During development, hemogenesis occurs invariably at sites of vasculogenesis. Between E9.5 and E10.5 in mouse, endothelial cells in the caudal portion of the dorsal aortae generate hematopoietic stem cells 1, 2 and are referred to as hemogenic endothelium 3-8. The mechanisms by which hematopoiesis is restricted to this domain, and how the morphological transformation from endothelial to hematopoietic is controlled are unknown. We show here that HoxA3, a gene uniquely expressed in the embryonic but not yolk sac vasculature, restrains hematopoietic differentiation of the earliest endothelial progenitors, and reverts the earliest hematopoietic progenitors into CD41-negative endothelial cells. This reversible modulation of endothelial-hematopoietic state is accomplished by targeting key hematopoietic transcription factors for downregulation, including Runx1, Gata1, Gfi1B, Ikaros, and PU.1. Through loss-of-function, and gain-of-function epistasis experiments and the identification of antipodally regulated targets, we show that among these factors, Runx1 is uniquely able to erase the endothelial program set up by HoxA3. These results suggest both why a frank endothelium does not precede hematopoiesis in the yolk sac and why hematopoietic stem cell generation requires Runx1 expression only in endothelial cells.
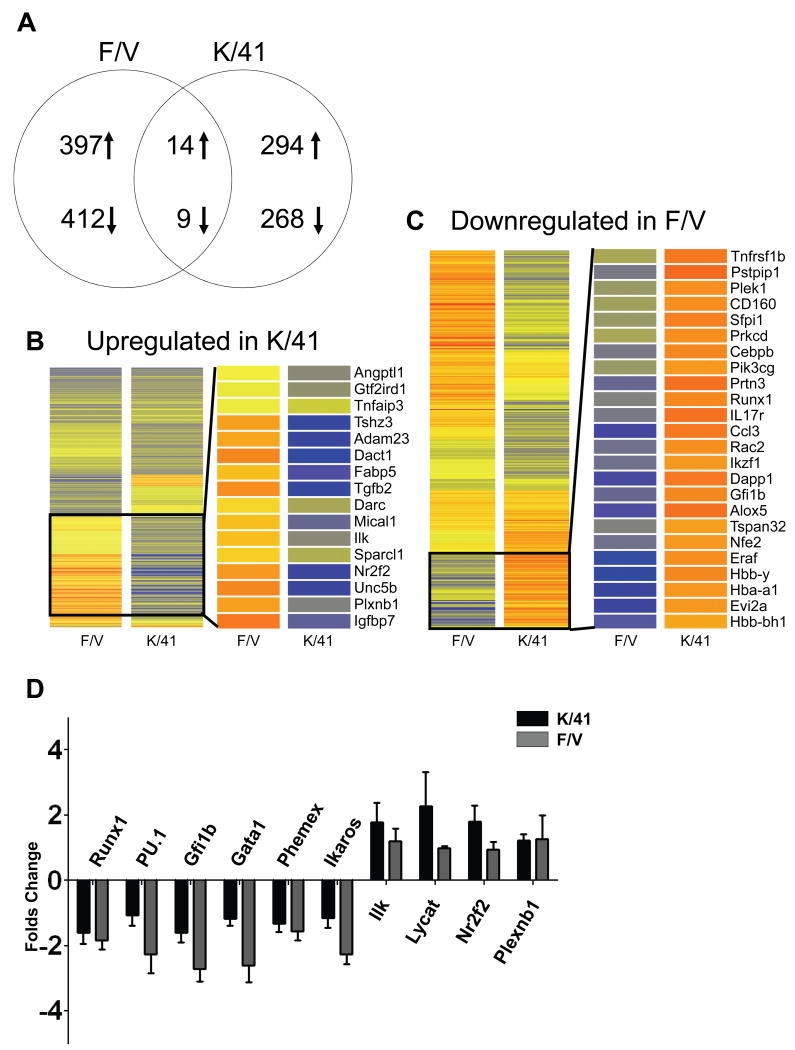
Homeobox genes play an important role during vertebrate development, determining cell identity along the rostral-caudal axis. In early development, they are expressed along this axis in a manner colinear with their order within the Hox cluster 9. In some tissues, these rostral-caudal expression patterns are reset during organogenesis; for example, several Hox genes have been identified in hematopoietic stem cells, and the expression patterns change with differentiation 10-13. The expression of members of Hox paralog group 4, including HoxB4, promotes self-renewal of the HSC 14-16 and allows engraftment of early embryonic hematopoietic progenitors produced in vitro from ES cells 17. Hox paralog group 3, the next anterior, is best known for specifying the identity of tissues that originate from the pharyngeal arches, however it also plays an important role in endothelial development. Mice lacking HoxA3 display cardiovascular abnormalities 18 and vascular expression of paralog group 3 members is associated with angiogenesis and wound repair 19-21. Growing evidence suggests that the embryonic vasculature gives rise to hematopoietic progenitors via a specialized hemogenic endothelium 3, 22, 23. The posterior dorsal aorta is such a specialized site that plays a particularly important role in the origin of the adult HSC pool through a poorly understood process that is dependent on Runx1 5. The vascular role of paralog group 3 prompted us to evaluate the temporal expression of HoxA3 in the hemogenic domains of the dorsal aorta. In early development (E7.5), HoxA3 expression is restricted to the embryo proper, while Runx1 expression marks hematopoiesis in the yolk sac (Sup. Fig. 1A, B). HoxA3 and Runx1 display a remarkable pattern of mutually exclusive expression at hematopoietic/vascular sites in the early embryo. At E8.25 and E8.5, HoxA3 is highly expressed throughout the neurectoderm and mesenchyme, is present at intermediate levels in the endothelium of both the anterior and posterior dorsal aortae, and remains absent from the yolk sac (Fig. 1A, C and Sup. Fig. 1C, E). By contrast, Runx1 is found in the yolk sac but not in the aortae or other embryonic vessels (Fig. 1B, D and Sup. Fig. 1D, F) with the exception of the omphalomesenteric artery, as seen previously 24, 25. Notably, HoxA3 is not expressed in the omphalomesenteric artery (Fig. 1C). At E9.5, and E10.5 the time point at which the posterior dorsal aortae first begin to express Runx1 and become hemogenic, HoxA3 expression is clearly lost in the aortic endothelial cells (Fig. 1E, G Sup. Fig. 1G, I), while Runx1 is expressed (Fig. 1F, H, Sup. Fig 1H,J).
Figure 1. Reciprocal expression of HoxA3 and Runx1 in embryonic endothelium.
In situ hybridization of E8.25-E10.5 embryonic tissues with HoxA3 and Runx1 probes. (A-B) HoxA3 is expressed in aortic endothelial cells, while Runx1 is not. Note that Runx1 is expressed in the yolk sac (black arrowheads) while HoxA3 is not. At E8.5, HoxA3 expression (C) begins to decline in aortic endothelium, while Runx1 (D) is still not detected. Note that the omphalomesenteric artery, oa, is negative for HoxA3 at this time but positive for Runx1. (E-H) In situ hybridization of dissected E9.5 and E10.5 AGMs (shown in left panels). At E9.5, HoxA3 (E) expression is barely detectable in aortic endothelial cells, while Runx1 (F) expression is now observed. At E10.5, HoxA3 (G) expression is completely extinguished in aortic endothelial cells. In contrast, Runx1 (H) expression has increased. a, aorta; g, gut tube; nt, neural tube; oa, omphalomesenteric artery. Stippled lines in E-H outline aorta. Scale bar = 50 μm for lower magnifications and AGM explants, = 10 μm for higher magnification panels.
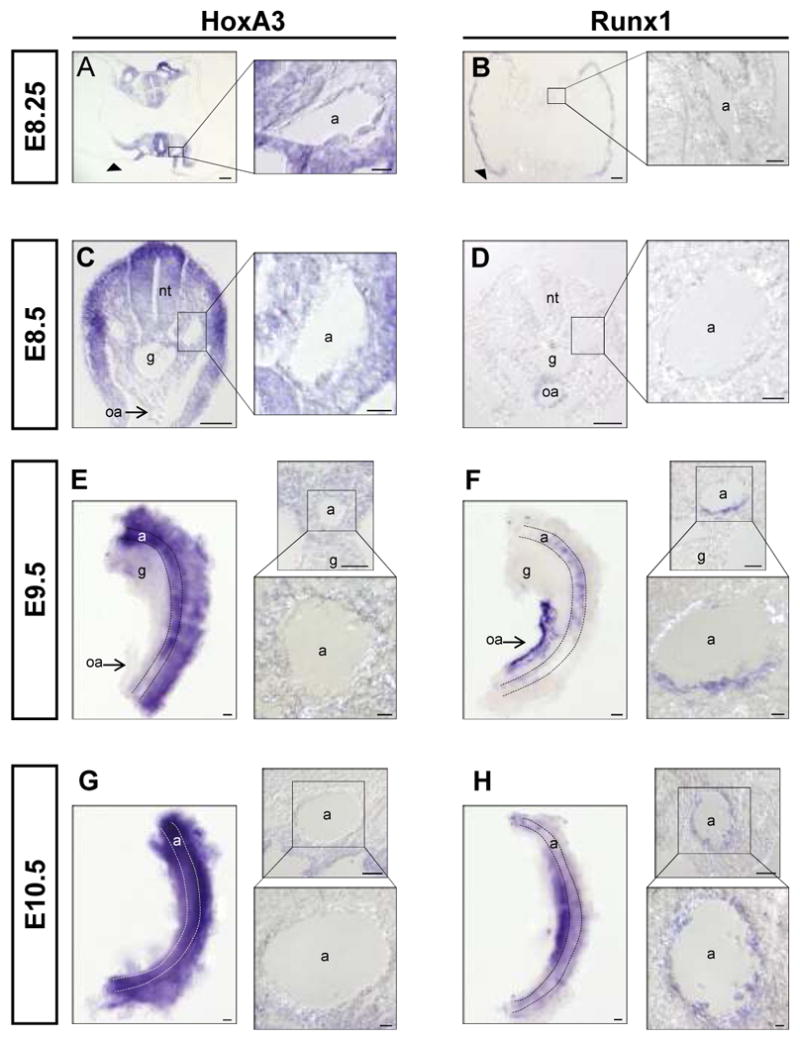
HoxA3 down-regulation thus marks the site of hemogenesis in the endothelium of the dorsal aorta. Does HoxA3 expression have any functional effect on the development of hemogenic endothelium? In order to answer this question, we generated a doxycycline (dox)-inducible HoxA3 murine ES cell line by cassette exchange recombination into a dox-inducible locus 16 and differentiated these cells as embryoid bodies (EBs). The kinetics of mesoderm differentiation in this system broadly mimics that of embryonic development 26 with bipotent hematopoietic-endothelial progenitors (hemangioblasts) identified in clonal assays as early as 2.75 days of differentiation 27, corresponding to embryonic bipotent progenitors of the posterior primitive streak, thought to contribute to yolk sac hematopoiesis 28. Vascular markers, eg. VE-cadherin, Tie2, and CD31, first appear two days later, coexpressed on many cells with the earliest hematopoietic marker, CD41 29, 30. The coexpressing population is capable of both endothelial and hematopoietic differentiation, thus defining it as hemogenic endothelium 31, 32. When we induced HoxA3 with dox just before this time (day 4-6), we noted a striking repression of the hematopoietic markers CD41+ and CD45+ (Fig. 2A, B). However, the total endothelial progenitor population identified as cells expressing both Flk1 and VE-cadherin 33, 34 (F/V population) was not reduced by HoxA3 expression (Fig. 2A, B). We assayed hematopoietic progenitor content in these EBs and found that HoxA3 dramatically suppressed hematopoietic colony-forming cell (CFC) content, (Fig. 2C) demonstrating that HoxA3 is not merely preventing expression of surface markers, but truly preventing hematopoietic differentiation. When hematopoietic progenitors (c-Kit CD41 double-positive cells) from uninduced EBs were sorted and plated in CFC assays, HoxA3 expression in the methylcellulose medium abolished hematopoietic colony-forming potential (Sup. Fig. 2A). To determine whether the hematopoietic repression of HoxA3 was due to cell death or a change in cell fate, hematopoietic (c-Kit+/CD41+; K/41), and endothelial (Flk1+/VE-cadherin+; F/V) fractions were purified from day 6 EBs and cultured on OP9 stromal cells, a system that support both hematopoiesis and endothelial development. In the absence of doxycycline both the F/V and K/41 fractions produced hematopoietic cells, consistent with the notion that the endothelial fraction is endowed with hemogenic capacity 32 (Fig. 2F, G, no dox). However when HoxA3 was upregulated, hematopoietic marker expression was significantly reduced (Figure 2F G, + dox). Remarkably, in the presence of doxycycline, not only were hematopoietic cells missing from the K/41 fraction, but colonies of cells with an epithelial morphology and expressing VE-cadherin were observed instead (Fig. 2E). The induction of endothelial markers and repression of hematopoietic markers was seen also in more committed progenitors already expressing the pan-hematopoietic marker CD45 (Sup. Fig. 2B). When HoxA3 expression was withdrawn, hematopoietic colonies developed again, in both K/41 and F/V-initiated cultures (Fig. 2D-G). This result shows that HoxA3 restrains hematopoietic development and maintains an endothelium, even in progenitors that have recently committed to hematopoiesis, indicated by expression of CD41 and CD45.
Figure 2. HoxA3 expression in early mesoderm and committed hemogenic endothelium restrains hematopoeisis.
(A) Representative flow cytometric profiles of EBs at day 6 without doxycycline (No Dox) or with 1 μg/mL doxycycline (+Dox) to induce HoxA3 expression from day 4 to day 6. VE-cadherin (VE-cad)/Flk-1 antibody staining or c-Kit/CD41 and c-Kit/CD45 staining were performed to identify vascular and hematopoietic progenitor populations. (B) Frequencies of cells expressing endothelial surface markers (Flk-1+/VE-cadherin+, F/V), hematopoietic markers CD41+ and CD45+ cells during EB differentiation in 7 independent experiments (for CD41 p=0.0004 and for CD45, p=0.0031). (C) 50,000 cells from day 6 EBs (induced with 1 μg/mL dox to express HoxA3 continually from EB day 4-6 or not) were plated in methylcellulose with hematopoietic cytokines. n=3. Black bar: no dox treatment, gray bar: dox treatment. Colonies: GEMM (granulocyte/erythrocyte/macrophage/megakaryocyte) GM (granulocyte/macrophage) M (macrophage only) Ery-D (definitive erythroid) p=0.032, Ery-P (primitive erythroid) p=0.0002 Ery-Meg (erythrocyte-megakaryocyte) p=0.0009. (D) Brightfield and fluorescence images showing both endothelial (+Dox) and hematopoietic colonies (No Dox or Dox removal) derived from Flk1+/VE-cadherin+ (F/V) endothelial progenitors from day 6 EBs. Immunofluorescence for VE-cadherin is shown in adherent cells growing in the presence of doxycycline. Bar 100 μm. (E) Equivalent analysis of cultures derived from day 6 EB c-Kit+/CD41+ (K/41) hematopoietic progenitors. (F) Representative flow cytometric profile of 100,000 Flk-1/VE-cadherin double positive cells or (G) c-Kit/CD41 double positive cells from day 6 uninduced EBs (left), cultured on OP9 for 5 days, in the presence or absence of 1 μg/mL doxycycline. Dox-induced cells were cultured for an additional 4 days in the absence of dox to test the effect of HoxA3 down-regulation. Hematopoietic surface markers, c-Kit, CD41 and CD45 and endothelial markers Flk-1 and VE-cadherin are plotted. (H) AGM tissue dissected from E10.5 embryos, dissociated and transduced with control ires-GFP or HoxA3-ires-GFP retrovirus, cultured on OP9 for 5 days. Bright field images are shown at left, GFP at right. Both hematopoietic and endothelial colonies that acquired GFP were observed with the control, but GFP segregated with endothelial colonies in the HoxA3-ires-GFP transduced sample, indicating skewing of differentiation towards endothelial by HoxA3. Bar 100 μm. (I) Representative flow cytometric profile of AGM cells co-cultured on OP9, and statistical analysis of 5 independent experiments (histogram CD41 p=0.053 CD45 p=0.02).
To test the effect of HoxA3 in bona fide hemogenic endothelium 6-8, we expressed HoxA3 with an ires-GFP reporter by retroviral transduction in disaggregated E10.5 AGM tissue cultured ex vivo. Both GFP+ hematopoietic and GFP+ adherent colonies could be detected in cultures transduced with control vector, however with the HoxA3 vector, only GFP-negative hematopoietic colonies were found while GFP+ colonies were adherent (Figure 2H). Flow cytometric analysis confirmed that cells expressing HoxA3 had elevated Flk-1 and VE-cadherin expression, and reduced CD41 and CD45 expression (Fig. 2I). Thus HoxA3 is able to repress hematopoietic development in both ES-derived hematopoieitic progenitors and in embryonic AGM-derived tissue.
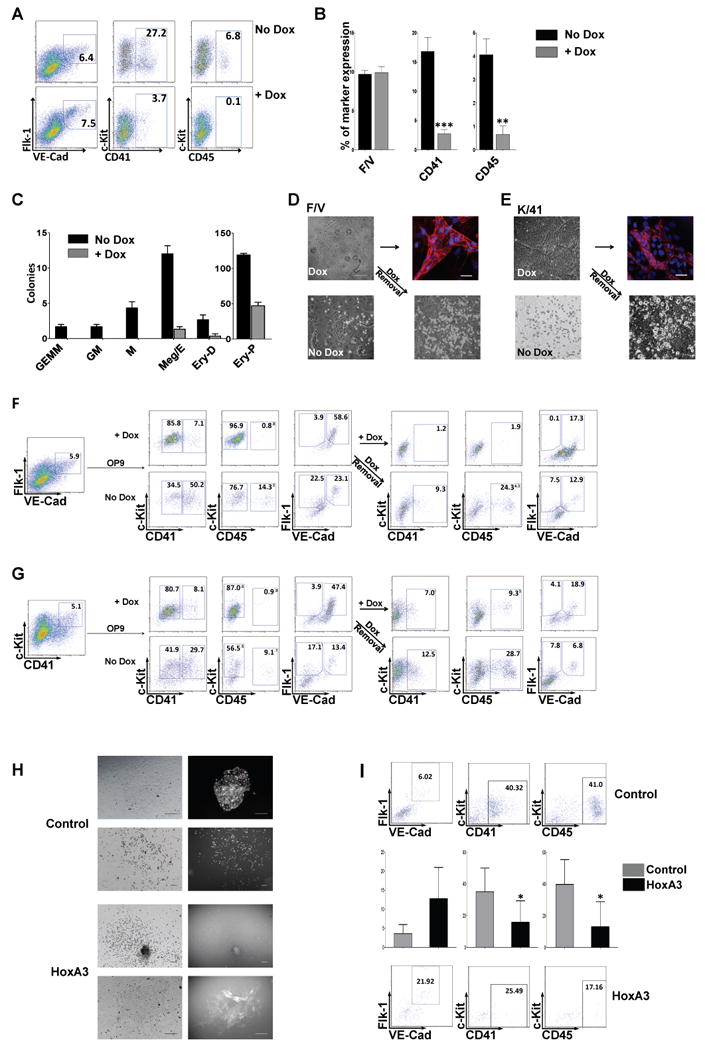
To gain insight into the mechanism by which HoxA3 regulates the hemogenic transition we performed transcriptional profiling on both the endothelial and hematopoietic progenitor fractions from day 6 EBs treated with doxycycline for 6 hours (Fig. 3). In the hematopoietic (K/41) fraction HoxA3 regulated 585 genes of which 277 were repressed and 308 were upregulated (1.5 fold p-value 0.05 cut-off Fig. 3A). Because HoxA3 promotes the endothelial phenotype in this fraction and inhibits hematopoiesis, we evaluated HoxA3 upregulated genes based on their expression in control hematopoietic or endothelial fractions. Genes involved in endothelial development clustered together, were expressed at higher levels in the control F/V fraction compared to the control K/41 fraction, and were generally upregulated by HoxA3 in the dox-treated K/41 fraction (Fig. 3B). We performed a similar analysis for the F/V fraction in which 832 genes were regulated (421 down and 411 up; 1.5 fold cut off p-value 0.05, Fig 3A). Genes repressed by HoxA3 included many known hematopoietic regulators most of which are expressed at higher levels in control K/41 cells compared to control F/V cells (Fig. 3C). Within this cluster HoxA3 significantly repressed the expression of Runx1, Gata1, Gfi1B, Ikaros and Phemx and PU.1, all validated by qPCR (Fig. 3D). HoxA3 thus coordinately regulates a large number of genes involved in endothelial and hematopoietic development, preventing the hematopoietic program from arising in endothelial progenitors and reactivating an endothelial program in nascent hematopoietic progenitors. It is the first regulatory factor identified to date to have this effect.
Figure 3. Global expression changes upon HoxA3 induction.
(A) Venn diagram of regulated genes in endothelial (F/V) and hematopoietic (K/41) progenitor cells. Arrow up upregulated genes, arrow down downregulated genes. (B) Clustering of genes upregulated in the c-Kit CD41 double-positive cells upon HoxA3 induction, during EB differentiation in the hematopoietic c-Kit CD41 double positive cells, based on their expression levels in the Flk-1 VE-cadherin double positive cells (F/V) and in the c-Kit CD41 double positive cells (K/41) of uninduced EBs. (C) Clustering of genes downregulated genes in the Flk-1 VE-cadherin double-positive population upon 6-hour HoxA3 induction, in day 6 EB cells, based on their baseline (control) levels in the Flk-1 VE-cadherin double-positive population (F/V). (D) Real time RTPCR measurements of gene expression changes following HoxA3 induction in sorted endothelial (F/V black bars) or hematopoietic (K/41 grey bars) progenitors. n=5 independent experiments.
One of the notable downregulated targets is Runx1. Loss of function analysis of Runx1 demonstrated it to be essential for definitive hematopoiesis 35, 36 and for the emergence of hematopoietic cells from the aorta 24. Conditional deletion of Runx1 has shown that its role is essential in VE-cadherin-positive cells of the aorta, but dispensable in the (Vav-1-expressing) hematopoietic stem cells themselves 5. To determine the functional relevance of Runx1 and other hematopoietic transcription factors repressed by HoxA3, we performed dominant epistasis experiments. Endothelial (F/V) progenitor cells from HoxA3-induced EBs were sorted and transduced with Runx1B, Ikaros, Pu.1, Gata1, Gfi1B, or control vectors bearing ires-GFP reporters, and cultured on OP9 stroma. Surface marker analysis of GFP-gated cells demonstrated that Runx1 strongly suppressed, GATA1 partially suppressed, and Gfi1B minimally suppressed the HoxA3 phenotype, while Pu.1 and Ikaros had no effect (Fig. 4A,B). Transcriptional analysis showed that Gfi1B, PU.1, Ikaros, and Phemx, were actually target genes of Runx1, but that GATA1 was not (Fig. 4C). To understand this epistasis, we sorted Runx1- or GATA1- transduced, HoxA3-expressing cells, and determined their global gene expression profiles in comparison to HoxA3-expressing cells (with control GFP vector), or cells in which HoxA3 expression was turned off by doxycycline withdrawal. Consistent with their ability to revert the HoxA3 phenotype, high level overexpression of both Runx1 and GATA1 reverted many of the gene expression changes induced by HoxA3, particularly those genes that were repressed by HoxA3, including Gfi1B and other hematopoietic transcription factors (Fig. 4D). However, Runx1 was more potent than GATA1 in repressing genes that were upregulated by HoxA3, including many key endothelial regulatory factors, as well as regulators of adhesion and polarity (Fig 4E).
Figure 4. Global expression changes upon reversion of HoxA3 by Runx1 or Gata1.
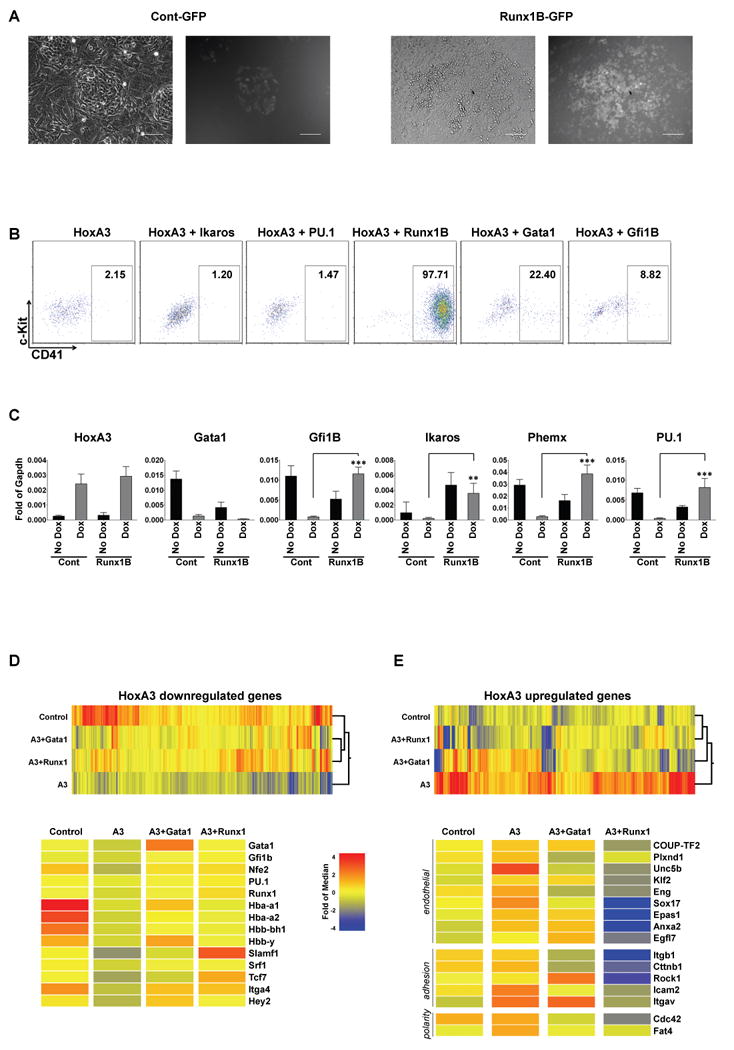
(A) Bright field (left) and fluorescent (right) images of HoxA3-induced day 6 EB-derived endothelial progenitors (F/V cells) transduced with control GFP vector or Runx1B-iresGFP retroviral vector and cultured on OP9 for 5 days. Scale bar =100 μm. (B) Representative flow cytometric profiles of F/V cells expressing HoxA3 and transduced with retroviral vectors expressing: ires-GFP (HoxA3), Ikaros (HoxA3+Ikaros), PU.1 (HoxA3+PU.1), Runx1-B (HoxA3+Runx1B), Gata1 (HoxA3+Gata1), Gfi1B (HoxA3+Gfi1B). GFP+ gated events are shown. X axis CD41, Y axis c-Kit. (C) Expression levels of hematopoietic marker genes (fold of Gapdh), without HoxA3 induction (No Dox, black bars) or with HoxA3 induction (+Dox grey bars) for control or Runx1B-transduced cells on OP9. n=3 independent experiments. Gfi1B: p=0.0003, Ikaros: p=0.0073, Phemx: p<0.0001, PU.1: p=0.0009. (D) Genes upregulated by HoxA3 clustered according to their expression in control uninduced, HoxA3+Runx1iresGFP, HoxA3+Gata1iresGFP, and HoxA3+GFP sorted cells. Both Runx1 and Gata1 significantly reverse these HoxA3-induced changes, indicated by their clustering together and near to the non-HoxA3 expressing control. Examples are shown on the heat map below. (E) Genes downregulated by HoxA3. Runx1 reverses downregulation of HoxA3-regulated changes more effectively than Gata1, indicated by its clustering closest to control. Among these genes are critical endothelial regulatory factors, and genes involved in adhesion and cell polarity.
We tested putative HoxA3 recognition sequences within the Runx1 locus by ChIP and found that several, including a conserved sequence upstream of the P2 enhancer, were sites of direct binding (Sup. Fig. 3A,B). We then tested whether the loss of HoxA3 would lead to derepression of Runx1 in vivo by performing in situ hybridization with Runx1 probe on HoxA3 mutant embryos 37. At E8.5, Runx1 expression was never detected in the dorsal aortae of wild-type (0/24) or heterozygous (0/41) embryos, however in a significant number of null embryos (14/29), we observed precocious expression of Runx1 in endothelial cells of the dorsal aorta, and occasionally in excess hematopoietic cells within the aortic lumen (Fig. 5A), demonstrating that HoxA3 represses Runx1 in vivo.
Figure 5. Regulation of Runx1 by HoxA3.
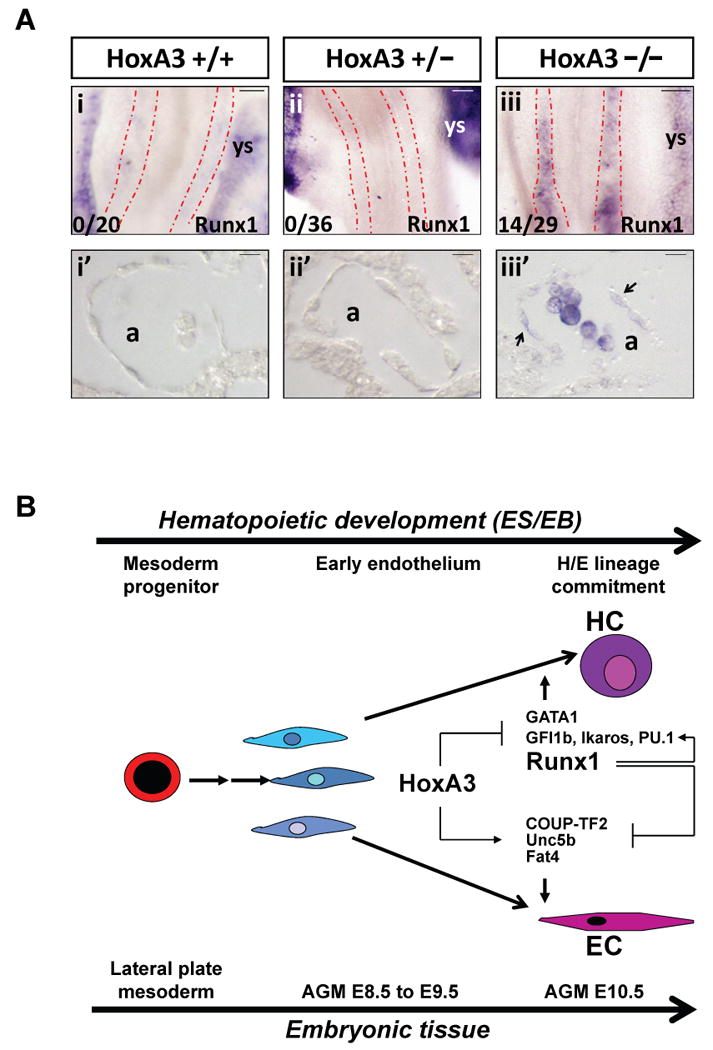
(A) In situ hybridization showing Runx1 expression in HoxA3 +/+, +/− and −/− E8.5 embryos. Runx1 expression is absent in the dorsal aortae of HoxA3 +/+ (i) and HoxA3+/− (ii) embryos, but robustly expressed in yolk sac. Runx1 is ectopically expressed in the dorsal aortae of HoxA3−/− (iii) embryos. Stippled red lines outline dorsal aortae. Penetrance of this phenotype is indicated at lower left. (i′-iii′) Sections of the embryos shown above. Both endothelial and hematopoietic cells are negative for Runx1 in wild-type or heterozygous embryos, while Runx1-expressing cells are found in HoxA3−/− embryos. Arrows indicate Runx1-expressing endothelial cells. a, aorta; ys, yolk sac. Scale bar = 50 μm for whole mounts, 10 μm for sections. (B) Model for regulation of endothelial hemogenesis by HoxA3 and Runx1. HoxA3 represses a cascade of transcription factors that promote hemogenesis and induces a set of genes that maintain endothelial character. Runx1 is a positive regulator of most of these transcription factors, and a negative regulator of genes essential for endothelial character, thus transient expression of Runx1 erases the endothelial program and initiates the hematopoietic.
These data demonstrate that HoxA3 sits at the apex of a cascade of regulatory factors, such that the spatiotemporal regulation of HoxA3 expression determines where and when hemogenic potential arises within embryonic vessels. HoxA3 represses hemogenesis in part by blocking the expression of Runx1, which would otherwise activate numerous downstream transcription factors to promote hematopoietic development. This finding has implications for the two modes of hematopoiesis in early embryonic development: yolk sac mesoderm, which does not express HoxA3, undergoes synchronous hematopoietic and endothelial differentiation, while hematopoietic differentiation from lateral plate mesoderm, which expresses HoxA3, is delayed, and occurs by budding from a well-defined endothelium. It is notable that Runx1 was shown to be required only transiently, and in the endothelial cells, not the derived hematopoietic cells, of the AGM 5. The expression profile of cells expressing both HoxA3 and Runx1 indicates that an essential role of Runx1 is to extinguish an endothelial program. This is a transient requirement, and not necessary in the yolk sac where endothelial and hematopoietic cells develop concurrently, not sequentially, but required in the AGM where hematopoietic cells emerge from an endothelium. Our model is summarized in Fig. 5B: endothelial progenitors produced in lateral plate mesoderm are inhibited from further differentiation towards hematopoeisis by HoxA3 which represses Runx1 together with other key hematopoietic transcription factors. HoxA3 is then extinguished within a subset of endothelium, allowing hemogenesis to occur. Although the presence of a hemogenic endothelium has been appreciated for almost a century 38, the spatiotemporal patterning of the embryonic endothelium into hemogenic and non-hemogenic domains, and the regulation of the endothelial-hematopoietic transition have remained inscrutable. The data presented here demonstrates that HoxA3 plays a critical role in both processes by acting as a gatekeeper at the apex of the transcriptional hierarchy restricting entry into the hematopoietic program.
Methods Summary
HoxA3 inducible ES cell generation culture and in vitro differentiation
HoxA3 cDNA (Y11717) was a gift from Dr Sarah Guthrie 39 and was subcloned into p2Lox, the targeting vector for the A2Lox ES cell line 16. HoxA3 inducible ES cells were generated by cassette exchange recombination into the doxycycline-inducible locus upstream of HPRT 1. ES cells were cultured on MEFs in DME supplemented with 15% FBS, 0.1 mM nonessential amino acids (GIBCO), 2 mM glutamax (Invitrogen), penicillin/streptomycin (Gibco), 0.1 mM β-mercaptoethanol, and 1000 U/mL LIF (Millipore), at 37°C in 5% CO2, and differentiated as EBs by preplating for 40 minutes to remove MEFs followed by suspension culture in hanging drops (100 cells per 10μL drop) in EBD medium: IMDM supplemented with 15% FBS, 200 μg/mL iron-saturated transferrin (Sigma), 4.5 mM monothiolglycerol (Sigma), 50 μg/mL ascorbic acid (Sigma), penicillin/streptomycin (Gibco), and 2 mM glutamax at 37°C in 5% CO2, 5% O2. After 48 hours, EBs were harvested from hanging drops by collecting and settling in IMDM, resuspended in 10mL of EBD and plated in nonadherent 10 cm dishes on a swirling rotator (1 rpm). EBs were fed after 48 hours by exchanging 50% of spent medium for fresh EBD medium.
Methylcellulose colony assays
For each colony assay, 50,000 cells were plated into 1.5 mL methylcellulose medium supplemented with IL3, IL6, Epo, and SCF (M3434, StemCell Technologies), and where indicated 1 μg/mL doxycycline. Primitive erythroid colonies were counted after 6 days, other colonies after 10 days.
OP9 cocultures
200,000 c-Kit+/CD41+ or Flk-1+/VE-cad+ cells from day 6 EBs were purified by flow cytometry and plated on OP9 monolayers (50,000 OP9 cells per well preplated in 6-well dishes one day prior) in IMDM supplemented with 10% FBS, 5 ng/mL VEGF, 40 ng/mL TPO, 40 ng/mL Flt-3 ligand, penicillin/streptomycin (from 100×, Gibco), 2 mM glutamax, and 0.5 ug/mL doxycycline at 37°C in 5% CO2, 5% O2. Semi-adherant cells were passaged by trypsinization every 4-5 days and replated plated at a density of 50,000 cells per well in 6-well dishes.
For ex vivo AGM cultures, 6 AGMs were pooled and dissociated with 0.25% Collagenase I. Cells were then transduced either with control (pMSCV-iresGFP) or HoxA3 retroviral vector (pMSCV-HoxA3-iresGFP) and cocultured on OP9 monolayers in IMDM supplemented with 10% FBS, 5 ng/mL VEGF, 40 ng/mL TPO, 40 ng/mL Flt-3 ligand, 5 ng/ml IL3, 50 ng/ml Ang1, 1000 U/mL LIF (Millipore), penicillin/streptomycin (Gibco), 2 mM glutamax, at 37°C in 5% CO2, 5% O2. Retroviral transduction of pMSCV-ires-GFP, MSCV-Runx1B, (kind gifts from Dr S. Tsuzuki) PU.1- (BC003815), Ikaros- (BC018349), Gfi1B- (BC052654) Gata1- (NM_008089.1) ires-GFP were performed on day 6 FV sorted progenitors. Retroviral transduction of pMSCV-ires-GFP, MSCV-Runx1B, (kind gifts from Dr S. Tsuzuki), MSCV-PU.1, MSCV-Ikaros, MSCV-Gata1 and MSCV-Gfi1B were performed as reported previously 17.
Embryo in situ hybridization
HoxA3 (Y11717) and Runx1 (BC069929) were used as templates for digoxigenin-labeled probes. Hybridizations were performed as described in 40. The HoxA3 knockout mice were kindly provided by Mario Capecchi.
Chromatin Immunoprecipitation
Chromatin Immunoprecipitation was performed by using Magna ChIP G protocol (Millipore). EBs were cultured as described above, and induced from day 4 to day 6. Between 107 and 2×107 disaggregated day 6 EB cells were crosslinked for 5 minutes with 1% formaldehyde and lysed. Chromatin was sheared to obtain DNA fragments between 200 and 500 bp. Immunoprecipitations used goat anti-mouse HoxA3 polyclonal (HoxA3G-14 SC22384 Santa Cruz) and IgG control (Chrompure goat IgG) antibodies. The following primer sets were used for qPCR: N1: F 5′-ttggaactcttagccttgggacc-3′ R 5′-tagatgcttcccagagaagtg-3′; N2: F 5′-tactctgggtagtccagtatttgg-3′ R 5′-cctatgacaaaggactaatcagagtg-3′; H1: F 5′-cctctcatttcacgttgcag-3′ R 5′-ggcttcacatttggaccagt-3′; H2: F 5′-ttccgtaatcctggcatgcag-3′ R: 5′-agtctttgctgtgcagtttc-3′; H4: F 5′agcagcagaagactgcagg-3′ R 5′-agtgcagatcactcgagg-3′; H5: F 5′-cctgaggatcaagctcgtgt-3′ R: 5′-tgggtgaaaaggaggtcatc-3′
Microarray experiments
HoxA3 was induced with 1 μg/mL doxycycline in day 5 + 18 hours EBs, and cells were harvested 6 hours later, at day 6. 3 independent experiments were performed. cRNA was hybridized to MouseWG-6 Bead Chip Arrays (Illumina) and raw data were processed using Beadstudio (Illumina) and analyzed on Genespring GX 7.3.1 (Agilent). For microarray experiments of inducible HoxA3 FV cells transduced with Gata1 or Runx1, cells were cultured on OP9, 5000 GFP+ cells were sorted, RNA was amplified by using SuperAmp (Miltenyi) amplification and Cy3- labeled cDNAs hybridized to Agilent Whole Mouse Genome Oligo Microarray 4 × 44K.
For qPCR validation, probes for HoxA3, Runx1, Gata1, PU.1, Ilk, Lycat, Nr2f2 and PlexinB1 were purchased from Applied Biosystems. Additional qPCR primers: Gfi1b 5′-CTAGAAAGGACCGTGGCATT-3′ 5′-CAGGGACAGTGTGGAGGTTC-3′; Phemx 5′-AGAATCTCCAGAAGGCCACC-3′ 5′-GAGCACCATAGCCACTGTGA-3′; Ikaros (Ikzf1) 5′-GCCTTTCTGGGTAAAGGAGG-3′ 5′-TGTCCACTACCTCTGGAGCA-3′.
Supplementary Material
Acknowledgments
We thank the Dr. Bob and Jean Smith Foundation for their generous support. This work was supported by the NIH grant 1R01HL081186-01 and the March of Dimes grant 5-FY2006-272. We thank Nardina Nash for genotyping and animal husbandry.
Footnotes
Author Contributions
Michelina Iacovino: experimental design and execution, wrote manuscript
Diana Chong: performed in situ hybridization studies
Istvan Szatmari: performed microarray studies
Lynn Hartweck: performed chromatin IP studies
Danielle Rux: performed chromatin IP experiments
Arianna Caprioli: performed in situ hybridization studies
Ondine Cleaver: experimental design, wrote manuscript
Michael Kyba: study and experimental design, wrote manuscript
References
- 1.Godin I, Dieterlen-Lièvre F, Cumano A. Emergence of multipotent hematopoietic cells in the yolk sac and para-aortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci USA. 1995;92:773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 3.de Bruijn MF, et al. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 4.North TE, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 8.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 9.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 10.Ivanova NB, et al. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 11.Taghon T, et al. Homeobox gene expression profile in human hematopoietic multipotent stem cells and T-cell progenitors: implications for human T-cell development. Leukemia. 2003;17:1157–1163. doi: 10.1038/sj.leu.2402947. [DOI] [PubMed] [Google Scholar]
- 12.Forsberg EC, et al. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauvageau G, et al. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acac Sci USA. 1994;91:12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cellot S, et al. Sustained in vitro trigger of self-renewal divisions in Hoxb4hiPbx1(10) hematopoietic stem cells. Exp Hematol. 2007;35:802–816. doi: 10.1016/j.exphem.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauvageau G, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- 16.Iacovino M, et al. A conserved role for Hox paralog group 4 in regulation of hematopoietic progenitors. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyba M, Perlingeiro RCR, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 18.Chisaka O, Capecchi MR. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991;350:473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- 19.Myers C, Charboneau A, Boudreau N. Homeobox B3 promotes capillary morphogenesis and angiogenesis. J Cell Biol. 2000;148:343–351. doi: 10.1083/jcb.148.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mace KA, Hansen SL, Myers C, Young DM, Boudreau N. HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. J Cell Sci. 2005;118:2567–2577. doi: 10.1242/jcs.02399. [DOI] [PubMed] [Google Scholar]
- 21.Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh DA. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–264. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Ferkowicz MJ, Johnson SA, Shelley WC, Yoder MC. Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. Stem Cells Dev. 2005;14:44–54. doi: 10.1089/scd.2005.14.44. [DOI] [PubMed] [Google Scholar]
- 23.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 24.North T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 25.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 26.Fehling HJ, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 27.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 28.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 29.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 30.Mitjavila-Garcia MT, et al. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development. 2002;129:2003–2013. doi: 10.1242/dev.129.8.2003. [DOI] [PubMed] [Google Scholar]
- 31.Lancrin C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita J, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 35.Okuda T, Deursen Jv, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, Is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabin FR. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contrib Embryol. 1920;9:213–262. [Google Scholar]
- 39.Guidato S, Prin F, Guthrie S. Somatic motoneurone specification in the hindbrain: the influence of somite-derived signals, retinoic acid and Hoxa3. Development. 2003;130:2981–2996. doi: 10.1242/dev.00496. [DOI] [PubMed] [Google Scholar]
- 40.Xu K, Chong DC, Rankin SA, Zorn AM, Cleaver O. Rasip1 is required for endothelial cell motility, angiogenesis and vessel formation. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.