thanks Robert G. Bergman for 28+ years of advice and friendship.
Footnotes
Dedicated to Robert G. Bergman
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ames BN. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Kreutzer DA, Essigmann JM. Mutat Res. 1998;400:99–115. doi: 10.1016/s0027-5107(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 3.Ames BN, Shigenaga MK, Hagen TM. Proct Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Clarendon Press; Oxford: 1989. [Google Scholar]
- 5.Bjelland S, Seeberg E. Mutat Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Pouget JP, Douki T, Richard MJ, Cadet J. Chem Res Toxicol. 2000;13:541–549. doi: 10.1021/tx000020e. [DOI] [PubMed] [Google Scholar]
- 7.Malins DC, Haimanot R. Cancer Res. 1991;51:5430–5432. [PubMed] [Google Scholar]
- 8.Cadet J, Sage E, Douki T. Mutat Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Hailer MK, Slade PG, Martin BD, Sugden KD. Chem Res Toxicol. 2005;18:1378–1383. doi: 10.1021/tx0501379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matter B, Malejka-Giganti D, Csallany AS, Tretyakova N. Nucl Acids Res. 2006;34:5449–5460. doi: 10.1093/nar/gkl596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gedik CM, Collins AF. FASEB J. 2005;19:82–84. doi: 10.1096/fj.04-1767fje. [DOI] [PubMed] [Google Scholar]
- 12.Steenken S, Jovanovic SV, Bietti M, Bernhard K. J Am Chem Soc. 2000;122:2373–2374. [Google Scholar]
- 13.Candeias LP, Steenken S. J Am Chem Soc. 1989;111:1094–1099. [Google Scholar]
- 14.Steenken S. Chem Rev. 1989;89:503–520. [Google Scholar]
- 15.Stemp EDA, Arkin MR, Barton JK. J Am Chem Soc. 1997;119:2921–2925. [Google Scholar]
- 16.Schiemann O, Turro NJ, Barton JK. J Phys Chem. 2000;104:7214–7220. [Google Scholar]
- 17.Misiaszek R, Crean C, Joffe A, Geacintov NE, Shafirovich V. J Biol Chem. 2004;279:32106–32115. doi: 10.1074/jbc.M313904200. [DOI] [PubMed] [Google Scholar]
- 18.Cadet J, Berger M, Buchko GW, Joshi PC, Raoul S, Ravanat JL. J Am Chem Soc. 2002;116:7403–7404. [Google Scholar]
- 19.Gasparutto D, Ravanat JL, Gerot O, Cadet J. J Am Chem Soc. 1998;120:10283–10286. [Google Scholar]
- 20.Raoul S, Berger M, Buchko GW, Joshi PC, Morin B, Weinfeld M, Cadet J. J Chem Soc Perkin Trans. 1996;2(2):371–381. [Google Scholar]
- 21.Luo W, Muller JG, Burrows CJ. Org Lett. 2001;3:2801–2804. doi: 10.1021/ol0161763. [DOI] [PubMed] [Google Scholar]
- 22.Shafirovich V, Dourandin A, Huang W, Geacintov NE. J Biol Chem. 2001;276:24621–24626. doi: 10.1074/jbc.M101131200. [DOI] [PubMed] [Google Scholar]
- 23.Kasai H, Yamaizumi Z, Berger M, Cadet J. J Am Chem Soc. 2002;114:9692–9694. [Google Scholar]
- 24.Vialas C, Pratviel G, Claparols C, Meunier B. J Am Chem Soc. 1998;120:11548–11553. [Google Scholar]
- 25.Vialas C, Claparols C, Pratviel G, Meunier B. J Am Chem Soc. 2000;122:2157–2167. [Google Scholar]
- 26.Shukla LI, Adhikary A, Pazdro R, Becker D, Sevilla MD. Nucl Acids Res. 2004;32:6565–6574. doi: 10.1093/nar/gkh989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadet J, Douki T, Gasparutto D, Ravanat JL. Mutat Res. 2003:5–23. doi: 10.1016/j.mrfmmm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Luo W, Muller JG, Rachlin EM, Burrows CJ. Org Lett. 2000;2:613–616. doi: 10.1021/ol9913643. [DOI] [PubMed] [Google Scholar]
- 29.Luo W, Muller JG, Rachlin EM, Burrows CJ. Chem Res Toxicol. 2001;14:927–938. doi: 10.1021/tx010072j. [DOI] [PubMed] [Google Scholar]
- 30.Neeley WL, Essigmann JM. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd DR, Phillips DH, Carmichael PL. Chem Res Toxicol. 1997;10:393–400. doi: 10.1021/tx960158q. [DOI] [PubMed] [Google Scholar]
- 32.Foote CS. Photochem and Photobiol. 1991;54:659–659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 33.Muller JG, Zheng P, Rokita SE, Burrows CJ. J Am Chem Soc. 1996;118:2320–2325. [Google Scholar]
- 34.Chen, Rokita SE, Burrows CJ. J Am Chem Soc. 1991;113:5884–5886. [Google Scholar]
- 35.Burrows CJ, Muller JG. Chem Rev. 1998;98:1109–1152. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- 36.Steenken S, Jovanovic SV. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- 37.Ye Y, Muller JG, Luo W, Mayne CL, Shallop AJ, Jones RA, Burrows CJ. J Am Chem Soc. 2003;125:13926–13927. doi: 10.1021/ja0378660. [DOI] [PubMed] [Google Scholar]
- 38.Muller JM, Duarte V, Hickerson RP, Burrows CJ. Nucl Acids Res. 1998;26:2247–2249. doi: 10.1093/nar/26.9.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vieira AJSC, Steenken S. J Am Chem Soc. 2002;112:6986–6994. [Google Scholar]
- 40.Kamiya H, Miura H, Murata-Kamiya N, Ishikawa H, Sakaguchi T, Inoue H, Sasaki T, Masutanl C, Hanaoka F, Nishimura S, Ohtsuka E. Nucl Acids Res. 1995;23:2893–2899. doi: 10.1093/nar/23.15.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaruga P, Dizdaroglu M. Nucl Acids Res. 1996;24:1389–1394. doi: 10.1093/nar/24.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawanishi S, Inoue S, Yamamoto K. Carcinogenesis. 1989;10:2231–2235. doi: 10.1093/carcin/10.12.2231. [DOI] [PubMed] [Google Scholar]
- 43.Nackerdien Z, Kasprzak KS, Rao G, Halliwell B, Dizdaroglu M. Cancer Res. 1991;51:5837–5842. [PubMed] [Google Scholar]
- 44.Kasprzak KS. Chem Res Toxicol. 2002;4:604–615. doi: 10.1021/tx00024a002. [DOI] [PubMed] [Google Scholar]
- 45.Wetterhahn K. New J Chem. 1996;20:199. [Google Scholar]
- 46.Ciccarelli RB, Wetterhahn KE. Cancer Res. 1982;42:3544–3549. [PubMed] [Google Scholar]
- 47.Ciccarelli RB, Hampton TH, Jennette KW. Cancer Lett. 1981;12:349–354. doi: 10.1016/0304-3835(81)90178-6. [DOI] [PubMed] [Google Scholar]
- 48.Muller JG, Chen X, Dadiz AC, Rokita SE, Burrows CJ. J Am Chem Soc. 1992;114:6407–6411. [Google Scholar]
- 49.Inoue S. Biochem and Biophys Res Commun. 1989;159:445–451. doi: 10.1016/0006-291x(89)90012-0. [DOI] [PubMed] [Google Scholar]
- 50.Datta K, Datta AK. Sci Total Environ. 1994;148:207–216. doi: 10.1016/0048-9697(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 51.Bal W, Lukszo J, Kasprzak KS. Chem Res Toxicol. 1996;9:535–540. doi: 10.1021/tx950157i. [DOI] [PubMed] [Google Scholar]
- 52.Muller JG, Hickerson RP, Perez RJ, Burrows CJ. J Am Chem Soc. 1997;119:1501–1506. [Google Scholar]
- 53.Burrows CJ, Rokita SE. Acc Chem Res. 1994;27:295–301. [Google Scholar]
- 54.Chen X, Burrows CJ, Rokita SE. J Am Chem Soc. 1992;114:322–325. [Google Scholar]
- 55.Wurdeman RL, Douskey MC, Gold B. Nucl Acids Res. 1993;21:4975–4980. doi: 10.1093/nar/21.21.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin RB. Acc Chem Res. 2002;18:32–38. [Google Scholar]
- 57.Muller JM, Chen X, Dadiz AC, Rokita SE, Burrows CJ. Pure & Appl Chem. 1993;65:545–550. [Google Scholar]
- 58.Alipazaga MV, Moreno RGM, Linares E, Medeiros MHG, Coichev N. Dalton Trans. 2005:3738–3744. doi: 10.1039/b507216f. [DOI] [PubMed] [Google Scholar]
- 59.Luo W. PhD Dissertation. University of Utah; UT, SLC: 2001. [Google Scholar]
- 60.Lapi A, Pratviel G, Meunier B. Metal Based Drugs. 2001;8:47–56. doi: 10.1155/MBD.2001.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pratviel G, Meunier B. Chem Eur J. 2006;12:6018–6030. doi: 10.1002/chem.200600539. [DOI] [PubMed] [Google Scholar]
- 62.Kupan A, Saulière A, Broussy S, Seguy C, Pratviel G, Meunier B. ChemBioChem. 2006;7:125–133. doi: 10.1002/cbic.200500284. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Karlin KD, Rokita SE. J Am Chem Soc. 2005;127:520–521. doi: 10.1021/ja044209e. [DOI] [PubMed] [Google Scholar]
- 64.Ye W, Sangaiah R, Degen DE, Gold A, Jayaraj K, Koshlap KM, Boysen G, Williams J, Tomer KB, Ball LM. Chem Res Toxicol. 2006;19:506–510. doi: 10.1021/tx0600144. [DOI] [PubMed] [Google Scholar]
- 65.Ye W, Sangaiah R, Degen DE, Gold A, Jayaraj K, Koshlap KM, Boysen G, Williams J, Tomer KB, Mocanu V, Dicheva N, Parker CE, Schaaper RM, Ball LM. J Am Chem Soc. 2009;131:6114–6123. doi: 10.1021/ja8090752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karn JL, Busch DH. Nature. 1966;211:160–162. [Google Scholar]
- 67.Holmes RE, Robins RK. J Am Chem Soc. 1965;87:1772–1776. doi: 10.1021/ja01086a028. [DOI] [PubMed] [Google Scholar]
- 68.Matsuda A, Shinozaki M, Suzuki M, Watanabe K, Miyasaka T. Synthesis. 1986:385–386. [Google Scholar]
- 69.Suzuki T, Friesen MD, Ohshima H. Chem Res Toxicol. 2003;16:382–389. doi: 10.1021/tx025638y. [DOI] [PubMed] [Google Scholar]
- 70.Maxam AM, Gilbert W. Proc Natl Acad Sci USA. 1977;74:560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.