Abstract
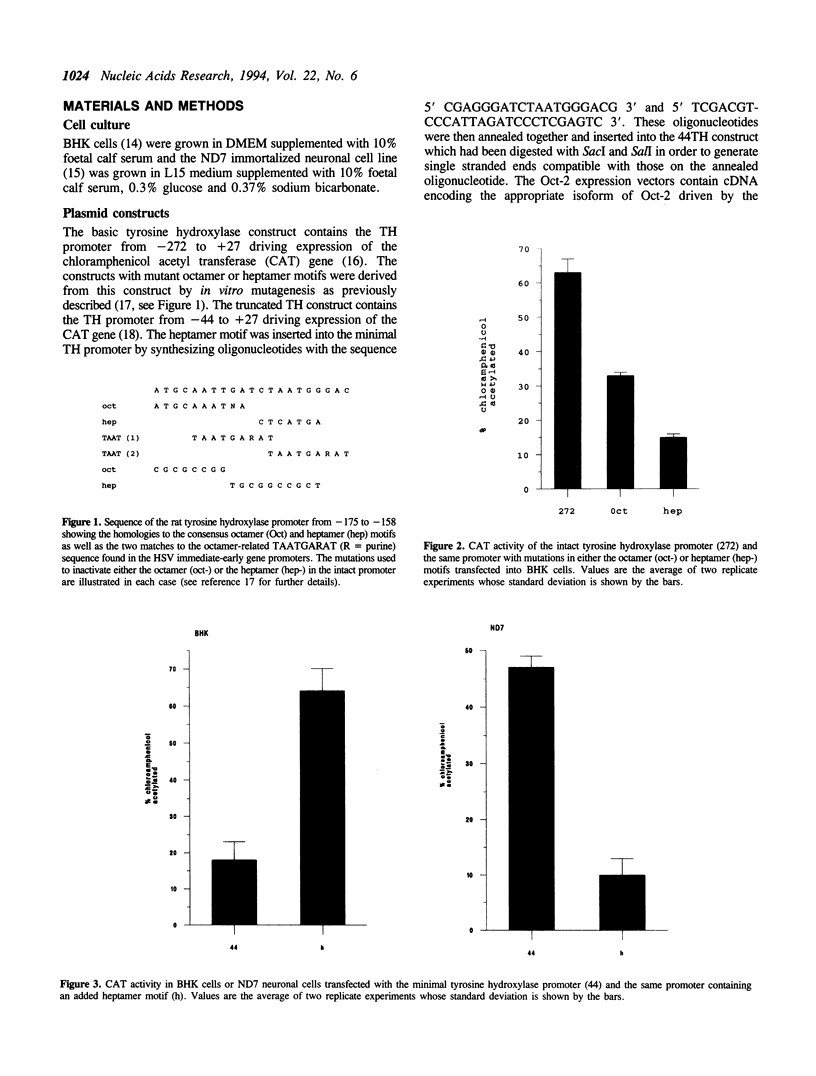
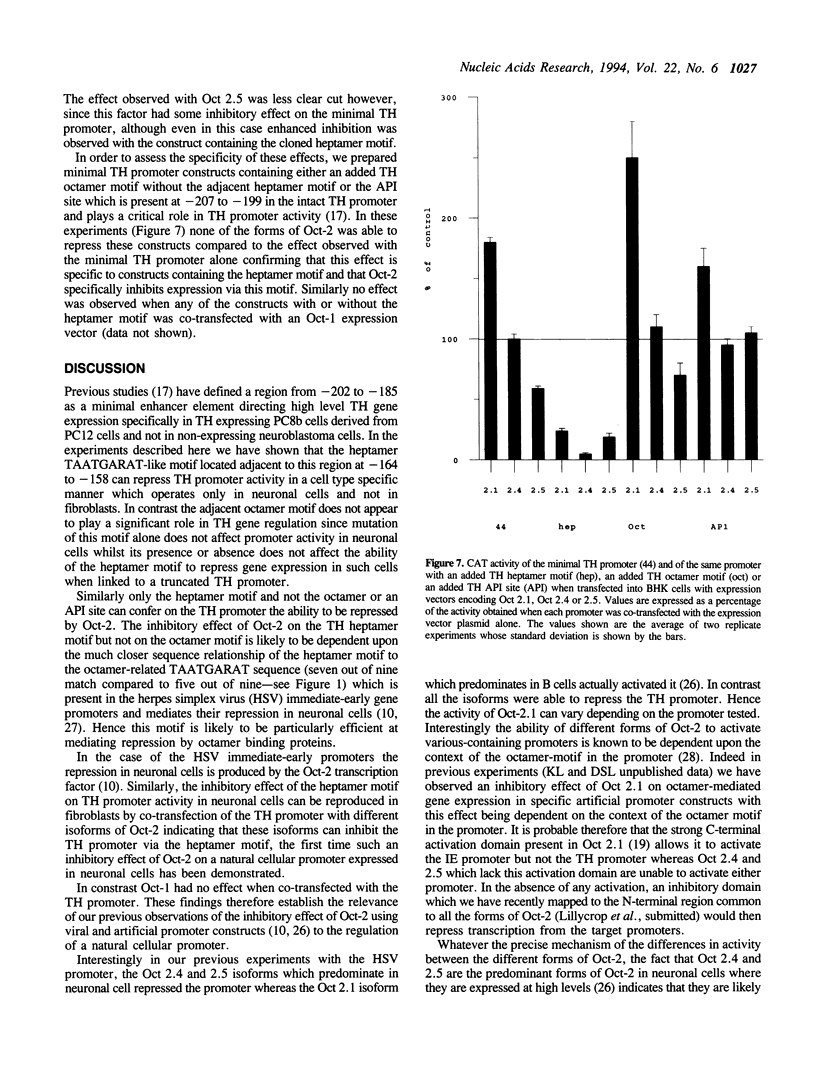
The tyrosine hydroxylase (TH) gene promoter contains adjacent octamer and heptamer motifs which act as target sites for octamer binding transcription factors. Mutation of the heptamer motif but not the octamer motif enhances TH promoter activity in neuronal cells expressing Oct-2 but not in non-expressing fibroblasts. Similarly addition of the heptamer motif to a minimal TH promoter represses gene expression in neuronal cells but not in fibroblasts. These effects can be reproduced by the artificial expression of neuronal isoforms of Oct-2 in fibroblasts which results in the repression of transfected TH promoters containing an intact heptamer motif but not those in which this motif has been mutated or deleted. The TH promoter thus represents the first example of a cellular promoter which is repressed by Oct-2. The significance of this effect is discussed in terms of the cell type specificity of the TH promoter and its induction by different physiological stimuli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abken H., Reifenrath B. A procedure to standardize CAT reporter gene assay. Nucleic Acids Res. 1992 Jul 11;20(13):3527–3527. doi: 10.1093/nar/20.13.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler A., Müller-Immerglück M., Wirth T. Oct2 transactivation from a remote enhancer position requires a B-cell-restricted activity. Mol Cell Biol. 1992 Jul;12(7):3107–3116. doi: 10.1128/mcb.12.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta C., Tognon M., Liboi E. Epidermal growth factor induces, in the EL alpha 4-2 cell line, herpes simplex virus-1 alpha 4 gene transcription in the absence of the viral trans-activator VP16. Virus Res. 1991 May;19(2-3):199–208. doi: 10.1016/0168-1702(91)90046-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cambi F., Fung B., Chikaraishi D. 5' flanking DNA sequences direct cell-specific expression of rat tyrosine hydroxylase. J Neurochem. 1989 Nov;53(5):1656–1659. doi: 10.1111/j.1471-4159.1989.tb08567.x. [DOI] [PubMed] [Google Scholar]
- Dent C. L., Lillycrop K. A., Estridge J. K., Thomas N. S., Latchman D. S. The B-cell and neuronal forms of the octamer-binding protein Oct-2 differ in DNA-binding specificity and functional activity. Mol Cell Biol. 1991 Aug;11(8):3925–3930. doi: 10.1128/mcb.11.8.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. P., Yoon S. O., Chikaraishi D. M. Sequences that direct rat tyrosine hydroxylase gene expression. J Neurochem. 1992 Jun;58(6):2044–2052. doi: 10.1111/j.1471-4159.1992.tb10945.x. [DOI] [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Ziff E. B. Nerve growth factor regulates tyrosine hydroxylase gene transcription through a nucleoprotein complex that contains c-Fos. Genes Dev. 1990 Apr;4(4):477–491. doi: 10.1101/gad.4.4.477. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989 Jul 6;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Kemler I., Schreiber E., Müller M. M., Matthias P., Schaffner W. Octamer transcription factors bind to two different sequence motifs of the immunoglobulin heavy chain promoter. EMBO J. 1989 Jul;8(7):2001–2008. doi: 10.1002/j.1460-2075.1989.tb03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp L. M., Dent C. L., Latchman D. S. Octamer motif mediates transcriptional repression of HSV immediate-early genes and octamer-containing cellular promoters in neuronal cells. Neuron. 1990 Feb;4(2):215–222. doi: 10.1016/0896-6273(90)90096-x. [DOI] [PubMed] [Google Scholar]
- Latchman D. S. Current status review: molecular biology of herpes simplex virus latency. J Exp Pathol (Oxford) 1990 Feb;71(1):133–141. [PMC free article] [PubMed] [Google Scholar]
- Leonard D. G., Ziff E. B., Greene L. A. Identification and characterization of mRNAs regulated by nerve growth factor in PC12 cells. Mol Cell Biol. 1987 Sep;7(9):3156–3167. doi: 10.1128/mcb.7.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. J., Chikaraishi D. M. Regulated expression of the tyrosine hydroxylase gene by epidermal growth factor. Mol Cell Biol. 1987 Sep;7(9):3332–3336. doi: 10.1128/mcb.7.9.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Budrahan V. S., Lakin N. D., Terrenghi G., Wood J. N., Polak J. M., Latchman D. S. A novel POU family transcription factor is closely related to Brn-3 but has a distinct expression pattern in neuronal cells. Nucleic Acids Res. 1992 Oct 11;20(19):5093–5096. doi: 10.1093/nar/20.19.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Dent C. L., Wheatley S. C., Beech M. N., Ninkina N. N., Wood J. N., Latchman D. S. The octamer-binding protein Oct-2 represses HSV immediate-early genes in cell lines derived from latently infectable sensory neurons. Neuron. 1991 Sep;7(3):381–390. doi: 10.1016/0896-6273(91)90290-g. [DOI] [PubMed] [Google Scholar]
- Lillycrop K. A., Latchman D. S. Alternative splicing of the Oct-2 transcription factor RNA is differentially regulated in neuronal cells and B cells and results in protein isoforms with opposite effects on the activity of octamer/TAATGARAT-containing promoters. J Biol Chem. 1992 Dec 15;267(35):24960–24965. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990 Feb 25;18(4):1068–1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Poellinger L., Roeder R. G. Octamer transcription factors 1 and 2 each bind to two different functional elements in the immunoglobulin heavy-chain promoter. Mol Cell Biol. 1989 Feb;9(2):747–756. doi: 10.1128/mcb.9.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Heguy A., Roeder R. G. Identification and purification of a human lymphoid-specific octamer-binding protein (OTF-2) that activates transcription of an immunoglobulin promoter in vitro. Cell. 1987 Dec 4;51(5):783–793. doi: 10.1016/0092-8674(87)90101-2. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Stachowiak M., Sebbane R., Stricker E. M., Zigmond M. J., Kaplan B. B. Effect of chronic cold exposure on tyrosine hydroxylase mRNA in rat adrenal gland. Brain Res. 1985 Dec 16;359(1-2):356–359. doi: 10.1016/0006-8993(85)91450-7. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Suburo A. M., Wheatley S. C., Horn D. A., Gibson S. J., Jahn R., Fischer-Colbrie R., Wood J. N., Latchman D. S., Polak J. M. Intracellular redistribution of neuropeptides and secretory proteins during differentiation of neuronal cell lines. Neuroscience. 1992;46(4):881–889. doi: 10.1016/0306-4522(92)90191-4. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Wheatley S. C., Dent C. L., Wood J. N., Latchman D. S. Elevation of cyclic AMP levels in cell lines derived from latently infectable sensory neurons increases their permissivity for herpes virus infection by activating the viral immediate-early 1 gene promoter. Brain Res Mol Brain Res. 1992 Jan;12(1-3):149–154. doi: 10.1016/0169-328x(92)90078-p. [DOI] [PubMed] [Google Scholar]
- Wirth T., Priess A., Annweiler A., Zwilling S., Oeler B. Multiple Oct2 isoforms are generated by alternative splicing. Nucleic Acids Res. 1991 Jan 11;19(1):43–51. doi: 10.1093/nar/19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Bevan S. J., Coote P. R., Dunn P. M., Harmar A., Hogan P., Latchman D. S., Morrison C., Rougon G., Theveniau M. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990 Sep 22;241(1302):187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- Yoon S. O., Chikaraishi D. M. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron. 1992 Jul;9(1):55–67. doi: 10.1016/0896-6273(92)90220-8. [DOI] [PubMed] [Google Scholar]





