Abstract
Functional connective tissues have been developed using tissue engineering approach by seeding cells on biodegradable scaffolds such as polyglycolic acid (PGA). However, a major drawback of tissue engineering approaches that utilize synthetic polymers is the persistence of polymer remnants in engineered tissues at the end of culture. Such polymer fragments may significantly degrade tissue mechanics and stimulate local inflammatory responses in vivo. In this study, several polymeric materials with a range of degradation profiles were developed and evaluated for their potential applications in construction of collagen matrix-rich tissues, particularly tissue-engineered blood vessels. The degradation characteristics of these polymers were compared as were their characteristics vis-à-vis cell adhesion and proliferation, collagen synthesis, and ability to support growth of engineered vessels. Under aqueous conditions at 37°C, Polymer I (comprising 84% glycolide and 16% trimethylene carbonate [TMC]) had a similar degradation profile to PGA, Polymer II (comprising 84% glycolide, 14% TMC, and 2% polyethylene succinate) degradedly more slowly, but Polymer III (comprising 87% glycolide, 7% TMC, and 6% polyethylene glycol) had a more extensive degradation as compared to PGA. All polymers supported cell proliferation, but Polymer III improved collagen production and engineered vessel mechanics as compared with PGA. In addition, more slowly degrading polymers were associated with poorer final vessel mechanics. These results suggest that polymers that degrade more quickly during tissue culture actually result in improved engineered tissue mechanics, by virtue of decreased disruption of collagenous extracellular matrix.
Introduction
Development of functional connective tissues, including small-diameter vascular grafts, is a rapidly growing field of endeavor.1,2 To create new biological tissues, cells are seeded onto polymer scaffolds that support cell proliferation, differentiation, and subsequently tissue regeneration.3,4 Many different types of polymer scaffolds have been tested for their use in the engineering of biological tissues, including naturally derived materials such as collagen gels and fibrin gels, and synthetic materials such as polyglycolic acid (PGA).5–8 For example, Weinberg and Bell described the development of a small-caliber vascular graft in vitro by seeding smooth muscle cells (SMCs) into a collagen gel.9 However, the use of collagen gels to generate connective tissues such as vascular grafts has been limited because collagen gels can inhibit cellular collagen production.10,11 Fibrin gels, on the other hand, have been shown to promote the deposition of collagen and other extracellular matrix,10,12,13 but they require careful modulation of gel breakdown using protease inhibitors14 and are difficult to form into long conduits.
In contrast to naturally derived biological gels, synthetic polymers can be easily fashioned into a variety of geometrical structures having reproducible properties. Our group has developed functional vessels with high collagen contents and sufficient mechanical properties for in vivo implantation using a bioreactor system where SMCs are seeded onto the absorbable nonwoven mesh made of PGA.15,16
However, although synthetic materials offer a number of advantages over naturally occurring materials, they also suffer from significant drawbacks, including low void volume and slow degradation rates. Persistence of artificial material in the final tissue can serve as a nidus for inflammation or infection after implantation. In addition, we have previously shown that even for rapidly degrading PGA, polymer remnants in the engineered vessels can substantially degrade tissue mechanics, as they cause stress concentrations in the collagenous tissue matrix.17
Ideally, a polymer scaffold material for constructing connective tissues should permit cell adhesion and proliferation, and support extracellular matrix synthesis and deposition. The polymer scaffold should also have high initial void volume and a rapid degradation profile, both of which serve to minimize the amount of polymeric material in the final, tissue-engineered product. PGA is one of the most widely used polymer scaffold materials in tissue engineering. It is synthesized by the ring-opening polymerization of glycolide and undergoes rapid degradation to soluble, safe by-products through chemical hydrolysis of the chain ester linkages. Nonwoven PGA meshes have been used extensively by our group15,16 and others to culture engineered vessels and other tissues such as cartilage,18 skin,19 tendon, and ligaments.20,21 However, PGA-based tissues often contain polymer fragments at the end of culture.15,16,22,23 To achieve a goal of creating biological connective tissues with minimal to no synthetic polymeric fragments in the final product, and hence maximal mechanical properties, there was an obvious need for a scaffold that degrades very rapidly, and yet supports cellular adhesion and collagenous matrix deposition.
We have developed a series of nonwoven meshes made of absorbable glycolide-based co-polyesters, which are structurally tailored to address this need in tissue engineering. These materials were designed with a range of degradation profiles. We systematically compared the hydrolysis/degradation profiles of these newly developed polymers, using PGA mesh as a standard control. The functions of these materials in supporting cell adhesion, proliferation, and collagen production were also determined and compared with PGA. Our studies suggest that one of the newly developed polymers might provide a better scaffold for generating collagen matrix-rich and mechanically strong constructs, than the commonly used PGA scaffold.
Materials and Methods
Polymer scaffolds
Polymer scaffolds including PGA and glycolide-based co-polyesters, denoted as Polymers I, II, and III (Tables 1 and 2), were supplied by Concordia Medical. The polymers and their respective multifilament yarns were made by Poly-Med, Inc., following previously reported procedures.24–26 Concordia Medical produced nonwoven scaffolds from multifilament yarns, which served as test materials herein. Polymer I was made from trimethylene carbonate (TMC) and glycolide (G), which have been used previously to prepare the polymeric precursors of a commercial absorbable monofilament suture. For the preparation of Polymer II, a prepolymer of polyethylene succinate was made and grafted with glycolide and TMC. Polyethylene succinate degrades into succinic acid and ethylene glycol. Polymer III was prepared by grafting TMC and glycolide onto a water-soluble polyethylene glycol (PEG). Compared with Polymers I and II, Polymer III was designed to have a higher propensity for absorbing water and higher rate of degradation. The physical characteristics of the PGA and Polymers I, II and III are listed in Tables 1 and 2.
Table 1.
Properties of Polymers
| Polymer | Structure | Monomers and prepolymers | Inherent viscositya(dL/g) | Glass transition temperature, Tg (°C) | Melting temperature, Tm (°C) |
|---|---|---|---|---|---|
| PGA | Poly-glycolic acid | 100% G | 0.91 | 38 | 225 |
| Polymer I | Tri-axial with pTMC central segment having basic monocentric nitrogen | 84% G, 16% TMC | 0.90 | 21 | 195 |
| Polymer II | Linear with slow-absorbing PES central segment | 84% G, 14% TMC, 2% PES | 0.93 | 50 | 213 |
| Polymer III | Linear with hydrophilic central PEG segment | 87% G, 7% TMC, 6% PEG | 1.09 | 34 | 207 |
Using hexafluoroisopropyl alcohol (HFIP) as a solvent.
TMC, trimethylene carbonate; PES, polyethylene succinate; PEG, polyethylene glycol.
Table 2.
Components of Polymers
| Glycolide (G) | |
| Trimethylene carbonate (TMC) | |
| Polyethylene succinate (PES) |  |
| Polyethylene glycol (PEG) |  |
| Polytrimethylene carbonate (pTMC) |  |
After polymerization, the polymers are melted and extruded into yarns through a multi-holed spinneret producing 30 or 35 filaments per end, depending on the spinneret. After extrusion, the yarn was oriented using heated godets to achieve the required tensile properties and single filament diameter of ∼15 μm. The multifilament yarns were subsequently made into nonwoven meshes through the BIOFELT™ process at Concordia Medical.
Surface hydrolysis of polymer scaffolds
To increase fiber wettability, similar to what has been reported for PGA polymer,27 polymer scaffolds in the form of nonwoven meshes were incubated in 1.0 N NaOH for 1 min to cleave ester linkages on the fiber surface. This step has previously been shown to produce alcohol and carboxylic acid groups in the fiber surface, and also significantly enhances cellular adhesion to PGA scaffolds.27 Since all of the polymers under investigation were between 84% and 100% PGA, treatment with NaOH in this manner should similarly cleave glycolide linkages. After NaOH treatment, mesh samples were rinsed extensively in distilled water to remove the residual NaOH, sterilized with 70% ethanol, and air-dried overnight under sterile conditions.
Scanning electron microscopy
Polymer mesh samples were processed for scanning electron microscopy (SEM) as described previously.28,29 Briefly, samples were fixed with 1% glutaraldehyde solution in 0.1 M sodium cacodylate buffer (pH 7.4; Polysciences) for 5 min, washed with distilled water for 5 min, and dehydrated with 5-min exchanges in each of 70%, 85%, and 95% aqueous ethanol and absolute ethanol. Samples were further immersed in hexamethyldisilazane (Polysciences) for 10 min and air-dried overnight. Dried samples were mounted, sputter-coated with gold (Cressington Sputter Coater 108 auto), and examined with a Phillips XL-30 environmental scanning electron microscope (FEI). The average diameter of the polymer fiber was determined from SEM images captured at 3000 × magnification using NIH ImageJ software. For each polymer sample, 20 fibers were randomly chosen and the diameters were measured.
Polymer mass degradation studies
Polymeric samples (∼100 mg) (W0) were placed in tubes containing 5 mL phosphate-buffered saline (PBS, pH 7.4; Invitrogen) supplemented with 1% (v/v) penicillin/streptomycin (Pen/Strep; Invitrogen) at 37°C, 5% CO2 for up to 8 weeks. Every 7 days, the liquid fraction was removed from the tubes and the tubes containing nondissolved polymeric fibers were lyophilized. The weight of the remaining polymer (Wt) was measured, and the mass remaining was determined as
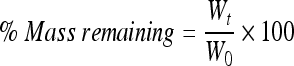 |
(1) |
For each polymer sample, at least three replicates were taken and averaged to determine the degradation profile.
SMC adhesion to scaffolds
Vascular SMCs were isolated from the medial layer of the thoracic aortas of Yucatan miniature pigs as described previously27 and cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% (v/v) fetal bovine serum (Hyclone), 10% (v/v) porcine serum (Gibco), and 1% (v/v) Pen/Strep at 5% CO2, 37°C.
To compare the cell adhesion on different polymer scaffolds, the polymer mesh was cut into small pieces (1 × 2 cm), treated with 1N NaOH for surface hydrolysis, and placed in an individual well of the six-well plate. About 0.2 mL of SMC suspension was seeded onto each mesh square at a seeding density of 10 × 106 cells/mL. Samples were incubated in 10 mL of medium at 5% CO2, 37°C for 24 h, and then mesh samples were assayed for total DNA content as a measure of cell adhesion to each mesh. At least three replicates were taken for each polymer sample.
Engineered vessel culture on scaffolds
Growth of functional connective tissues, including blood vessels, is critically dependent upon collagen production by constituent cells, as well as cell growth and scaffold biocompatibility.15,30 Vessels were cultured from porcine SMC using techniques similar to those described previously.15–17 Porcine SMC were expanded in flasks to passage 3 or 4, and were seeded onto tubular scaffolds of 3 mm diameter that were mounted inside of sterilized glass bioreactors. Vessels were cultured at 37°C, 5% CO2 in vessel growth medium to support collagen synthesis15,17 for 8 weeks. The vessel growth medium is Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 10% porcine serum, penicillin G (100 U/mL; Sigma), ascorbic acid (50 μg/mL), CuSO4 (3 ng/mL), 5 mM HEPES, proline, alanine, glycine, basic fibroblast growth factor (10 ng/mL), and platelet-derived growth factor (10 ng/m).15,17 The culture medium was refreshed weekly, and ascorbic acid was supplemented every 2 days.
Determination of polymer remnants
The areas of polymer remnants were measured from high-resolution histological images (n = 6 for each sample) of engineered vessels using NIH ImageJ. The area of polymer remnants in the vessel grown on PGA was set as 1, and other areas of polymer remnants were expressed relative to PGA area.
Immunostaining and immunoblotting
SMC phenotypes in engineered vessels were determined by immunostaining for α-smooth muscle actin (α-SMA) as described previously.29 α-SMA antibody (Dako) was used at a dilution of 1:1000. Expression of SMC markers in engineered vessels was also determined by immunoblotting performed similarly to previously described.31 Concentrations for monoclonal antibodies used are 1:8000 for β-actin (Sigma-Aldrich), 1:400 for α-SMA (Dako), and 1:200 for calponin (Dako).
Quantification of cell density by DNA assay
Cell-seeded mesh samples were digested in papain buffer and the DNA content was determined fluorometrically using Hoechst 33258 dye (DNA Quantitation Kit, Fluorescence Assay; Sigma) as describe previously.32 SMC density was calculated on the basis of 8 pg DNA per porcine SMC.27
Quantification of collagen content by hydroxyproline assay
The collagen content of engineered vessel samples was determined by measuring hydroxyproline levels, as described previously.33 Collagen was calculated as 10 times the amount of hydroxyproline.34
Measurement of vessel mechanics
Vessel mechanical characteristics, including maximal modulus and maximal wall stress, were measured as described previously.16,17 Vessels were attached using sutures to a perfusion system that allowed manual application of intraluminal fluid at controlled pressures. Vessel outer diameter was measured from high-resolution camera images, and inner diameter and wall thickness were calculated from the outer diameter measurements and vessel cross-sectional areas obtained from histology. Wall stress and maximal modulus were calculated as previously described.17
Statistics
Data are expressed as mean ± standard deviation. Statistical significance was determined by one-way analysis of variance. Difference (p-value) <0.05 was considered significant.
Results
Polymer physical characteristics and scaffold morphology
The polymers under study were grossly similar to PGA with respect to inherent viscosity and melting temperature, though the glass transition temperature for Polymer II was higher than the other materials. PGA had an inherent viscosity of 0.91 dL/g (Table 1), and the other polymers had similar or higher inherent viscosities. While the glass transition temperature (Tg) of Polymer II was 50°C (Table 1), the other polymers (PGA and Polymers I and III) had a Tg that was around or lower than 37°C, suggesting that Polymer II but not the other polymers might be glassy under tissue culture conditions. However, polymer fiber meshes for all of the materials were compliant at room temperature and at 37°C. (The word “compliant” is used to describe the physical properties of the polymeric materials as being “not brittle” in our studies.)
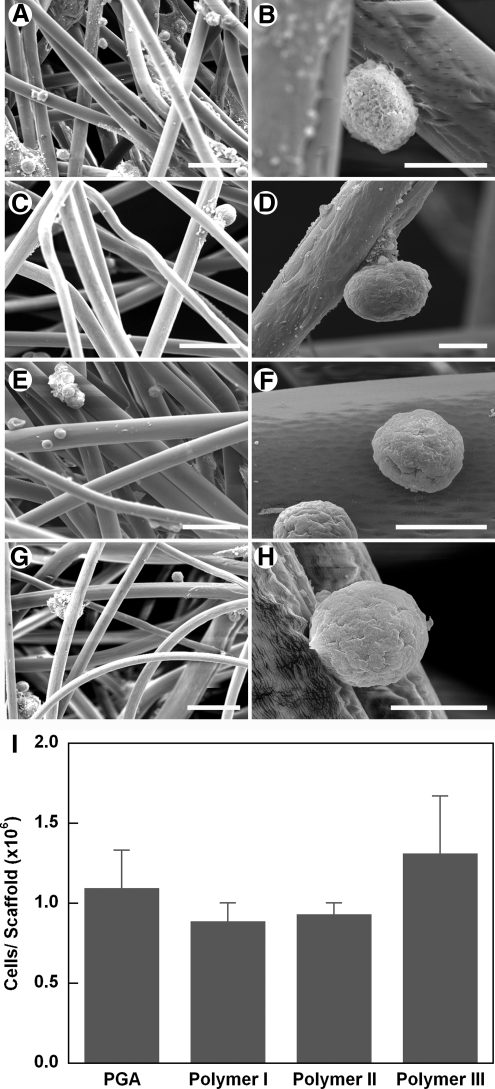
The PGA and Polymers I, II, and III scaffolds for this study were all 1 mm thick with a density of 50 mg/mL. SEM was used to examine the scaffold structure and surface morphology. Polymers I, II, and III all had fibrous mesh structures similar to PGA and were highly porous (Fig. 1A–D). The diameter of fibers in each type of mesh was fairly uniform and average fiber size ranged from 13.5 to 16.0 μm, with no significant differences between any of the polymer samples (Fig. 1E).
FIG. 1.
(A–D) SEM images of polymeric fibers. (A) PGA; (B) Polymer I; (C) Polymer II; (D) Polymer III. Scale bar, 500 μm. The insets show representative high-magnification SEM images. Scale bar, 10 μm. (E) Fiber diameter of polymers. Data are represented as mean ± s.d. from 20 random measurements. PGA, polyglycolic acid; SEM, scanning electron microscopy; s.d., standard deviation.
Polymer degradation
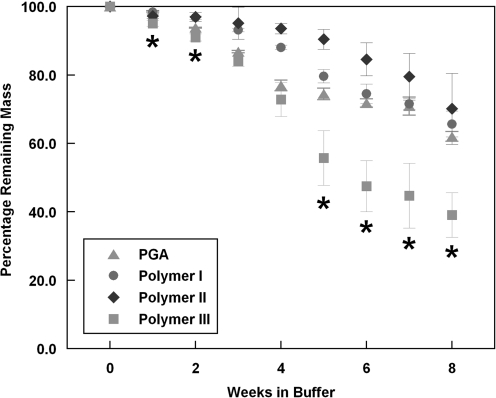
In light of the importance of scaffold degradation rate and minimization of polymeric fragments in the final engineered product, the degradation rates of the various polymers were compared at 37°C under aqueous conditions in PBS. To obtain an estimate of polymer degradation behavior over an 8-week vessel culture period, polymer mesh samples were incubated in pH 7.4 PBS at 37°C and 5% CO2 for up to 8 weeks. As shown in Figure 2, a 13.2% mass loss of PGA was observed after 3 weeks of incubation in PBS, and ∼40% of its original mass was lost at the end of 8 weeks. This result is roughly comparable to previous reports of PGA mesh degradation under aqueous conditions.22 Polymer I had a similar degradation profile to PGA, with a mass loss of about 35% after 8 weeks. By comparison, Polymer II degraded very slowly at the first 5 weeks, and still had 70% of its original mass at the end of the 8 weeks. Polymer III showed a similar amount of mass loss as PGA at the 3-week and 4-week time points, but degraded significantly faster than PGA thereafter with over 60% of its original mass being lost at the end of 8 weeks (Fig. 2), indicating a more extensive degradation as compared to PGA. These data suggest that Polymer III might result in fewer polymer fragments than PGA in engineered tissues after prolonged culture periods.
FIG. 2.
Hydrolytic degradation profiles of polymer fibers in phosphate-buffered saline at 37°C, 5% CO2. Data are represented as mean ± s.d. of at least three determinations. *Polymer III is significantly different from PGA, p < 0.0005.
Cell adhesion on polymer meshes
To compare cell adhesion on the scaffolds, porcine SMC were seeded onto the surface-hydrolyzed polymer meshes and incubated at 37°C and 5% CO2 for 24 h. Six hours after seeding, a sub-set of samples were examined by SEM and were observed to adhere to the surfaces of all of the polymer fibers, both as individual cells and as cell aggregates (Fig. 3A–H). No substantial differences in cell morphology were observed between PGA and other polymers. Quantification of cell seeding density indicated that about 1.1 ± 0.2 × 106 cells were attached to the 1 × 2 cm PGA mesh at 24 h after seeding, which equals ∼55% of the total number of cells exposed to the mesh (Fig. 3I). These results were similar to the reported cell adhesion efficiency of 49% on 1N NaOH-treated PGA polymers at 2 h.27 There was no significant difference in cell seeding density between any of the polymer scaffolds (Fig. 3). These data indicate that, for early time points, all of the scaffolds achieved similar cell seeding efficiencies as compared to PGA mesh.
FIG. 3.
SEM images of porcine SMCs cultured on polymer scaffolds for 6 h. (A, B) PGA; (C, D) Polymer I; (E, F) Polymer II; (G, H) Polymer III. Scale bars, 50 μm in (A, C, E, G) and 10 μm in (B, D, F, H). (I) Cell density of porcine SMCs cultured on polymer scaffolds for 24 h. Data are presented as mean ± s.d. of at least three determinations. SMC, smooth muscle cell.
Vessel culture on polymer scaffolds–Pilot study
To evaluate the effects of the different polymer scaffolds on cell proliferation, collagen production and tissue formation over longer time periods, SMCs were cultured on scaffolds for 8 weeks to form engineered blood vessels as described in the Materials and Methods section. Vessel culture experiments were conducted in two phases. In the first phase, pilot studies were conducted to evaluate which (if any) novel scaffold resulted in tissue-engineered porcine vessels that were comparable or superior to those cultured on PGA mesh. For the pilot studies, three bioreactors were fitted to each contain two engineered vessels. In each bioreactor, one vessel was cultured on PGA mesh, whereas the other vessel was cultured on scaffold made from Polymer I, II, or III (Fig. 4). In this way, cells and culture medium were controlled within each bioreactor between the PGA and experimental scaffolds, allowing for differences resulting from the scaffold per se to be assessed.
FIG. 4.
Gross photos of porcine engineered vessels cultured in bioreactors for 8 weeks. (A) Polymer I and PGA; (B) Polymer II and PGA; (C) Polymer III and PGA. Vessels are 3 mm internal diameter, and 8 cm in length. Color images available online at www.liebertonline.com/tea
After 8 weeks of culture, vessels grown on all of the scaffolds appeared grossly similar (Fig. 4). However, histological analysis using Masson's Trichrome stain, which stains collagen blue and polymer fragments white, revealed obvious differences between resultant engineered vessels (Fig. 5). Vessels cultured on PGA (Fig. 5A, B) and Polymer III (Fig. 5G, H) showed much smaller and substantially fewer polymer fragments as compared to those grown on Polymers I (Fig. 5C, D) and II (Fig. 5E, F). The engineered vessel grown on Polymer III contained the least amount of visible polymer fragments after 8 weeks' culture (Fig. 5I), as predicted by the hydrolysis study at 37°C. These results suggest that simple in vitro tests of scaffold degradation—in buffer at 37°C—have some predictive value for polymer residuals in engineered tissues.
FIG. 5.
Masson's trichrome stain of porcine vessels grown on PGA (A, B), Polymer I (C, D), Polymer II (E, F), or Polymer III (G, H). Scale bars, 50 μm in (A, C, E, G) and 20 μm in (B, D, F, H). Arrowheads indicate polymer remnants. (I) Relative polymer remnants in porcine vessels after 8 weeks' culture. Color images available online at www.liebertonline.com/tea
SMC markers, including α-SMA and calponin, were found to be expressed by vessels grown on all polymer scaffolds at the end of 8 weeks (Fig. 6). By immunohistochemistry, α-SMA-positive cells were found to be distributed across the vessel wall for all polymers (Fig. 6A–D). By immunoblotting, the level of α-SMA expression was no different among polymers, but vessels grown on Polymer II expressed substantially less calponin as compared to PGA and Polymers I and II (Fig. 6E).
FIG. 6.
Immunostaining for α-SMA of engineered vessels grown on PGA (A), Polymers I (B), II (C), or III (D). Scale bar, 50 μm. (E) α-SMA and calponin immunoblotting of vessels grown on polymers as shown in (A–D). β-actin staining is used as a loading control. α-SMA, α-smooth muscle actin. Color images available online at www.liebertonline.com/tea
In the pilot experiment, the maximal wall stress and maximal modulus of the vessel grown on Polymer III (2.0 and 16.3 MPa, respectively, n = 1) were not substantially different from those of the three PGA vessels (2.1 ± 0.4 and 19.7 ± 4.5 MPa, respectively, n = 3). Collagen content as a percentage of dry weight, which (other things being equal) is generally a good predictor of maximal elastic modulus in engineered vessels,16 was 42.3%, 34.2%, and 43.7%, respectively, for pilot vessels grown on Polymers I, II, and III. For vessels grown on PGA, collagen was 45.0% ± 0.6% of dry weight, which was not substantially different from that observed with either Polymer I or III. However, the vessel that was cultured on Polymer I (Fig. 5C, D) had a lower maximal wall stress and modulus (1.3 and 10.2 MPa, respectively, n = 1) than those of vessels grown on PGA. The maximal wall stress and modulus of the Polymer II vessel (1.0 and 5.1 MPa, respectively, n = 1) was also substantially lower than those of PGA vessels.
Vessel culture on polymer scaffolds: Follow-on study
Based upon these observations, we elected to study vessels that were grown on Polymer III in more depth, since this polymer appeared to produce the highest quality engineered vessel in the pilot study. We cultured three more vessels each for Polymer III and for PGA. The Polymer III scaffold had a density of 50 mg/mL and a filament size of 1.8 dpf (filament size ∼13 μm), whereas the PGA scaffold had the same density and a filament size of 2.0 dpf (filament size ∼14 μm). Porcine vessels were cultured as described for 8 weeks. Vessels grown on PGA versus Polymer III had similar levels of cellular density (Fig. 7A) and collagen (Fig. 7B). However, vessels grown on Polymer III trended toward increased maximal wall stress and maximal modulus as compared to PGA vessels (Fig. 7C, D). Although the difference in maximal wall stress did not achieve statistical significance (perhaps due to n = 3, p = 0.09), there was evidence that vessels cultured on Polymer III have improved mechanics as compared to PGA, despite similar cell densities and collagen contents.
FIG. 7.
Characteristics of porcine engineered vessels grown on PGA versus Polymer III. (A) DNA content, (B) Collagen content, (C) Maximal wall stress, and (D) Maximal modulus. Data are presented as mean ± s.d. of three determinations.
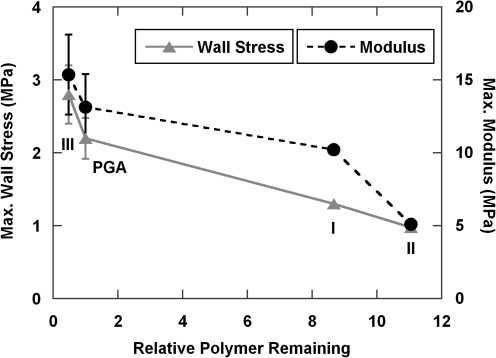
Interestingly, both wall stress and modulus are inversely related to relative polymer remnants in the vessel walls (Fig. 8). Polymers I and II had substantially more polymer fragments at the end of culture than did polymer III and PGA. Even when collagen contents of vessels were similar (42.3% for Polymer I vs. 45.0% ± 0.6% for PGA), the vessel grown on Polymer I had lower modulus and maximal wall stress than PGA vessels. Lower modulus of the vessels grown on Polymers I and II, in the setting of higher amounts of polymer residuals than PGA, supports the hypothesis that residual polymer fragments can cause stress concentrations that degrade the bulk mechanics of engineered tissues. By extension, reducing polymer fragments at the end of culture should be important for optimizing engineered tissue mechanics.17
FIG. 8.
Relationship of maximal wall stress and modulus to the relative polymer remnants in porcine vessels at 8 weeks. Data were from both pilot and follow-on experiments. Both wall stress and modulus are inversely related to relative polymer remnants in the vessel walls. For PGA and Polymer III, n = 3 and data are represented as mean ± s.d. For Polymers I and II, n = 1.
These data support our hypothesis that fast-degrading polymer scaffold supports the construction of connective tissues with maximal mechanical properties. Thus, Polymer III may represent a superior scaffolding material over PGA for the culture of engineered connective tissues.
Discussion
The goals of the present study were to evaluate the utility of three novel biodegradable polymers as scaffold materials for constructing connective tissues, specifically vascular tissues, in comparison with PGA. We chose PGA as the gold standard because so much previous work has been done with this nonwoven scaffold, and because our laboratory has extensive experience with tissues grown on this material. Assessment of polymer hydrolysis under aqueous conditions indicated that Polymer III had a faster and more extensive degradation than PGA, even though Polymer III contains some 87% of PGA in its backbone. It has been reported previously that PGA undergoes bulk hydrolysis (i.e., erosion from both the inside and outside of the fibers) under tissue culture conditions.35 Since Polymer III contains an additional hydrophilic central PEG segment and has higher propensity for absorbing water than PGA, we anticipated that Polymer III would degrade faster than PGA. We observed no significant difference in the physical appearance (including fiber size and surface morphology) between Polymer III and PGA, suggesting that the addition of PEG segment in Polymer III might be the possible mechanism by which Polymer III degrades more rapidly and completely than PGA at 37°C under aqueous conditions.
Vessels grown on Polymer III showed little evidence of polymer fragments, as did PGA vessels at the end of the 8-week culture period. However, 40% of the original mass for Polymer III or 60% for PGA were still maintained after 8 weeks in PBS at 37°C. On the other hand, vessels cultured with Polymers I and II had markedly more fragments at 8 weeks than did PGA vessels. However, no distinct difference was observed between PGA and Polymers I and II at the end of the hydrolysis study at 37°C in PBS. It has been suggested that cells could promote polymer degradation via enzyme-catalyzed hydrolysis.36 Thus, the discrepancy of polymer degradation profiles in PBS versus in bioreactors might result from the different cellular effects on the hydrolytic degradation of these individual polymers. Therefore, these data suggest that degradation in buffer might be grossly correlated with, but is not completely predictive of, degradation under tissue culture conditions.
We observed that Polymer III, and also Polymers I and II, supported initial SMC adhesion similarly to PGA. All of the polymers under study had PGA as a major constituent. Previous studies have shown that PGA breakdown products, such as glycolic acid monomers and oligomers, induce SMC de-differentiation, switching cells from a quiescent, contractile state to a proliferative, matrix-synthesis state.15,16,37 Hence, all of the materials under study would be expected to support collagen synthesis similarly in SMC.
All connective tissues that support tensile or compressive loads rely on collagen for their mechanical properties. For the engineering of connective tissues, providing a substrate that allows collagen deposition and evolution of excellent tissue mechanics is critical. Whether an engineered tissue is designed to remodel in the body and acquire adequate mechanics in situ, or whether it is designed to possess requisite collagen-based mechanics at the time of implantation, the scaffolding material must support the development of necessary mechanical properties. As such, an ideal scaffold for connective tissue growth likely has a high void volume, combined with a rapid degradation profile that allows cells to deposit extracellular matrix in an uninterrupted environment. Although a polymer that degrades too quickly will dissolve before the tissue can form, it is clear that a material that degrades too slowly also has adverse effects on tissue mechanics and organization. Novel polymers that degrade more quickly than current alternatives, while also stimulating collagen synthesis (e.g., by elaborating glycolic or lactic acid upon degradation), will greatly facilitate the development of engineered connective tissue substitutes.
Conclusion
An optimal polymer scaffold plays an important role in the successful construction of biological tissues by providing proper surface for cell attachment, proliferation, differentiation, and tissue regeneration. Herein, we systematically compared three polymers with PGA and demonstrated that Polymer III degraded faster and more completely, and also resulted in somewhat improved characteristics in tissue-engineered blood vessels. These findings make Polymer III possibly as a superior scaffold for preparation of tissue-engineered vascular grafts, as well as other engineered connective tissues.
Acknowledgments
This work was supported by NIH R01 HL083895 and R01 EB008366 (to L.E.N.).
Disclosure Statement
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Niklason L.E. Langer R. Prospects for organ and tissue replacement. JAMA. 2001;285:573. doi: 10.1001/jama.285.5.573. [DOI] [PubMed] [Google Scholar]
- 2.Niklason L.E. Langer R.S. Advances in tissue engineering of blood vessels and other tissues. Transpl Immunol. 1997;5:303. doi: 10.1016/s0966-3274(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 3.Langer R. Vacanti J P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Vacanti J.P. Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):SI32. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 5.Isenberg B.C. Williams C. Tranquillo R.T. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W.J. Liu W. Cui L. Cao Y. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11:945. doi: 10.1111/j.1582-4934.2007.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nerem R.M. Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 8.Vacanti J.P. Langer R. Upton J. Marler J.J. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev. 1998;33:165. doi: 10.1016/s0169-409x(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg C.B. Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 10.Clark R.A. Nielsen L.D. Welch M.P. McPherson J.M. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995;108(Pt 3):1251. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- 11.Thie M. Schlumberger W. Semich R. Rauterberg J. Robenek H. Aortic smooth muscle cells in collagen lattice culture: effects on ultrastructure, proliferation and collagen synthesis. Eur J Cell Biol. 1991;55:295. [PubMed] [Google Scholar]
- 12.Jockenhoevel S. Zund G. Hoerstrup S.P. Chalabi K. Sachweh J.S. Demircan L. Messmer B.J. Turina M. Fibrin gel—advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001;19:424. doi: 10.1016/s1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 13.Grassl E.D. Oegema T.R. Tranquillo R.T. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60:607. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 14.Ye Q. Zund G. Benedikt P. Jockenhoevel S. Hoerstrup S.P. Sakyama S. Hubbell J.A. Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17:587. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 15.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 16.Niklason L.E. Abbott W. Gao J. Klagges B. Hirschi K.K. Ulubayram K. Conroy N. Jones R. Vasanawala A. Sanzgiri S. Langer R. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 17.Dahl S.L. Rhim C. Song Y.C. Niklason L.E. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed L.E. Vunjak-Novakovic G. Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cell Biochem. 1993;51:257. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- 19.Hansbrough J.F. Cooper M.L. Cohen R. Spielvogel R. Greenleaf G. Bartel R.L. Naughton G. Evaluation of a biodegradable matrix containing cultured human fibroblasts as a dermal replacement beneath meshed skin grafts on athymic mice. Surgery. 1992;111:438. [PubMed] [Google Scholar]
- 20.Liu W. Chen B. Deng D. Xu F. Cui L. Cao Y. Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model. Tissue Eng. 2006;12:775. doi: 10.1089/ten.2006.12.775. [DOI] [PubMed] [Google Scholar]
- 21.Cao D. Liu W. Wei X. Xu F. Cui L. Cao Y. In vitro tendon engineering with avian tenocytes and polyglycolic acids: a preliminary report. Tissue Eng. 2006;12:1369. doi: 10.1089/ten.2006.12.1369. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar V. Grinstaff M.W. Alarcon J. Knors C. Solan A.K. Niklason L.E. Engineering porcine arteries: effects of scaffold modification. J Biomed Mater Res A. 2003;67:303. doi: 10.1002/jbm.a.10603. [DOI] [PubMed] [Google Scholar]
- 23.Poh M. Boyer M. Solan A. Dahl S.L. Pedrotty D. Banik S.S. McKee J.A. Klinger R.Y. Counter C.M. Niklason L.E. Blood vessels engineered from human cells. Lancet. 2005;365:2122. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 24.Shalaby S.W. Lindsey J.M., III. Bioswellable sutures. Poly-Med, Inc (Anderson, SC) United States Patent Patent Application Serial No. 11/596,545, 2006.
- 25.Shalaby S.W. Copolyesters with minimized hydrolytic stability and crystalline absorbable copolymers thereof. Poly-Med, Inc (Pendleton, SC) United States Patent 2001.
- 26.Shalaby S.W. Amorphous polymeric polyaxial initiator and compliant crystalline copolymers therefrom. Poly-Med, Inc (Pendleton, SC) United States Patent 2002.
- 27.Gao J. Niklason L. Langer R. Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J Biomed Mater Res. 1998;42:417. doi: 10.1002/(sici)1097-4636(19981205)42:3<417::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Nation J.L. A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol. 1983;58:347. doi: 10.3109/10520298309066811. [DOI] [PubMed] [Google Scholar]
- 29.Gui L. Muto A. Chan S.A. Breuer C.K. Niklason L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A. 2009;15:2665. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armentano R.L. Levenson J. Barra J.G. Fischer E.I. Breitbart G.J. Pichel R.H. Simon A. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am J Physiol. 1991;260:H1870. doi: 10.1152/ajpheart.1991.260.6.H1870. [DOI] [PubMed] [Google Scholar]
- 31.Gui L. Chan S.A. Breuer C.K. Niklason L.E. Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods. 2010;16:173. doi: 10.1089/ten.tec.2009.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 33.Woessner J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 34.Piez K. Likins R.C. The nature of collagen. In: Sognnaes R.F., editor. American Association for the Advancement of Science; Calcification in Biological Systems. A symposium presented at the Washington meeting of the American Association for the Advancement of Science; Dec 29;1958 ; 1960. p. 411. [Google Scholar]
- 35.Freed L.E. Vunjak-Novakovic G. Biron R.J. Eagles D.B. Lesnoy D.C. Barlow S.K. Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (NY) 1994;12:689. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 36.Park K. Shalaby W.S.W. Park H. Biodegradable Hydrogels for Drug Delivery. Lancaster, PA: Technomic Publishing Company, Inc.; 1993. [Google Scholar]
- 37.Higgins S.P. Solan A.K. Niklason L.E. Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J Biomed Mater Res A. 2003;67:295. doi: 10.1002/jbm.a.10599. [DOI] [PubMed] [Google Scholar]