Abstract
Background
There is a need for a reliable and inexpensive noninvasive marker of hepatic fibrosis in nonalcoholic fatty liver disease (NAFLD).
Aim
To compare the performance of the FIB4 index (based on age, aspartate and alanine aminotransferase and platelet counts) with six other non-invasive markers of fibrosis in patients with NAFLD.
Methods
Using a nation-wide database of 541 adults with NAFLD, jackknife-validated areas under receiver operating characteristic curves (AUROC) of FIB4 and seven other markers were compared. The sensitivity at 90% specificity, 80% positive predictive value, and 90% negative predictive values were determined along with cutoffs for advanced fibrosis.
Results
The median FIB4 score was 1.11 (IQR=0.74–1.67). The jackknife-validated AUROC for FIB4 was 0.802 (95% CI: 0.758, 0.847) which was higher than that for the NAFLD fibrosis score (0.768 CI:0.720–0.816, p= 0.09), Goteburg University Cirrhosis Index (0.743, CI:0.695–0.791, p< 0.01), AST:ALT ratio (0.742, CI:0.690–0.794, p< 0.015), AST to platelet ratio index (0.730, CI:0.681–0.779, p< 0.001), AST to platelet ratio (0.720, 0.669–0.770, p< 0.001), BARD score (0.70, p< 0.001) and cirrhosis discriminant score (0.666, CI:0.614–0.718, p< 0.001). For a fixed specificity of 90% (FIB4 = 1.93), the sensitivity of identifying advanced fibrosis was only 50% (95% CI: 46, 55). A FIB4 ≥ 2.67 had an 80% positive predictive value and a FIB4 index ≤ 1.30 had a 90% negative predictive value.
Conclusions
The FIB4 index is superior to seven other non-invasive markers of fibrosis in patients with NAFLD; however its performance characteristics highlight the need for even better non-invasive markers.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease in North America. The clinical-histological phenotype of NAFLD extends from a nonalcoholic fatty liver (NAFL) alone to nonalcoholic steatohepatitis (NASH) 1. About 30% of the general population has NAFLD and up to 5% of the population has NASH 2–4. Approximately 15% and 5% percent of those with NASH and NAFL respectively will progress to cirrhosis 5, 6. It is thus estimated that about 6 million individuals in the United States are at risk for development of cirrhosis from NAFLD over the next two decades.
A liver biopsy is currently the gold standard for the staging of NAFLD. Liver biopsies suffer several shortcomings including their invasive nature, association with discomfort, potential risks including rare deaths, and sampling variability 7, 8. It is also unlikely that there will be enough medical manpower available to perform a liver biopsy on all subjects with NASH. These considerations underscore the need for simple noninvasive methods for assessing fibrosis.
Numerous noninvasive panels of tests have been developed to stage liver disease 9. These include a combination of clinical and routine laboratory parameters as well as specialized tests such as direct markers of fibrosis and elastography 10–17. Of these, the BAAT (BMI, age, ALT, triglycerides), European liver fibrosis (ELF) score, Fibrotest, Fibroscan, hyaluronic acid, BARD (BMI, AST:ALT, Diabetes), NASH score and the NAFLD fibrosis score have been tested in subjects with NAFLD 10, 11, 14,16, 18–21. Several of these require additional tests which incur costs. Other tests have not been validated in other liver diseases. Therefore, there is still a need for a simple and inexpensive/cost-free measure of hepatic fibrosis which can be used across many liver diseases.
The FIB4 index was developed as a noninvasive panel to stage liver disease in subjects with HIV-hepatitis C virus (HCV) co-infection 22 It relies on the age, aspartate- and aminotransferase levels and the platelet count, which are routinely measured and available for virtually all subjects with liver disease. This index has been independently validated in subjects with HCV infection alone as well 23. The objective of the current study was to evaluate the utility of the FIB4 index as a marker of advanced fibrosis (bridging fibrosis or cirrhosis) in NAFLD and compare it to existing noninvasive panels that do not require additional laboratory testing.
MATERIALS AND METHODS
Study Population
The study population consisted of subjects with histologically proven NAFLD who were enrolled in the NIH NASH Clinical Research Network (CRN). The NASH CRN has three sets of subjects: (1) those enrolled in a natural history database, (2) those enrolled in a randomized clinical trial of pioglitazone or vitamin E versus placebo (PIVENS) in adults, and (3) a randomized clinical trial of metformin or vitamin E versus placebo (TONIC) in pediatric subjects with NAFLD. The current study included baseline data from the first two groups of subjects. Also, only those subjects with a complete available data set were included.
Pediatric subjects were excluded from this analysis because the pattern of fibrosis in children sometimes varies from that in adults with NASH 24. Also, subjects with other concomitant causes of liver disease e.g. hepatitis B or C, hemochromatosis, Wilson disease, a1-antitrypsin deficiency, primary biliary cirrhosis etc were excluded. In subjects with a positive anti-nuclear antibody test, the presence of piecemeal necrosis or other histologic features of autoimmune hepatitis as well as hypergammaglobulinemia were considered to be exclusionary criteria. Alcohol use was assessed both by the alcohol use disorders identification test (AUDIT) and lifetime alcohol consumption (Alcohol time-line follow back) 25–27. The nonalcoholic nature of the disease was established by an alcohol consumption level of < 20 gm/day for women and < 30 gm/day for men over at least 5 years prior to entry in to the database.
Laboratory and Clinical parameters
These were obtained from the data closest to the liver biopsy at the time of entry into the NASH CRN. The following data were obtained for each patient: gender, age at liver biopsy, height, weight, hemoglobin, WBC, platelets, total bilirubin, direct bilirubin, AST, ALT, alkaline phosphatase, albumin, INR, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, glucose, insulin, BMI, presence or absence of HTN and Type 2 diabetes, and liver histology.
Liver Histology
All patients in this dataset had a liver biopsy in the 12 months prior to enrollment. If a patient had multiple biopsies during that 12 month period, the biopsy closest to the time of enrollment was selected. Liver histology was assessed and scored by the Pathology Committee of the NASH CRN in a blinded manner. Fatty liver was defined as the presence of ≥ 5% steatosis while steatohepatitis was diagnosed by steatosis, inflammation and ballooning 28. Hepatic fibrosis was assessed from trichrome-stained sections which were all performed in a central laboratory. The individual parameters of NASH histology including fibrosis were scored independently using the NASH CRN scoring system that was developed by the NASH CRN 28. Advanced fibrosis was classified as those with stage 3 or 4 disease (bridging fibrosis or cirrhosis).
Selection of noninvasive panels that did not require specialized testing
A Pubmed search was performed for noninvasive markers of hepatic fibrosis that did not require additional testing. Based on the review of the literature, the following scores were calculated for each patient: FIB4 22, AST/ALT ratio, Cirrhosis Determinant Score 29 AST/Platelet ratio 30, Goteburg University Cirrhosis Index 31, AST to Platelet Ratio Index 32, BARD score 33 and NAFLD fibrosis score 16. The values for the upper limit of normal were set according to the International Federation of Clinical Chemistry: AST 35 U/L for men, 30 U/L for women, and were comparable to the values used in other analyses 34. The specific formulae used to determine these scores are shown in Table 1.
Table 1.
Formulae of noninvasive panels for detection of fibrosis used for the study
| Formula | Equation | ||
|---|---|---|---|
| AST to ALT Ratio (AAR) | AST/ALT | ||
| AST to Platelet Ratio | AST/Platelet Count × 100 | ||
| AST to Platelet Ratio Index (APRI) | [(AST/upper limit of normal)/Platelet Count (109/l)] × 100 | ||
| Cirrhosis Discriminant Score (CDS) | Platelet count | AST/ALT ratio | INR |
| >340=0 | >1.7=0 | <1.1=0 | |
| 280–339=1 | 1.2–1.7=1 | 1.1–1.4=1 | |
| 220–279=2 | 0.6–1.19=2 | >1.4=2 | |
| 160–219=3 | <0.6=3 | ||
| 100–159=4 | Score is the sum of three (0–11) |
||
| 40–99=5 | |||
| <40=6 | |||
| FIB4 Index | (Age[years] × AST[U/L])/(platelet [109] X√ALT[U/L]) | ||
| NAFLD Fibrosis Score | −1.675 + (0.037 × Age[years]) + (0.094 × BMI) + (1.13 × IFG/diabetes [yes=1, no=0]) + (0.99 × AST/ALT) - (0.013 × platelet[109/l]) - (0.66 X albumin[g/dl]) |
||
| Goteborg University Cirrhosis Index (GUCI) | (AST/ Upper limit of normal ) × INR × 100(Platelet Count) | ||
| BARD score | Scale 0–4 | ||
| BMI ≥ 28 kg/m2 = 1 point | |||
| AST to ALT Ratio ≥ 0.8 = 2 points | |||
| Diabetes mellitus = 1 point | |||
ULN for AST: 30 in women, 35 in men
Plan of analysis
Characterization of the cohort
Descriptive statistics were obtained to characterize the cohort and are described in Table 2 and Table 3.
Table 2.
Characteristics of Population by Histologic Status*
| Non-NASH |
NASH |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Fibrosis Stage 0–2 N=200 |
Fibrosis Stage 3–4 N=24 |
Total N=224 |
Fibrosis Stage 0–2 N=216 |
Fibrosis Stage 3–4 N=101 |
Total N=317 |
p- value§ |
| Age (yrs) | 46 ± 12 | 55 ± 7 | 47 ± 12 | 46 ± 12 | 53 ± 11 | 48 ± 12 | <0.001 |
| Males | 94 (47) | 10 (42) | 104 (46) | 86 (40) | 26 (26) | 112 (35) | 0.005 |
| Caucasian | 142 (71) | 20 (83) | 162 (72) | 160 (74) | 80 (79) | 240 (76) | 0.112 |
| BMI (kg/m2) | 34 ± 6.4 | 36 ± 7.6 | 34 ± 6.6 | 34 ± 6.4 | 35 ± 5.6 | 34 ± 6.1 | 0.683 |
| Hypertension | 78 (39) | 10 (42) | 88 (39) | 92 (43) | 59 (58) | 151 (48) | 0.013 |
| Type 2 diabetes | 29 (15) | 7 (29) | 36 (16) | 40 (19) | 29 (29) | 69 (22) | 0.017 |
| Hemoglobin (g/dl) | 15 ± 1.3 | 14 ± 1.3 | 15 ± 1.3 | 15 ± 1.5 | 14 ± 1.5 | 14 ± 1.5 | 0.017 |
| WBC (×109/l) | 7.0 ± 1.9 | 5.8 ± 1.9 | 6.8 ± 2.0 | 7.1 ± 2.1 | 6.8 ± 2.1 | 7.0 ± 2.1 | 0.970 |
| Platelet (×109/l) | 253 ± 67 | 166 ± 59 | 244 ± 71 | 253 ± 62 | 208 ± 72 | 239 ± 69 | <0.001 |
| AST (IU/l) | 35 | 46 | 36 | 50 | 56 | 53 | <0.001 |
| (27–49) | (35–57) | (28–50) | (36–73) | (42–79) | (37–74) | ||
| ALT (IU/l) | 57 | 47 | 57 | 74 | 62 | 68 | <0.001 |
| (36–86) | (34–75) | (35–83) | (49–111) | (45–96) | (47–103) | ||
| Alkaline | 76 | 100 | 78 | 79 | 91 | 82 | <0.001 |
| Phosphatase (IU/l) | (62–95) | (71–115) | (63–100) | (65–96) | (70–118) | (67–103) | |
| Bilirubin (mg/dl) | 0.7 | 0.8 | 0.7 | 0.6 | 0.7 | 0.6 | 0.076 |
| (0.5–0.9) | (0.6–1.2) | (0.5–1.0) | (0.5–0.9) | (0.5–0.9) | (0.5–0.9) | ||
| Albumin (g/dl) | 4.2 ± 0.4 | 4.0 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.4 | 0.724 |
| INR | 1.00 ± 0.18 | 1.09 ± 0.12 | 1.01 ± 0.17 | 1.01 ± 0.12 | 1.05 ± 0.12 | 1.02 ± 0.12 | 0.019 |
| Fasting glucose | 95 | 96 | 96 | 96 | 100 | 97 | 0.341 |
| (mg/dl) | (87–109) | (87–112) | (87–110) | (86–108) | (87–118) | (86–111) | |
| Fasting insulin | 16 | 25 | 17 | 17 | 24 | 20 | <0.001 |
| (µU/ml) | (11–23) | (17–37) | (11–24) | (12–26) | (16–40) | (13–30) | |
| Triglycerides | 140 | 110 | 137 | 158 | 143 | 153 | 0.008 |
| (mg/dl) | (100–203) | (92–163) | (100–200) | (112–238) | (105–179) | (109–218) | |
| LDL-cholesterol | 123 ± 34 | 97 ± 30 | 120 ± 34 | 123 ± 34 | 111 ± 33 | 119 ± 34 | 0.056 |
| (mg/dl) | |||||||
| HDL-cholesterol | 44 ± 11 | 48 ± 14 | 44 ± 12 | 43 ± 12 | 43 ± 12 | 43 ± 12 | 0.715 |
| (mg/dl) | |||||||
| Biopsy length | 18 ± 8.7 | 17 ± 8.1 | 18 ± 8.7 | 20 ± 9.3 | 21 ± 9.9 | 20 ± 9.5 | 0.001 |
| (mm) | |||||||
| Steatohepatitis | 0 (0) | 0 (0) | 0 (0) | 216 (100) | 101 (100) | 317 (100) | n/a |
Values are means ± SD, medians (IQR), or counts (%), as appropriate
P values from univariate ordinal logistic regression, Mann-Whitney, or chi-square test, as appropriate
Non-Hispanic white
Table 3.
Characteristics of Population*
| Variable | Fibrosis Stage 0–2 N= 416† |
Fibrosis Stage 3–4 N= 125‡ |
Total N= 541 |
P value§ |
|---|---|---|---|---|
| Age (yrs) | 46 ± 12 | 53 ± 11 | 48 ± 12 | <0.001 |
| Males | 180 (43) | 36 (29) | 216 (40) | 0.004 |
| Caucasian ¶ | 302 (73) | 100 (80) | 402 (74) | 0.105 |
| BMI (kg/m2) | 34 ± 6.4 | 35 ± 6.0 | 34 ± 6.3 | 0.335 |
| Hypertension | 170 (41) | 69 (55) | 239 (44) | 0.005 |
| Type 2 diabetes | 69 (17) | 36 (29) | 105 (19) | 0.003 |
| Hemoglobin (g/dl) | 15 ± 1.4 | 14 ± 1.4 | 14 ± 1.4 | 0.002 |
| WBC (×109/l) | 7.1 ± 2.0 | 6.6 ± 2.1 | 7.0 ± 2.1 | 0.028 |
| Platelet (×109/l) | 253 ± 64 | 200 ± 72 | 241 ± 70 | <0.001 |
| AST (IU/l) | 41 (30–61) | 54 (40–76) | 43 (32–64) | <0.001 |
| ALT (IU/l) | 64 (42–97) | 58 (40–94) | 63 (42–96) | 0.316 |
| Alkaline Phosphatase (IU/l) | 78 (64–96) | 92 (70–117) | 80 (66–101) | <0.001 |
| Bilirubin (mg/dl) | 0.7 (0.5–0.9) | 0.7 (0.5–1.0) | 0.7 (0.5–0.9) | 0.337 |
| Albumin (g/dl) | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.4 | 0.004 |
| I.N.R. | 1.01 ± 0.15 | 1.06 ± 0.12 | 1.02 ± 0.15 | <0.001 |
| Fasting glucose (mg/dl) | 95 (87–109) | 99 (87–115) | 96 (87–110) | 0.113 |
| Fasting insulin (µU/ml) | 17 (12–25) | 24 (16–38) | 19 (12–29) | <0.001 |
| Triglycerides (mg/dl) | 151 (105–217) | 139 (104–178) | 148 (105–207) | 0.067 |
| LDL-cholesterol (mg/dl) | 123 ± 34 | 108 ± 33 | 120 ± 34 | <0.001 |
| HDL-cholesterol (mg/dl) | 44 ± 12 | 44 ± 13 | 44 ± 12 | 0.517 |
| Biopsy length (mm) | 19 ± 9.1 | 20 ± 9.7 | 19 ± 9.2 | 0.167 |
| Steatohepatitis | 216 (52) | 101 (81) | 317 (59) | <0.001 |
Values are means ± SD, medians (IQR), or counts (%), as appropriate
Stage 0 (n=140), stage 1 (n=159), stage 2 (n=117)
Stage 3 (n=85), stage 4 (n=40)
P values from t-test, Mann-Whitney or chi-square test, as appropriate
Non-Hispanic white
ROC Curves
To determine the clinical utility for detecting fibrosis, Receiver Operating Characteristic (ROC) curves were developed for each of the non-invasive scoring systems. From a clinical perspective, an important goal of a noninvasive marker is to identify those with a high probability of having advanced fibrosis. Therefore, stages 3 and 4 were considered positive, while stages 0 to 2 were negative. The ROC curves were subsequently superimposed, and the statistical significance between the curves was examined 35. Positive and negative predictive values were obtained for each point on the FIB4 ROC curve. These were used to determine a lower cutoff point to detect those without advanced fibrosis and an upper cutoff point to detect those with advanced fibrosis. Alternatively, in order to evaluate the utility of the test to identify those with none to minimal fibrosis, additional analyses where stages 0–1 were considered to be positive were performed. Separate analyses comparing the utility of this test in those with fatty liver versus steatohepatitis and in those who had a liver biopsy within the previous 6 months versus 6–12 months were also performed.
Validation analysis
A prediction model validation was performed using a leave-one-out cross validation procedure, or “jackknife” 36–38. For this procedure, the model was fit once for each observation in the dataset (each time omitting a different observation) and used to predict the omitted observation. The AUROC from all of these models was then computed. Split sample (50:50 and 80:20) validations were also performed by randomly selecting a portion of the observations to estimate the model and then applying it to the remaining observations. Each validation procedure was run 5 times and the average AUROC reported.
RESULTS
A total of 541 subjects were included for this analysis. Of these, 325 (60%) were women and 400 (74%) were Caucasian (Table 2); 105 (19%) had type 2 diabetes mellitus and 239 (44%) were hypertensive. A total of 317 subjects had steatohepatitis; of these, 101 subjects had advanced fibrosis. A total of 224 subjects who had fatty liver disease but did not meet the NASH CRN definition of steatohepatitis were also included. 24 of these 224 subjects had advanced fibrosis. As expected, subjects with more advanced fibrosis were older, more likely to have diabetes, higher AST but lower ALT levels and higher alkaline phosphatase. The median (IQR) FIB-4 score was 1.11 (0.74–1.67) (Table 3). Of the individual components of the FIB-4 score, the mean (± S.D.) or median (IQR) values were as follows: age (48±12 yrs), AST (43, 32–64 IU/L), ALT (63, 42–96 IU/L) and platelets (241±70 × 1000 cells/mm3) (Table 4).
Table 4.
| Fibrosis Panel | Fibrosis Stage 0–2 N= 416 |
Fibrosis Stage 3–4 N= 125 |
Total N= 541 |
|---|---|---|---|
| FIB4 Index | 0.97 (0.68–1.37) | 1.98 (1.28–3.08) | 1.11 (0.74–1.67) |
| NAFLD Fibrosis Score | 1.68 (2.56–0.73) | 0.11 (1.19–0.85) | 1.38 (2.37–0.29) |
| Goteborg University Cirrhosis Index (GUCI) | 0.53 (0.35–0.83) | 0.96 (0.59–1.53) | 0.59 (0.39–0.97) |
| AST/ALT Ratio | 0.67 (0.54–0.82) | 0.94 (0.69–1.19) | 0.71 (0.56–0.91) |
| APRI | 0.53 (0.35–0.83) | 0.93 (0.57–1.43) | 0.59 (0.40–0.96) |
| AST to Platelet Ratio | 18 (12–26) | 29 (18–46) | 19 (13–30) |
| Cirrhosis Discriminant Score (CDS) | 5 (4–6) | 4 (3–5) | 5 (4–5) |
| BARD Score | |||
| 0 | 38 (9) | 5 (4) | 43 (8) |
| 1 | 215 (52) | 29 (23) | 244 (45) |
| 2 | 61 (15) | 16 (13) | 77 (14) |
| 3 | 76 (18) | 52 (42) | 128 (24) |
| 4 | 26 (6) | 23 (18) | 49 (9) |
Values are medians (IQR). Values for BARD Score are N (%)
The Mann-Whitney test comparing stage 0–2 to stage 3–4 calculated a p value <0.001 for each fibrosis panel.
Liver histology
The mean biopsy length was 19 ± 9.2 mm. While the biopsy length of those with steatohepatitis was somewhat greater than those without steatohepatitis (20 vs 18 mm), there were no significant differences between those with or without advanced fibrosis in either group. The distribution of fibrosis stages included stage 0 (n=140), stage 1 (n=159), stage 2 (n=117), stage 3 (n=85) and stage 4 (n=40).
Receiver Operating Curve (ROC) Analysis
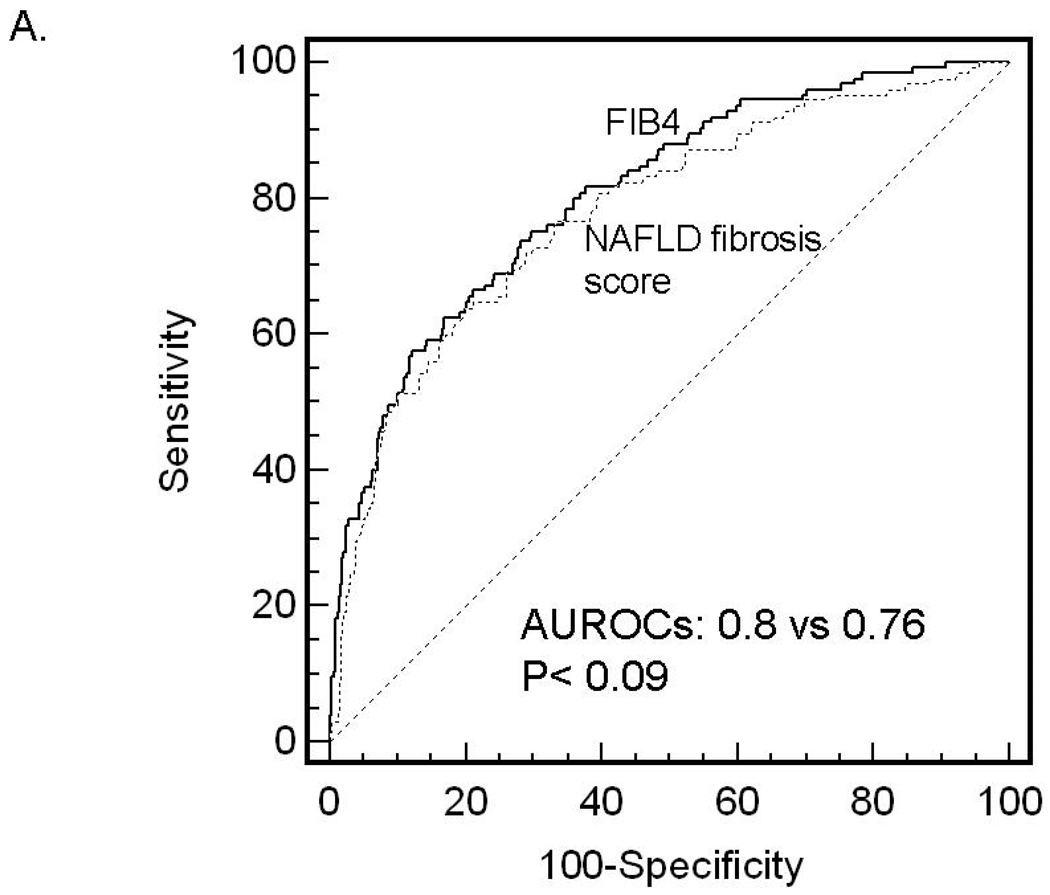
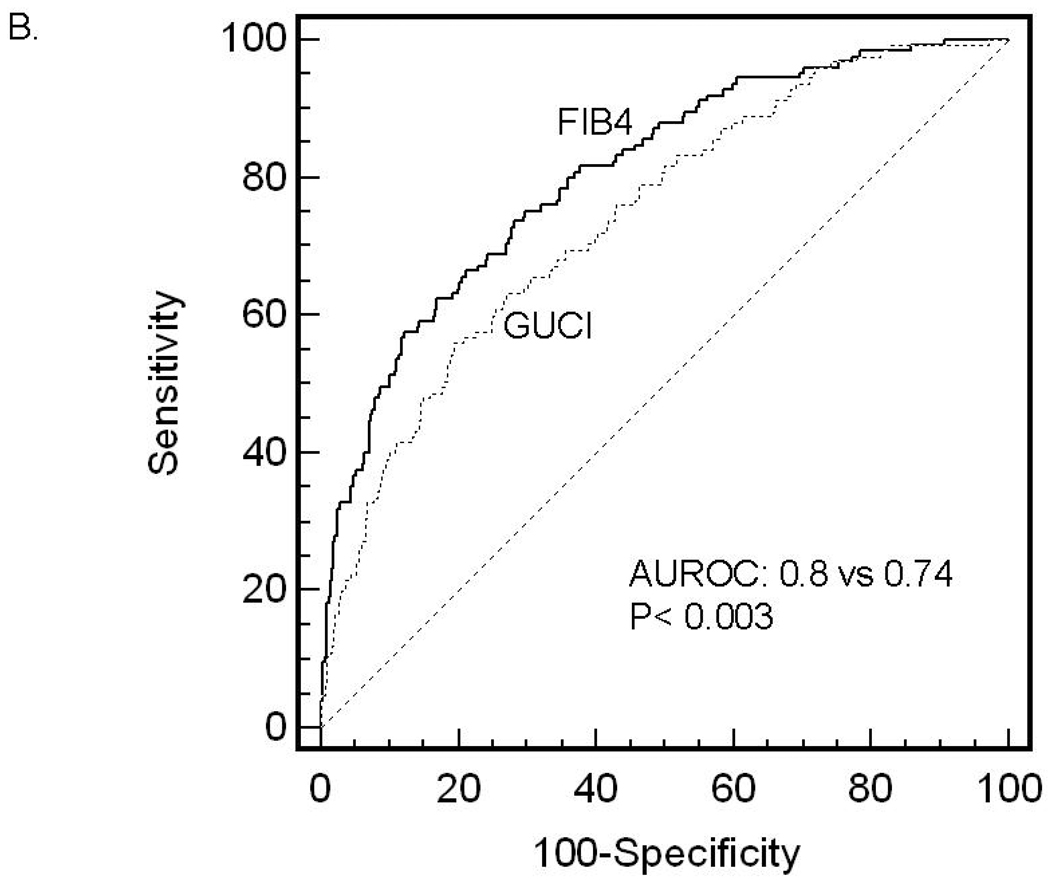
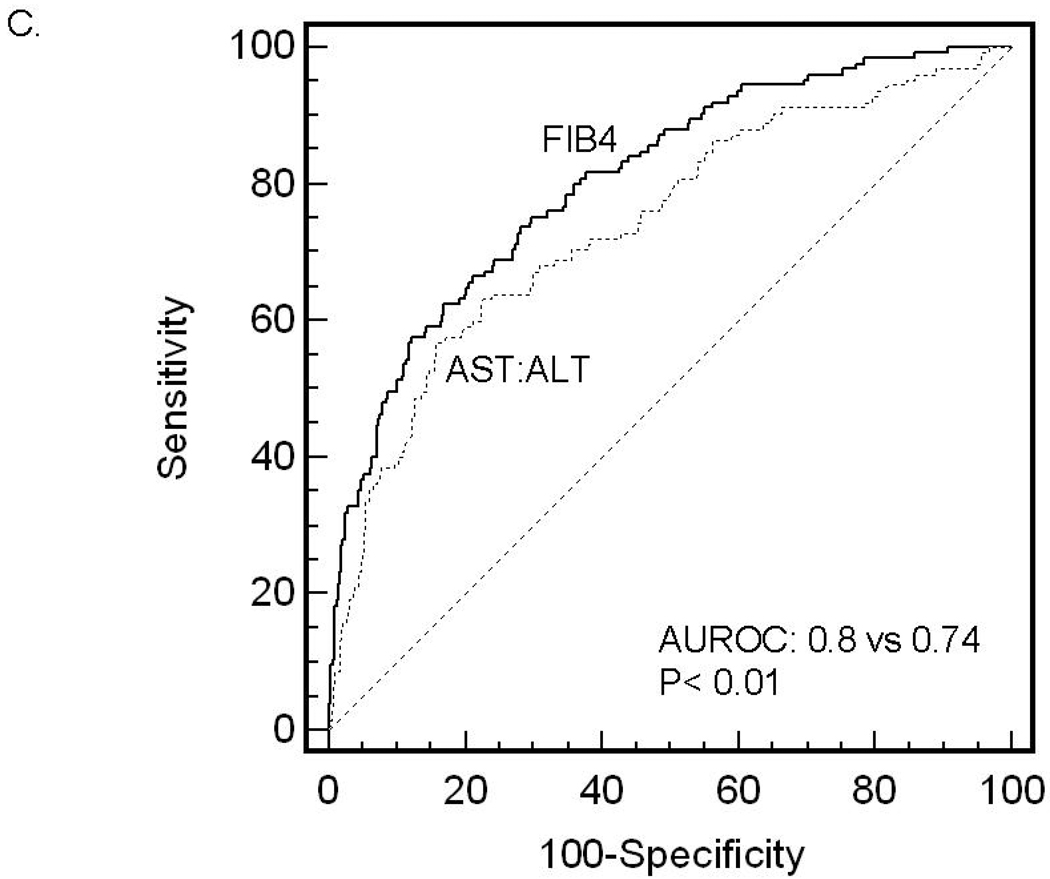
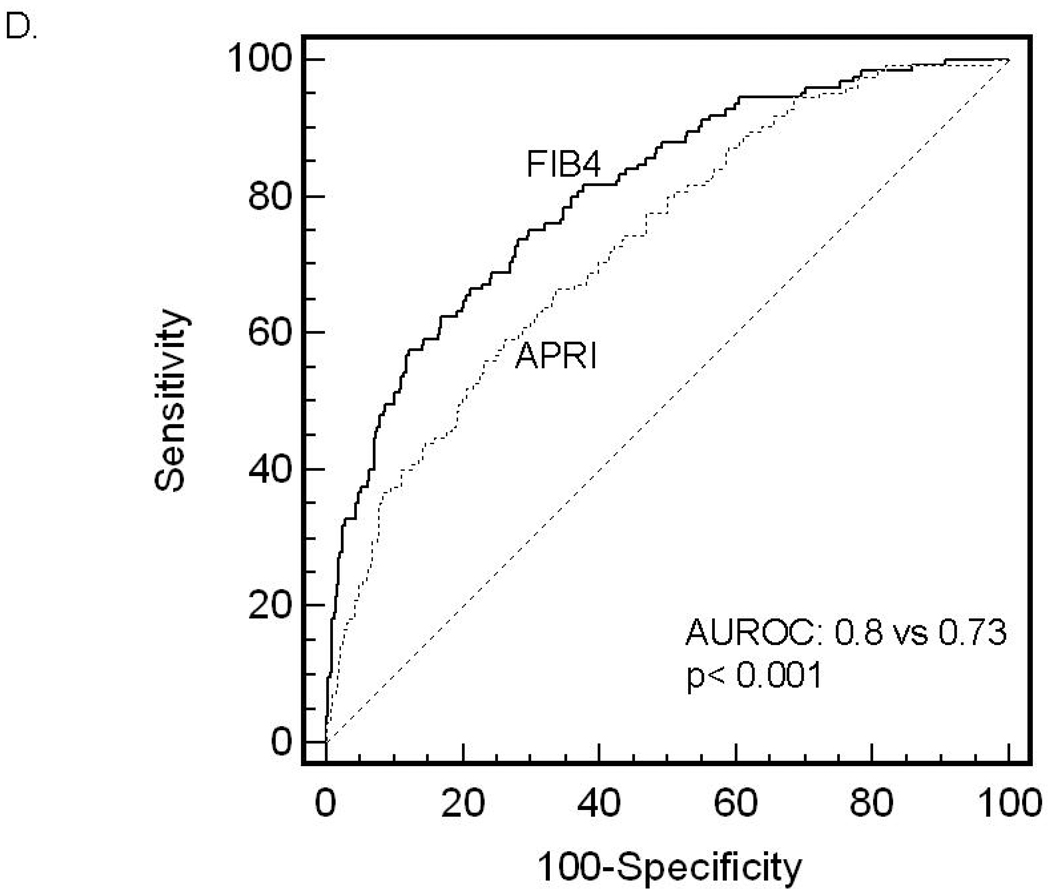
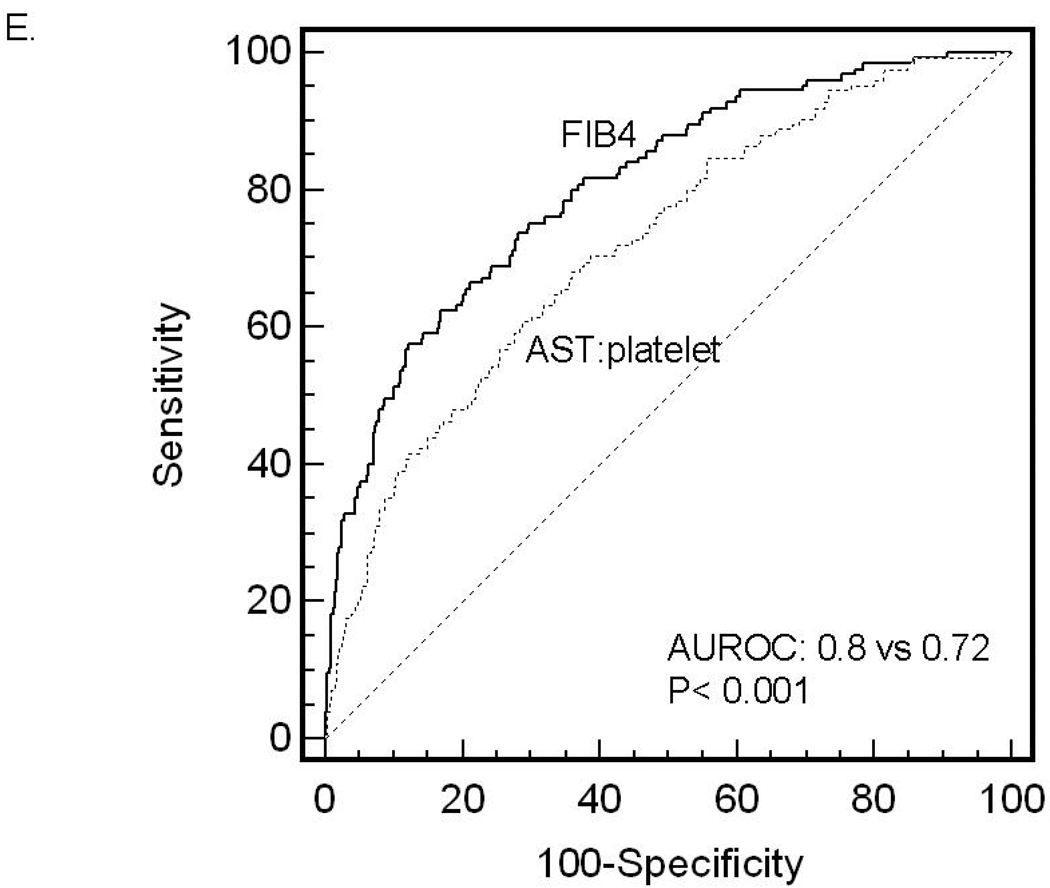
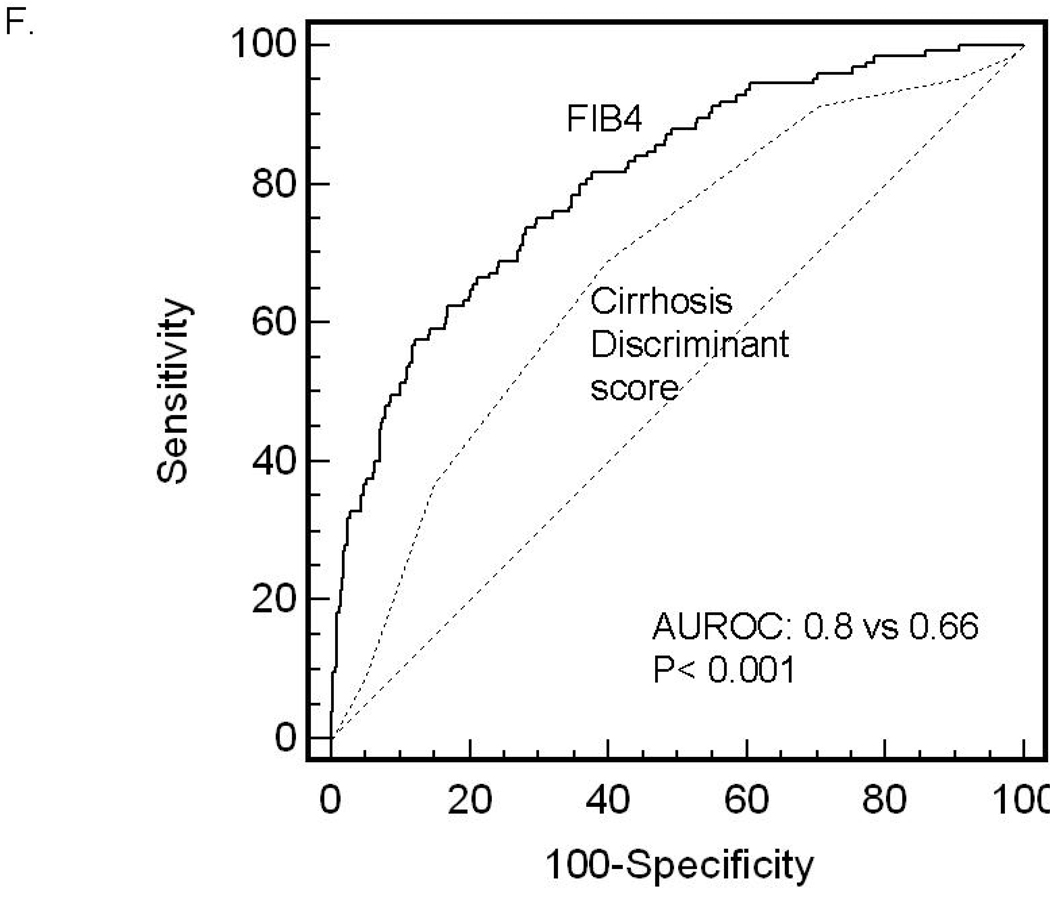
ROC curves were then developed for each of the non-invasive marker panels and superimposed to determine which score would have the most clinical utility (Figure 1). The ROC curves were created to determine the utility of these indices for prediction of advanced fibrosis (stage 3 and 4 vs lower scores), the clinical question of interest. The area under ROC curve (AUROC, 95% CI) was greatest for FIB4 (0.802, 0.758–0.847), then NAFLD fibrosis score (0.768, 0.720–0.816), followed by GUCI (0.743, 0.695–0.791), AST to ALT ratio (0.742, 0.690–0.794), APRI (0.730, 0.681–0.779), AST to platelet ratio (0.720, 0.669–0.770), BARD score (0.70, 0.64–0.75) and Cirrhosis Discriminant Score (0.666, 0.614–0.718). Furthermore, there was a statistically significant difference between the AUROC for FIB4 and the AUROCs for all of the other non-invasive screening tests (p<0.015) except the NAFLD fibrosis score where it approached significance (p=0.092) 35. These data indicate that of the noninvasive panels studied, FIB4 significantly outperformed the others, with the exception of the NAFLD fibrosis score where it approached significance, for prediction of bridging fibrosis and cirrhosis versus lower stages of fibrosis.
Figure 1.
An alternate analysis where the ability of the FIB4 to identify those with stage 0–1 fibrosis versus higher stages was tested, an overall AUROC of 0.75 (p<0.0001) was obtained. At a fixed sensitivity of 80%, the specificity was only 56%. In this analysis also, the FIB4 significantly (p< 0.03 for all) outperformed the other scores (NAFLD fibrosis score (0.69, 0.65–0.73), BARD (0.68, 0.62–0.72), cirrhosis discriminant score (0.63, 0.59–0.67), GUCI (0.71, 0.63–0.74). APRI (0.70, 0.67–0.74). The AUROCs for the FIB4 scores in subjects with a biopsy within 6 months was not significantly different from those who had a biopsy between 6–12 months prior to the calculation of the score (0.8 vs 0.78, p= not significant).
Clinical Utility of the FIB-4 index for prediction of fibrosis (Table 5)
Table 5.
Predictive Values of FIB-4 Index Scores for Advanced Fibrosis (stage 3–4)*
| Low cutoff point (<1.30) |
Indeterminate (1.30–2.67) |
High cutoff point (>2.67) |
Total | |
|---|---|---|---|---|
| Total | 327 | 163 | 51 | 541 |
| No advanced fibrosis | 294 | 112 | 10 | 416 |
| Advanced fibrosis | 33 | 51 | 41 | 125 |
| Sensitivity | 74% | 33% | ||
| Specificity | 71% | 98% | ||
| Positive predictive value | 43% | 80% | ||
| Negative predictive value | 90% | 83% | ||
| Interpretation | Absence of advanced fibrosis |
Presence of advanced fibrosis |
||
Prevalence of advanced fibrosis in study sample is 23%
The sensitivity and specificity of FIB4 along the ROC was first assessed. At a sensitivity of 90% the specificity was 45% while at a specificity of 90%, the sensitivity was 52%. The ROC curve was used to determine FIB4 cutoff points that best discriminated between the presence (2.67) and absence (1.3) of advanced fibrosis. A total of 327 subjects had a FIB4 score < 1.3; of these 294 were correctly classified as not having advanced fibrosis (true negatives) while 33 subjects were falsely classified (false negatives). The upper cutoff point of 2.67 correctly identified 41 of the 125 patients with advanced fibrosis (true positives) and misclassified only 10 out of 416 patients (false positives) without advanced fibrosis as having advanced fibrosis. The specificity was 31% for a sensitivity of 92% (threshold value −2.3) for the NAFLD fibrosis score and conversely the sensitivity was 35% when the specificity was fixed at 90%.
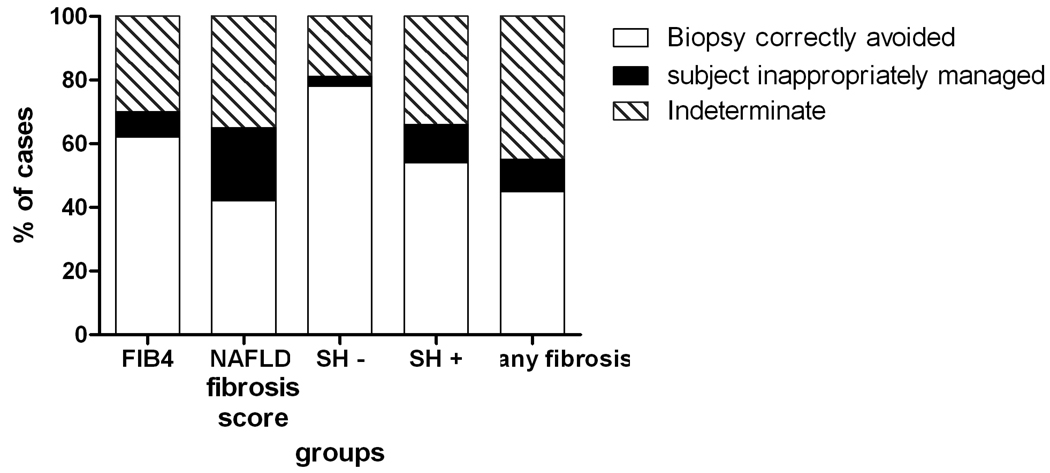
378 patients (72% of the cohort) had a FIB4 index below 1.30 or above 2.67; in these 378 subjects, the FIB4 identified the absence or presence of advanced fibrosis with 89% accuracy. 163 subjects (30 %) had FIB4 values in the indeterminate range (1.3–2.67). Assuming that a liver biopsy could be appropriately avoided in those who had a true negative or true positive test result, 62% versus 42% of biopsies (p< 0.007 by Fisher’s Exact test) would have been appropriately avoided with the FIB4 versus the NAFLD score if one was evaluating subjects for advanced fibrosis (Figure 2). 8% vs 23% of subjects would have been inappropriately managed by the FIB4 and NAFLD scores respectively (p< 0.005). If the objective was to search for any fibrosis at all, 45% of cases would be appropriately identified by the FIB4 scores while 45% would be in the indeterminate range.
Figure 2.
The positive and negative predictive values of these cutoff points would be expected to vary depending on the prevalence of advanced fibrosis in the population being studied. To study this, the clinical utility of the FIB4 test in those without steatohepatitis (11% (24/224) prevalence of advanced fibrosis) was studied. In this population the overall AUROC was 0.88, p< 0.0001 and a threshold value of 1.79 provided a sensitivity of 79% and specificity of 91.5%. Using the threshold values of 1.3 and 2.67 for the absence and presence of advanced fibrosis respectively, a liver biopsy would have been appropriately avoided in 78% of subjects (Figure 2). In subjects with steatohepatitis (32% prevalence of advanced fibrosis), the overall AUROC was somewhat lower at 0.75, p< 0.0001. The negative predictive value of a cutoff value of 1.3 for identification of advanced fibrosis was 83% while the positive predictive value for a threshold of 2.67 was 80%. In this population, a liver biopsy would have been appropriately avoided in 54% of cases.
Validation of the model
A “jack-knife” procedure was performed to determine the robustness of the model 39. The AUROC from the FIB4 jack-knife validation model was 0.797, which is very close to the full-sample estimate of 0.802. The AUROCs from the split-sample validation (50/50: 0.795, and 80/20: 0.794) were also virtually identical to the full sample estimate of 0.802.
DISCUSSION
An ideal non-invasive test for assessment of hepatic fibrosis would be one that is sensitive, specific, free of additional cost to the patient and applicable across all chronic liver diseases. In the context of NAFLD, such a test should also be able to distinguish between a fatty liver and steatohepatitis. Unfortunately, none of the currently available tests meet these criteria and the search for such a marker goes on.
In the absence of an ideal marker, the utility of any marker should take in to consideration both the clinical question being asked and the performance metrics of the marker in that clinical context. NAFLD often presents as abnormal liver enzymes without markers of other common liver diseases e.g. hepatitis C, persistent hepatomegaly without obvious cause or abnormal hepatic imaging with or without abnormal liver enzymes. None of the existing non-invasive panels have evaluated their performance in specific subsets of NAFLD based on clinical presentation. It is well known that a proportion of subjects with NAFLD with even advanced fibrosis have normal liver enzymes 40. It is thus likely that tests based on liver enzyme elevation will not work well in such a situation.
The FIB4 does not distinguish between a fatty liver and steatohepatitis and it should not be used to diagnose NASH. The potential use of FIB4 should be restricted to subjects with suspected NAFLD to evaluate the likelihood of having advanced or no fibrosis. While some studies have tried to focus on those with stage 0–1 fibrosis by trying to separate them from higher degrees of fibrosis 10, we elected to focus mainly on those with stage 3–4 fibrosis and separate them from the rest as has been done in other studies 11. The rationale for this was that, in the absence of an approved treatment for NASH, this would help identify those who should undergo a biopsy because the confirmation of advanced fibrosis would lead to closer follow up and screening for hepatocellular cancer.
The AUROC of FIB4 was 0.8 for diagnosis of advanced fibrosis and was superior to other noninvasive panels that that were tested. This is somewhat inferior to those reported for the fibrotest and the ELF score where an AUROC of 0.9 have been reported 10, 11. However, the range of AUROCS for the FIB4 for varying degrees of fibrosis and prevalence of advanced fibrosis (0.75–0.88) in the population overlap substantially with the range reported for fibrotest and the ELF scores 41. Importantly, the ELF score has a reported AUROC of 0.76 for the diagnosis of no fibrosis which was almost identical to that seen with FIB4 (0.75) 11.
The shape of the ROC and the sensitivity and specificity of specific diagnostic thresholds should also be considered. Unfortunately, for all existing markers for which there are published data, there is a marked drop off in sensitivity when the specificity is fixed at 90% and vice versa. The sensitivity of FIB4 drops to 52 % at a specificity of 90% and further decreases of 38% when the specificity increases to 95%. Of note, the sensitivity of the fibrotest and ELF scores are 25% and 57% respectively when the specificity is set at 96% and 97% respectively 10, 11. This highlights the limitations of the FIB4 and also holds true for other similar noninvasive panels. The clinical utility of the FIB4 is thus in the general range reported for other published panels for NAFLD including the ELF score and Fibrotest which all require additional testing and add cost to the care provided to patients 11,14, 42.
Another important guide to the clinical utility of a test is the proportion of the study population where the diagnosis can be made or excluded with confidence and the residual proportion where the test results are indeterminate. This is critically linked to the prevalence of advanced fibrosis in the population being studied. 30% of subjects in this study had indeterminate test results with FIB4. In those with a low prevalence of fibrosis, only 19% of subjects remained in the indeterminate range. Although the AUROC for the NAFLD score and FIB4 were not significantly different, the performance of the NAFLD score was significantly inferior to that of the FIB4 score based on the percent of biopsies correctly avoided and those inappropriately denied. These data are also somewhat higher than but overlap with those reported with the ELF score 11. The published data with the fibrotest and fibroscan do not permit this analysis.
It is well known that there is considerable sampling variability in fibrosis staging in liver biopsies 8. This may be one reason why the AUROC for most validated panels, including the FIB4, are in the 0.8–0.9 range and not higher. Another potential limitation to our study was the inclusion of subjects who had a biopsy up to 12 months prior to FIB4 calculation. However, the AUROC for those who had their biopsy within 6 months was similar to those who had the biopsy between 6–12 months. Study groups with highly polarized distributions of fibrosis stages also provide greater sensitivities and specificities than those with a more even distribution of fibrosis stages. The current study used a large data set for NAFLD and had a broad distribution of fibrosis stages. The use of such a data set and the reproducibility of the data with both the “jackknife” validation test and split-sample validation test attest to the relative robustness of the FIB4 as a marker of advanced fibrosis in subjects with NAFLD.
When the goal is to identify those with advanced fibrosis, the use of the FIB4 scores should take in to account the a priori risk of fibrosis and the predictive values associated with these risks. For example, in a young adult with obesity and no features of the metabolic syndrome who has a relatively low risk of advanced fibrosis, a score < 1.3 may allow one to predict the absence of advanced fibrosis with confidence (Table 6). However, in an older subject with diabetes and other features of the metabolic syndrome with a high a priori risk of advanced fibrosis, the negative predictive value of a value < 1.3 is much lower (Table 6). In such a population, a high score (> 2.67) has a high positive predictive value.
Table 6.
Predictive Values for Cutoff Points for Different Prevalences of Advanced Fibrosis
| Prevalence | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Cutoff 1.30 | ||||
| 10% | 74 | 71 | 22 | 96 |
| 20% | 74 | 71 | 39 | 91 |
| 50% | 74 | 71 | 72 | 73 |
| Cutoff 1.93 | ||||
| 10% | 50 | 90 | 36 | 94 |
| 20% | 50 | 90 | 56 | 88 |
| 50% | 50 | 90 | 84 | 65 |
| Cutoff 2.67 | ||||
| 10% | 34 | 98 | 60 | 93 |
| 20% | 34 | 98 | 77 | 85 |
| 50% | 34 | 98 | 93 | 59 |
An important question relates to whether changes in FIB4 scores correspond to changes in fibrosis over time. The sensitivity of the FIB4 to changes in fibrosis was not formally tested in this study and now needs to be studied to determine if changes in FIB4 can be used to monitor fibrosis in subjects with NAFLD. Another potential application of the FIB4 score could be as a screening tool for silent liver disease with advanced fibrosis in the general population. These potential applications require validation in appropriately designed studies.
In summary, the current study demonstrates that the FIB4 score is a simple, relatively inexpensive method that correlates with the stage of fibrosis in adult subjects with NAFLD. It has the potential advantage of having been validated for both HCV and NAFLD two common chronic liver diseases. Its performance characteristics for the diagnosis of advanced fibrosis in NAFLD are better than other similar panels that do not require additional testing and comparable to several other existing tests that require additional tests. The FIB4 test also has several serious limitations as do other non-invasive tests of fibrosis and none of these tests, including the FIB4 test can be used to replace the need for a liver biopsy yet.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Contos MJ, Choudhury J, Mills AS, Sanyal AJ. The histologic spectrum of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:481–500. doi: 10.1016/j.cld.2004.04.013. vii. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 5.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 9.Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95–106. doi: 10.1038/ncpgasthep1025. [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF, Le Bail B, de Ledinghen V, Poynard T. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6(6) doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD, Aithal GP, Day CP, Rosenberg WM. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 12.Huwart L, Sempoux C, Salameh N, Jamart J, Annet L, Sinkus R, Peeters F, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto M, Mizuguchi T, Katsuramaki T, Nagayama M, Oshima H, Kawasaki H, Nobuoka T, Kimura Y, Hirata K. Assessment of liver fibrosis by a noninvasive method of transient elastography and biochemical markers. World J Gastroenterol. 2006;12:4325–4330. doi: 10.3748/wjg.v12.i27.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H, Kobayashi N, Kirikoshi H, Abe Y, Inamori M, Kubota K, Saito S, Tamano M, Hiraishi H, Maeyama S, Yamaguchi N, Togo S, Nakajima A. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2007 doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Poynard T, Imbert-Bismut F, Munteanu M, Ratziu V. FibroTest-FibroSURE: towards a universal biomarker of liver fibrosis? Expert Rev Mol Diagn. 2005;5:15–21. doi: 10.1586/14737159.5.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25:779–786. doi: 10.1111/j.1478-3231.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakugawa H, Nakayoshi T, Kobashigawa K, Yamashiro T, Maeshiro T, Miyagi S, Shiroma J, Toyama A, Nakayoshi T, Kinjo F, Saito A. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2005;11:255–259. doi: 10.3748/wjg.v11.i2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 22.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 23.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 24.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 25.Cervantes EA, Miller WR, Tonigan JS. Comparison of Timeline Follow-Back and Averaging Methods for Quantifying Alcohol Consumption in Treatment Research. Assessment. 1994;1:23–30. doi: 10.1177/1073191194001001004. [DOI] [PubMed] [Google Scholar]
- 26.Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Alcohol Use Disorder Identification Test. Addiction. 1995;90:1349–1356. doi: 10.1046/j.1360-0443.1995.901013496.x. [DOI] [PubMed] [Google Scholar]
- 27.Kokotailo PK, Egan J, Gangnon R, Brown D, Mundt M, Fleming M. Validity of the alcohol use disorders identification test in college students. Alcohol Clin Exp Res. 2004;28:914–920. doi: 10.1097/01.alc.0000128239.87611.f5. [DOI] [PubMed] [Google Scholar]
- 28.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- 30.Bourliere M, Penaranda G, Renou C, Botta-Fridlund D, Tran A, Portal I, Lecomte L, Castellani P, Rosenthal-Allieri MA, Gerolami R, Ouzan D, Deydier R, Degott C, Halfon P. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat. 2006;13:659–670. doi: 10.1111/j.1365-2893.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 31.Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005;40:867–872. doi: 10.1080/00365520510015674. [DOI] [PubMed] [Google Scholar]
- 32.Borroni G, Ceriani R, Cazzaniga M, Tommasini M, Roncalli M, Maltempo C, Felline C, Salerno F. Comparison of simple tests for the non-invasive diagnosis of clinically silent cirrhosis in chronic hepatitis C. Aliment Pharmacol Ther. 2006;24:797–804. doi: 10.1111/j.1365-2036.2006.03034.x. [DOI] [PubMed] [Google Scholar]
- 33.Blonsky JJ, Harrison SA. NAFLD and HCV: partners in crime. Aliment Pharmacol Ther. 2008 doi: 10.1111/j.1365-2036.2008.03672.x. [DOI] [PubMed] [Google Scholar]
- 34.Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–656. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- 35.Hanley J, Mcneill BJ. A method for comparing the area under the receiver operating curves derived from the same cases. Radiology. 1983;148(839) doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 36.Escudero J, Hornero R, Poza J, Abasolo D, Fernandez A. Assessment of classification improvement in patients with Alzheimer's disease based on magnetoencephalogram blind source separation. Artif Intell Med. 2008 doi: 10.1016/j.artmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Sahiner B, Chan HP, Hadjiiski L. Classifier performance estimation under the constraint of a finite sample size: Resampling schemes applied to neural network classifiers. Neural Netw. 2008;21:476–483. doi: 10.1016/j.neunet.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valkenborg D, Van Sanden S, Lin D, Kasim A, Zhu Q, Haldermans P, Jansen I, Shkedy Z, Burzykowski T. A cross-validation study to select a classification procedure for clinical diagnosis based on proteomic mass spectrometry. Stat Appl Genet Mol Biol. 2008;7 doi: 10.2202/1544-6115.1363. Article12. [DOI] [PubMed] [Google Scholar]
- 39.Rivera-Fernandez R, Vazquez-Mata G, Bravo M, Aguayo-Hoyos E, Zimmerman J, Wagner D, Knaus W. The Apache III prognostic system: customized mortality predictions for Spanish ICU patients. Intensive Care Med. 1998;24:574–581. doi: 10.1007/s001340050618. [DOI] [PubMed] [Google Scholar]
- 40.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 41.Poynard T, Halfon P, Castera L, Munteanu M, Imbert-Bismut F, Ratziu V, Benhamou Y, Bourliere M, de Ledinghen V. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53:1615–1622. doi: 10.1373/clinchem.2007.085795. [DOI] [PubMed] [Google Scholar]
- 42.Ratziu V, Giral P, Munteanu M, Messous D, Mercadier A, Bernard M, Morra R, Imbert-Bismut F, Bruckert E, Poynard T. Screening for liver disease using non-invasive biomarkers (FibroTest, SteatoTest and NashTest) in patients with hyperlipidaemia. Aliment Pharmacol Ther. 2007;25:207–218. doi: 10.1111/j.1365-2036.2006.03182.x. [DOI] [PubMed] [Google Scholar]