Abstract
Immature thymocytes that are positively selected based upon their response to self-peptide-MHC complexes develop into mature T cells that are not overtly reactive to those same complexes. Developmental tuning is the active process through which TCR-associated signaling pathways of single-positive thymocytes are attenuated to respond appropriately to the peptide-MHC molecules that will be encountered in the periphery. In this study, we explore the mechanisms that regulate the tuning of CD4+ single-positive T cells to MHC class II encountered in the thymic medulla. Experiments with murine BM chimeras demonstrate that tuning can be mediated by MHC class II expressed by either thymic medullary epithelial cells or thymic dendritic cells. Tuning does not require the engagement of CD4 by MHC class II on stromal cells. Rather, it is mediated by interactions between MHC class II and the TCR. To understand the molecular changes that distinguish immature hyperactive T cells from tuned mature CD4+T cells, we compared their responses to TCR stimulation. The altered response of mature CD4 single-positive thymocytes is characterized by the inhibition of ERK activation by low-affinity self-ligands and increased expression of the inhibitory tyrosine phosphatase SHP-1. Thus, persistent TCR engagement by peptide-MHC class II on thymic medullary stroma inhibits reactivity to self-Ags and prevents autoreactivity in the mature repertoire.
Thymic selection generates a diverse repertoire of T cells that can respond to the universe of foreign Ags while maintaining tolerance to self. Positive selection generates a repertoire of T cells that recognize self-MHC-peptide complexes (1–3); negative selection purges thymocytes with strong reactivity to self (4). The peripheral repertoire is composed of T cells that recognize self-Ags, yet autoreactivity is quite rare. Thus, mechanisms for preventing the activation of T cells by self-ligands in the periphery must exist. We and others have previously termed this “developmental tuning” (5–8). Early studies by two different groups showed that developmental maturation of T cells decreases TCR reactivity to weak ligands without significantly changing the response to high-affinity cognate ligands (9, 10). We previously showed that interactions between CD4 single-positive (SP)3 thymocytes and MHC class II (MHC II) molecules on thymic medullary stroma are necessary to avoid hyper-reactivity of the mature repertoire (5). Thus, maturation during thymic medullary residency “developmentally tunes” the TCR to self-ligands, and this tuning is mediated by MHC II-positive thymic stroma.
Developmental tuning of CD4+ T cells is an active process requiring MHC II expression (5); yet, the molecular and cellular regulation of this process have not been characterized. Our previous results show that decreased responsiveness to TCR engagement is mediated by alterations in proximal TCR signaling pathways. We proposed that changes in the subcellular localization of the tyrosine kinase Lck regulate the threshold for activation of the TCR (5). However, developmental changes in the expression level or the activation state of other signaling molecules that regulate proximal TCR signaling may also occur during tuning. For example, increased expression of regulatory tyrosine phosphatases that inhibit Lck or its downstream targets would result in inhibition of TCR signaling; protection from the activity of phosphatases would enhance signaling (11). Neither the expression pattern of MHC II that regulates tuning nor the secondary molecular changes that occur are known.
In this study, we have used a series of bone marrow (BM) chimeras and genetically modified mouse models to further dissect mechanisms of developmental tuning. Our results show that MHC II expression on either medullary thymic epithelial cells (mTECs) or thymic dendritic cells (DCs) is sufficient to reduce TCR responsiveness to weak ligands. Moreover, we have found that tuning requires peptide-MHC II-specific interactions with the polymorphic TCR rather than the nonpolymorphic CD4 coreceptor. MHC II-specific interactions with the thymic medullary stroma inhibit ERK activation by weak ligands and elevate Src homology region 2 domain-containing phosphatase 1 (SHP-1) expression in the maturing SP thymocytes. These results show that developmental tuning by interactions with peptide-MHC II in the thymic medulla introduce negative regulatory mechanisms in the TCR signaling pathway and prevent self-reactivity in mature T cells.
Materials and Methods
Animals
C57BL/6 (B6), B10.BR, AND TCR transgenic (Tg) (12), RAG-1-deficient, and H2-DM-deficient (H2-DM−/−) (13) mice were purchased from The Jackson Laboratory. H2-DM−/− mice were backcrossed to B6 mice at least eight generations and used with H2-DM+/− littermate controls. EA137/VA142 MHC II mutant (MUT) (EA137/VA142 Aβ MUT) and control wild-type (WT) I-Ab Tg (Aβ WT Tg) mice were previously described (14). K14/Aβb (2) and CD11c/Aβb (15) transgenic mice were used together with Aβb +/− littermate controls. NG-BAC (RAG2/GFP) Tg reporter mice (16) were provided by Dr. Michel Nussenzweig (Rockefeller University, New York, NY). MHC II-deficient (Aβb −/−) mice (17) were backcrossed to B6 mice at least 26 generations. All animals were maintained and used in accordance with the institutional animal care and use guidelines of the University of Pennsylvania (Philadelphia, PA).
Abs and peptides
The fluorochrome-conjugated anti-mouse CD4, CD8, CD69, TCRβ, Vβ3, diphosphorylated ERK1/2 (ppERK1/2) mAbs, and isotype controls were purchased form BD Pharmingen. Anti-CD3ε (clone 145-2C11; American Type Culture Collection (ATCC)) mAb was purified from culture supernatant. Anti-SHP-1 (clone C19) Ab (18) was purchased from Santa Cruz Biotechnology. Rabbit IgG (staining control) and secondary donkey anti-rabbit IgG (allophycocyanin conjugated) Abs were from Jackson Immuno-Research Laboratories. Hybridomas for the anti-mouse CD4 (clone GK1.5), CD8 (clone 2.43), CD16/32 (clone 2.4G2), MHC II (clone M5114), and Thy1.2 (clone MMT1) mAbs used for blocking and cell depletion experiments were obtained from ATCC. Pigeon cytochrome c (PCC) 88–104 peptide was purchased from AnaSpec. The altered peptide ligand (APL) P99 (APL P99) (10, 19) has an Leu → Pro substitution at position 99 and was synthesized by the University of Pennsylvania Protein Chemistry Laboratory (Philadelphia, PA).
Cell isolation and intrathymic transfers
CD4 SP thymocytes were enriched by depleting CD8 and MHC II-positive cells using a previously described protocol (5). APCs from B10.BR or Aβb−/− mice were isolated from spleens by depleting T cells with anti-Thy1.2 mAb and rabbit complement (Cedarlane Laboratories). CD4 SP thymocytes (107) were injected into surgically exposed thymi (5). Transferred cells were distinguished from endogenous cells by either CFSE labeling or congenic markers. Mice were euthanized 4–5 days after the cell transfer.
Radiation BM chimeras
Mononuclear BM cells collected from the femurs and tibias of the donor mice were T cell depleted with anti-CD4 and anti-CD8 mAbs followed by sorting with anti-rat IgG-conjugated magnetic beads. T cell-depleted BM cells (1–2 × 106) were transferred i.v. into lethally irradiated (950 rad) recipients. Chimeras were given antibiotic-containing water for 2 wk and used for experiments at 6–8 wk.
T cell activation assays
Equal numbers (105) of CD4 SP thymocytes and Aβb −/− APCs were cocultured at 37°C in 96-well U-bottom tissue culture plates with graded concentrations of anti-CD3ε mAbs in RPMI 1640 medium supplemented with 10% (v/v) FBS, penicillin/streptomycin, 2-ME, and L-glutamine. AND TCR Tg CD4 SP (AND CD4 SP) thymocytes were stimulated in vitro with graded concentrations of PCC 88–104 or APL P99 peptides and B10.BR APCs. Cultured cells were harvested at 4 h to analyze TCR down-regulation or at 6–16 h to analyze CD69 up-regulation as described previously (5). Endogenous or intrathymically transferred cells that matured in the K14/Aβb (5) or Aβb−/− mice were used as positive controls for tuning.
Flow cytometry
Single-cell suspensions were incubated on ice for 20 min with anti-CD16/32 mAb (αFcγR) followed by staining with fluorochrome-conjugated anti-mouse mAbs for 30 min. Cells were washed thoroughly and acquired using a FACSCalibur flow cytometer (BD Biosciences). Data was analyzed using FloJo (Tree Star) software.
Intracellular staining
SP thymocytes were fixed with 1.5% formaldehyde (Electron Microscopy Sciences) for 10 min at room temperature followed by surface staining with anti-CD4 and anti-CD8. Cells were then permeabilized with ice-cold methanol, washed thoroughly in staining buffer (PBS with 5% BSA and 0.01% sodium azide), and stained with either directly conjugated ppERK1/2 mAbs or with anti-SHP-1 (C-19) Ab, followed by washing and staining with secondary donkey anti-rabbit IgG.
Quantitative RT-PCR
Total cellular RNA was extracted with the mirVan microRNA isolation kit (Applied Biosystem) and converted to cDNA with SuperScript II reverse transcriptase and random hexamers (Invitrogen). Quantitative PCR was performed on an Applied Biosystems PRISM 7500 sequence detection system. For SHP-1 and GAPDH, standard commercial TaqMan probes were used (Applied Biosystems). Samples were normalized to GAPDH and represented as fold change compared with double-positive (DP) thymocyte controls for SHP-1. Data are the mean ± SEM of three independent experiments done in triplicate.
Statistical analysis
Data were analyzed by a paired Student’s t test using GraphPad Prism 5.0 software. Statistical comparisons were made between individual mutant mice and their WT or heterozygous control groups only. Values of p < 0.05 (*) or p < 0.001 (**) were considered significant.
Results
Interactions between CD4 SP thymocytes and MHC II on mTECs change TCR sensitivity
We previously asked whether developmental tuning of CD4+ T cells is an active event requiring TCR-MHC II interactions. To answer this question, the postselection maturation of CD4+ T cells was examined in K14/Aβb transgenic mice, in which MHC II expression is restricted to cortical thymic epithelial cells. Neither mTECs nor thymic DCs are MHC II positive in K14/Aβb mice. Therefore, CD4 SP T cells can be positively selected on MHC II+ cortical thymic epithelial cells but undergo no further interactions with MHC II in the thymic medulla. We found that postselection CD4 SP thymocytes maturing in the K14/Aβb thymic medulla were hyperresponsive to proximal TCR signaling (5). This suggested that interactions between maturing CD4 SP cells and MHC II-positive medullary stroma altered the TCR responsiveness to ligands. Multiple cell types express MHC II in the thymic medullary stroma. Our first set of experiments asked which MHC II-positive stromal cells mediate developmental tuning.
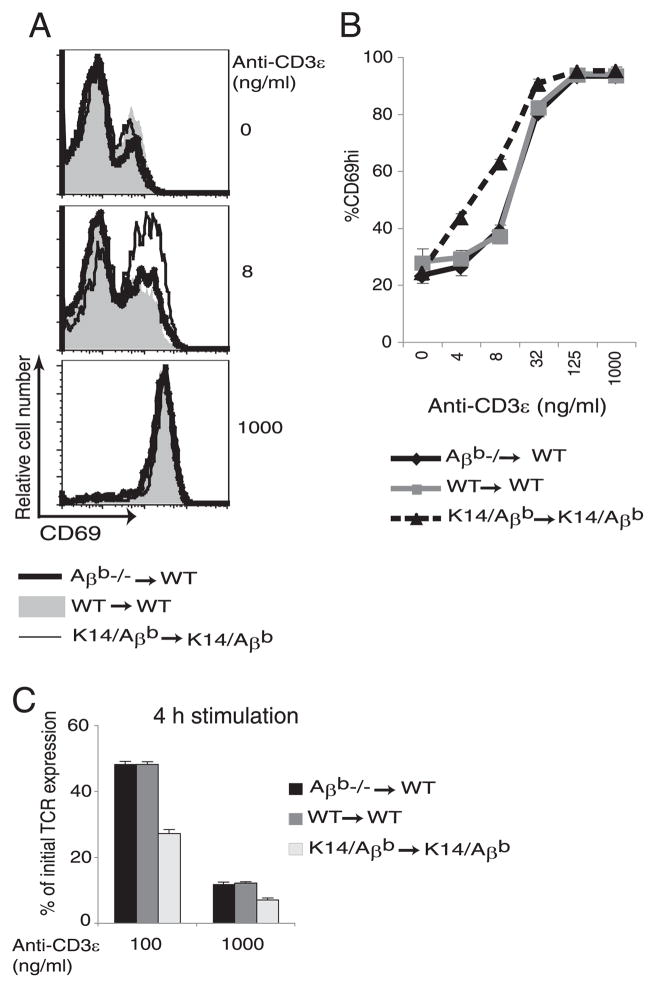
To study the requirement for MHC II expression on various medullary stromal cells for tuning, a series of BM chimeras (BMC) were generated. We compared the requirement for MHC II on radiation-resistant thymic epithelial cells and radiation-sensitive, BM-derived hematopoietic cells. To do this, lethally irradiated B6 WT recipient mice were injected with T cell-depleted Aβb −/− BM (Aβb −/− → WT). In these chimeras, radiation-resistant epithelial cells are the only MHC II-positive cells in the thymus. Control WT → WT and K14/Aβb→ K14/Aβb chimeras were generated and analyzed in parallel. Positive selection is mediated by MHC II on thymic cortical epithelial cells (2), so CD4 SP thymocytes were generated in each of these chimeras (data not shown). CD4 SP thymocytes from the chimeras were enriched and stimulated with graded doses of anti-CD3ε mAb in the presence of Aβb −/− APCs (5). Up-regulation of the early T cell activation marker CD69 (20, 21) and down-regulation of surface TCR (5, 22, 23) were measured. When compared with CD4 SP cells from WT → WT BMC, CD4 SP thymocytes from K14/Aβb→K14/Aβb chimeras were hyperactive to stimulation with low concentrations of anti-CD3ε mAb with increased up-regulation of CD69 (Fig. 1, A and B) and down-regulation of TCR (Fig. 1C). In contrast, the responses of the CD4 SP thymocytes maturing in Aβb −/− → WT chimeras and the control WT → WT chimeras were the same (Fig. 1). Thus, MHC II expression restricted to radiation-resistant thymic epithelial cells is sufficient for the “developmental tuning” of maturing CD4 SP thymocytes.
FIGURE 1.
MHC II expression on thymic epithelial cells tunes maturing CD4 SP thymocytes. CD4 SP thymocytes were purified from Aβb−/− → WT, WT → WT, or K14/Aβb→ K14/Aβb BMCs. Purified CD4 SP thymocytes were stimulated in vitro with an equal number of Aβb−/− splenic APCs and increasing doses of anti-CD3ε mAb for 16 h followed by surface staining and FACS analysis for relative CD69 expression. A, Representative histograms of CD69 expression at two different doses of anti-CD3ε. B, Cumulative data of CD69 expression at multiple doses of anti-CD3ε stimulation. Shown are representative data from four experiments. C, TCR down-regulation was quantified by stimulating CD4 SP thymocytes from the chimeras with Aβb −/− APCs conjugated with 100 or 1000 ng/ml anti-CD3 ε mAb for 4 h, followed by surface staining for TCRβ. To calculate the percentage of initial TCR expression, the mean fluorescence intensity of stimulated cells was divided by that of unstimulated cells. Data are representative of three experiments.
MHC II expression on thymic DCs can reduce the TCR reactivity of CD4 SP thymocytes
Our results demonstrate that MHC II-positive mTECs could induce thymocyte tuning. We therefore asked whether this function is unique to thymic epithelial cells or whether interactions with MHC II-positive thymic DCs also alter TCR sensitivity. To address this question, we used CD11c/Aβb mice in which MHC II expression is restricted to CD11c+ DCs (Ref. 15 and data not shown). CD11c promoter-driven MHC II expression in thymic DCs of CD11c/Aβb mice is lower than that of control Aβb +/− mice (supplemental Fig. 1).4 In the absence of MHC II on thymic epithelium, there are no endogenous CD4 SP thymocytes in CD11c/Aβb mice (15). We therefore used the previously described method of intrathymic adoptive transfer (5) of polyclonal WT or monoclonal AND CD4 SP thymocytes to study tuning. CD4 SP thymocytes lose their responsiveness to low-affinity peptides as they mature while maintaining responsiveness to high-affinity cognate ligands (9, 10), and we have shown that this change in TCR sensitivity is MHC II dependent (5). The high-affinity agonist ligand for AND CD4 T cells is the PCC 88–104 peptide, and the low-affinity ligand is the APL P99 peptide (10, 19). CFSE-labeled polyclonal WT or AND CD4 SP thymocytes (107) were injected intrathymically into CD11c/Aβb, K14/Aβb, or control Aβb +/− mice. Equivalent numbers of transferred SP cells were recovered from host thymi 5 days after transfer (data not shown). The polyclonal WT cells recovered after transfer were in vitro stimulated with anti-CD3ε mAb presented by Aβb −/− APCs. AND CD4 SP cells were stimulated with increasing concentrations of PCC 88–104 or APL P99 peptides presented by I-Ek-positive B10.BR APCs and assayed for up-regulation of CD69. Results are shown in Fig. 2. In comparison to transferred WT CD4 SP cells maturing in Aβb +/− thymi, cells maturing in K14/Aβb thymi responded to lower concentrations of anti-CD3ε. CD4 SP cells maturing in CD11c/Aβb thymi required higher doses of anti-CD3ε than did cells maturing in K14/Aβb thymi (Fig. 2A). AND CD4 SP cells recovered from CD11c/Aβb, K14/Aβb, and control Aβb +/− thymi up-regulated CD69 equivalently in response to the high-affinity cognate ligand PCC 88–104 (Fig. 2B). In contrast, the AND CD4 SP cells recovered from CD11c/Aβb thymi were less reactive to the weak APL P99 stimulation (Fig. 2B) than the same cells recovered from K14/Aβb thymi. Thus, interactions with MHC II on thymic DCs can decrease the TCR sensitivity of CD4 SP thymocytes. However, this effect is less complete than the changes mediated by MHC II expressed by the thymic epithelium.
FIGURE 2.
MHC II-positive thymic DCs alter the reactivity of CD4 SP thymocytes. CFSE-labeled polyclonal WT or AND CD4 SP thymocytes (107) were intrathymically transferred to CD11c/Aβb, K14/Aβb, or Aβb +/− mice. CD4 SP cells were purified 5 days after transfer. A, Transferred polyclonal CD4 SP cells were stimulated in vitro with an equal number of Aβb −/− APCs and increasing concentrations of anti-CD3ε mAb. After 16 h, CD69 expression was determined by FACS. B, Transferred AND CD4 SP thymocytes were stimulated in vitro with equal numbers of B10.BR splenic APCs and increasing concentrations of agonist PCC 88–104 or partial agonist APL P99 peptides for 16 h and then assayed by FACS for CD69 expression. Data represent five (A) and six (B) experiments. *, p < 0.05; **, p < 0.001 between CD11c/Aβb and Aβb +/− control groups. C, AND CD4 SP thymocytes were intrathymically transferred to WT → Aβb −/−, Aβb −/− → Aβb −/−, or WT → WT BMCs. Transferred cells recovered on day 5 were in vitro stimulated with PCC 88–104 or APL P99 peptides and measured for surface expressions of CD69. Data represent five experiments. *, p < 0.05 between WT → Aβb −/− BMCs and their WT control groups.
The reduced tuning of CD4+ SP cells transferred into CD11c/Aβb thymi suggests that DCs are less efficient than mTECs at tuning. However, we were concerned about whether lower levels of MHC II on the transgenic DCs, when compared with WT DCs (supplemental Fig. 1), resulted in reduced tuning in the CD11c/Aβb mice. To rule out this possibility, we generated WT → Aβb −/− BMCs in which only thymic hematopoietic cells express MHC II. In these chimeras, thymic DCs have WT levels of MHC II expression. Control Aβb −/− → Aβb −/− and WT → WT chimeras were generated and analyzed in parallel. Intrathymically transferred AND CD4 SP thymocytes that matured in the different chimeric thymi were stimulated in vitro with PCC 88–104 or APL P99 and analyzed for surface CD69 levels. As shown in Fig. 2C, the response to the agonist peptide PCC 88–104 was the same in all of the chimeras. Similar to the changes mediated by DCs in the CD11c/Aβb thymus, interactions with MHC II on thymic hematopoietic APCs in WT → Aβb −/− BMCs reduced the TCR reactivity of maturing AND CD4 SP cells to APL P99. However, a larger percentage of transferred thymocytes that matured in the WT → WT BMCs lost their responsiveness to weak stimulation by APL P99. Thus, interactions with thymic DCs expressing a WT level of MHC II alone are also not sufficient to tune all the maturing CD4 SP thymocytes, and DCs are therefore less efficient than mTECs in developmental tuning.
Developmental tuning is peptide dependent
Interactions between SP thymocytes and MHC II on thymic stromal cells could be mediated by either the polymorphic TCR or the nonpolymorphic CD4 coreceptor. We first asked whether changes in TCR reactivity are dependent upon interactions between the TCR and self-peptide-MHC complexes on thymic stromal cells. MHC II molecules in H2-DM−/− mice predominantly present one self-peptide, the invariant chain-derived CLIP (13, 24, 25). Polyclonal WT or monoclonal TCR Tg CD4 T cells are deprived of self-peptide-specific MHC II-TCR interactions after transfer to H2-DM−/− mice (26, 27). To define the requirement for self-peptide-MHC II-TCR interactions in tuning, CFSE-labeled polyclonal WT or monoclonal AND CD4 SP thymocytes were transferred intrathymically to H2-DM−/−, K14/Aβb, or control H2-DM+/− thymi. CD4 SP thymocytes were enriched 5 days after thymic transfer and stimulated in vitro. CD69 levels were analyzed by FACS. Results are summarized in Fig. 3. To ensure that H2-DM deficiency does not inherently disrupt tuning, we first examined the endogenous CD4 SP cells. Endogenous CD4 SP thymocytes from H2-DM−/− and H2-DM+/− thymi responded equally to stimulation with anti-CD3 ε mAb and were less responsive than hyperactive K14/Aβb CD4 SP cells (Fig. 3A). Thus, H2-DM deficiency did not affect the TCR reactivity of endogenous CD4 SP thymocytes. In contrast, intrathymically transferred WT CD4 SP thymocytes maturing in both H2-DM−/− and K14/Aβb thymi were activated by much lower doses of anti-CD3ε stimulation than transferred cells recovered from control H2-DM+/− thymi (Fig. 3B). Similarly, AND CD4 SP cells transferred into H2-DM−/−, K14/Aβb, and control H2-DM+/− thymi responded equally to the strong cognate peptide PCC 88–104 (Fig. 3C; left). However, the same transferred cells maturing in H2-DM−/−, but not in control H2-DM+/− thymi, retained their reactivity to the weak ligand APL P99. This enhanced responsiveness to APL P99 was similar to that of transferred cells maturing in K14/Aβb thymi that completely lack MHC II expression in the thymic medulla (Fig. 3C, right). In parallel with increased CD69 expression, AND CD4 SP thymocytes maturing in H2-DM−/− thymi also had increased down-regulation of the TCR following APL stimulation (data not shown). Thus, when maturing thymocytes are unable to interact with peptide-MHC complexes in the thymic medullary stroma, tuning does not proceed normally.
FIGURE 3.
Developmental tuning is peptide dependent. CFSE-labeled WT or AND CD4 SP thymocytes (107) were intrathymically transferred to H2-DM−/−, K14/Aβb, or control H2-DM+/− mice. CD4 SP thymocytes were purified 5 days after the transfer. WT CD4 SP cells were stimulated in vitro with Aβb −/− splenic APCs and anti-CD3ε mAb for 16 h and assayed for CD69 surface expression by FACS. A, CD69 expression on endogenous CD4 SP cells (CFSE+). B, CD69 expression on intrathymically transferred WT cells (CFSE+). Data represent four experiments. C, AND CD4 SP thymocytes purified 5 days after intrathymic transfer into the indicated hosts were stimulated with B10.BR APCs and increasing concentrations of PCC 88–104 or APL P99 peptides and assayed for CD69 surface expression. Data are representative of six independent experiments. *, p < 0.05; **, p < 0.001 between H2-DM−/− and control H2-DM+/− groups).
CD4 coreceptor engagement with MHC II is not required for tuning
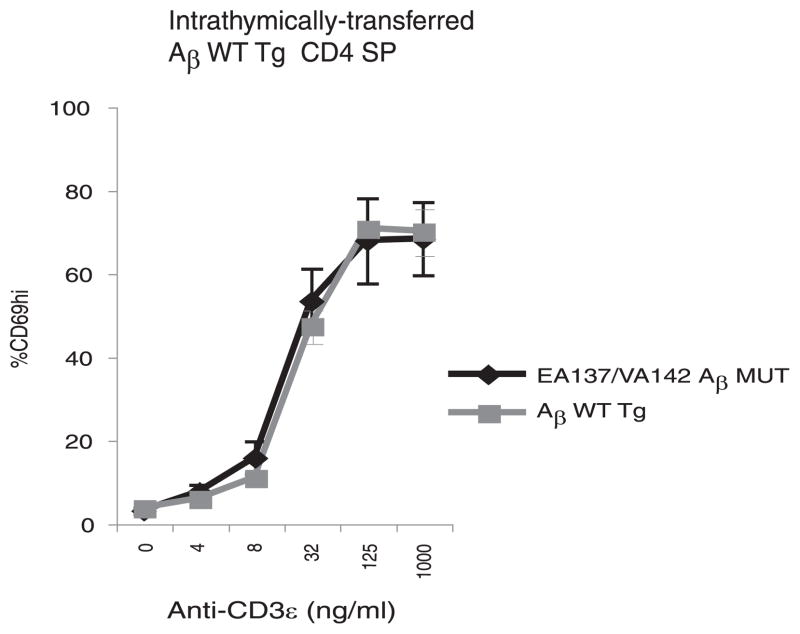
These results suggest that the tuning of CD4+ T cells depends on TCR-peptide-MHC II interactions. We next asked whether CD4-MHC II interactions are also required for tuning. To do so, we used EA137/VA142 Aβ MUT mice, which have two amino acid mutations in the β-chain of the MHC II I-Ab molecule that disrupt the CD4 binding site (14). Control AβWT Tg mice with matched MHC II expression were examined in parallel (14). CFSE-labeled CD4 SP thymocytes (107) from AβWT Tg mice were intrathymically injected into EA137/VA142 Aβ MUT or control AβWT Tg mice. Five days after transfer, CD4 SP thymocytes were recovered and stimulated in vitro with Aβb −/− APCs and increasing concentrations of anti-CD3ε mAb. As shown in Fig. 4, CD4 SP thymocytes maturing in the presence or absence of functional CD4-MHC II interactions reacted identically to TCR stimulation. Thus, the changes in TCR reactivity in maturing SP thymocytes do not require functional CD4-MHC II interactions but are mediated by peptide-MHC II-specific interactions with the TCR.
FIGURE 4.
Developmental tuning is independent of CD4 coreceptor engagement with MHC II. EA137/VA142 Aβ UT and control AβWT Tg mice were intrathymically injected with 107 CFSE-labeled CD4 SP thymocytes purified from AβWT Tg mice. On day 5, CD4 SP thymocytes were enriched followed by in vitro stimulation with Aβb −/− APCs and increasing concentrations of anti-CD3ε mAb. After 16 h, the relative surface expression of CD69 was assayed by FACS. Data represent four experiments.
Developmental tuning alters ERK activation
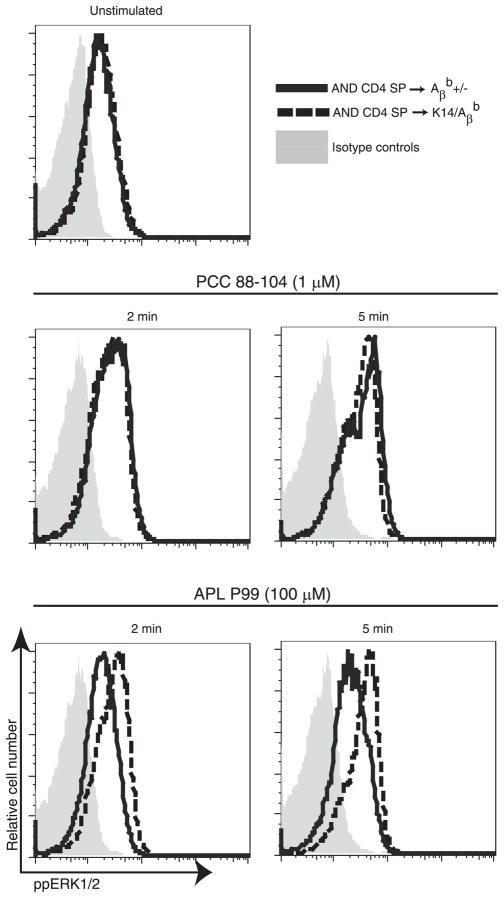
Our results shows that interactions between CD4 SP thymocytes and self-peptide-MHC II complexes on mTECs and thymic DCs decrease TCR responsiveness in maturing thymocytes to weak ligands such as APLs without affecting the response to high-affinity ligands. Previous studies of mature peripheral CD4+ T cells have shown that the loss of TCR responsiveness to weak ligands is mediated by decreased activation of ERK, a positive regulator of the proximal TCR signaling molecule Lck (11). We therefore asked whether MHC II-dependent changes in CD4 SP thymocyte responsiveness were also associated with the loss of ERK activation by weak ligands. ERK activation was assayed by flow cytometry in AND CD4 SP thymocytes maturing in different thymic environments. Representative results are shown in Fig. 5. Basal levels of ppERK1/2 were the same in AND CD4 SP thymocytes that matured in the MHC II-negative thymic medulla of K14/Aβb and the control MHC II-positive Aβb +/− thymic medulla (data not shown). Following stimulation with the high-affinity cognate peptide PCC 88–104, CD4 SP cells recovered from K14/Aβb and control Aβb +/− thymi had equivalent phosphorylation of ERK. In contrast, AND CD4 SP cells that matured in the MHC II-negative K14/Aβb thymic medulla had increased levels of phosphorylated ERK in response to low affinity APL P99 stimulation compared with cells maturing in control Aβb +/− thymi. Indeed, in untuned SP thymocytes in K14/Aβb thymi, ERK activation in response to APL P99 persisted at least 30 min following stimulation (data not shown). Similarly, AND CD4 SP thymocytes maturing in H2-DM−/− thymi, but not in control H2-DM+/− thymi, phosphorylated ERK following APL P99 stimulation (supplemental Fig. 2). Thus, interactions between the maturing CD4 SP thymocytes and peptide-MHC II on thymic medullary stromal tissues that mediate tuning prevent ERK activation by weak ligands.
FIGURE 5.
Thymic tuning alters ERK activation. CFSE-labeled AND CD4 SP cells were purified 5 days after intrathymic transfer to K14/Aβb or Aβb +/− mice. B10.BR APCs were pulsed with 1 μM PCC 88–104 or 100 μM APL P99 for 3 h. Equal numbers of prewarmed AND CD4 SP cells and peptide-pulsed APCs were incubated for the indicated time points at 37°C. AND CD4 SP thymocytes incubated with APCs without peptides served as control. Cells were fixed, permeabilized, and stained for ppERK1/2 and assayed by FACS. Data represent two independent experiments.
Developmental tuning is associated with up-regulation of SHP-1 expression
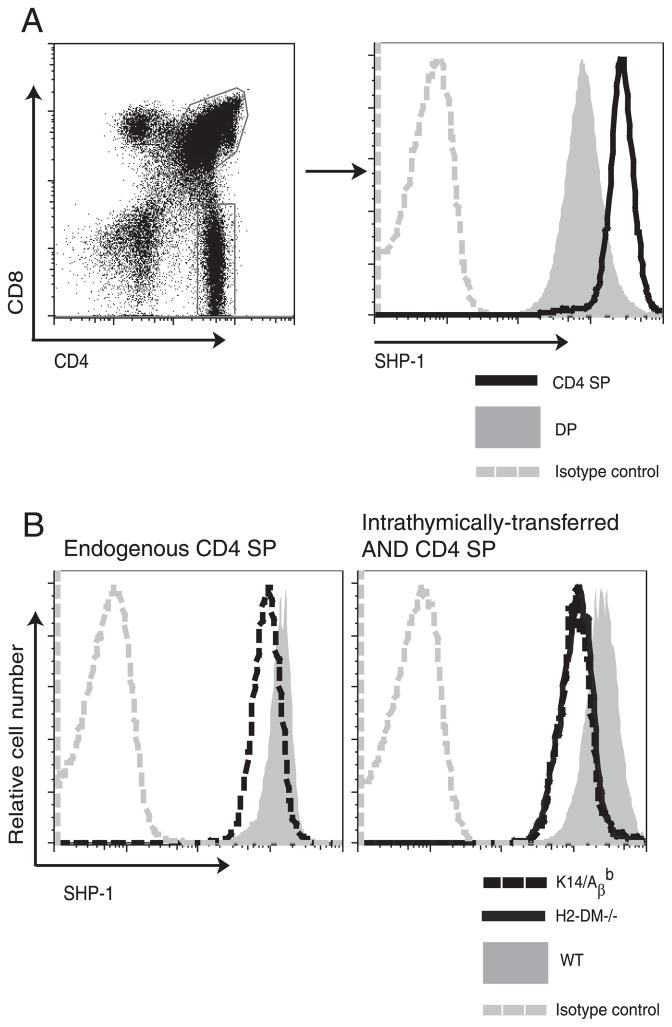
The decrease in TCR responsiveness that accompanies stimulation could be regulated by diminished activation of positive regulators such as ERK or by increasing negative regulators such as tyrosine phosphatases (11, 18). We focused on the tyrosine phosphatase SHP-1, as the lack of SHP-1 results in hyperphosphorylation of both the proximal TCR complex and the downstream signaling molecules that couple the activated TCR to the Ras/MAPK response pathway (28, 29). Interestingly, CD4+ T cells from SHP-1 heterozygous mice can respond to weak ligands such as MHC variant peptides (30), a phenotype reminiscent of immature untuned thymocytes. We therefore used flow cytometry to track the level of SHP-1 during thymocyte maturation. Mature CD4 SP thymocytes express more SHP-1 than do immature DP cells (Fig. 6A) (10). We next asked whether the induction of SHP-1 expression on maturing CD4 SP thymocytes depended upon TCR-peptide-MHC II interactions in the thymic medulla. To address this question, we first analyzed the SHP-1 expression levels in CD4 SP thymocytes from K14/Aβb and the control Aβb +/− mice. As can be seen in Fig. 6B (left), CD4 SP thymocytes from K14/Aβb mice express less SHP-1 than control Aβb +/− CD4 SP thymocytes. To verify this observation, SHP-1 expression was assayed in intrathymically transferred AND CD4 SP thymocytes maturing in H2-DM−/−, K14/Aβb, or control H2-DM+/− thymi. CD4 SP thymocytes maturing without peptide-MHC II interactions in either H2-DM−/− or K14/Aβb thymi had less SHP-1 than transferred cells maturing in control WT thymi (Fig. 6B, right). We observed the same results with intrathymically transferred polyclonal WT CD4 SP thymocytes (data not shown). Thus, the up-regulation of SHP-1 expression in maturing SP thymocytes is mediated by TCR-peptide-MHC II interactions with thymic medullary stroma.
FIGURE 6.
Thymocyte tuning correlates with increased SHP-1 expression. A, Thymocytes from WT B6 mice were stained for SHP-1, CD4, and CD8 and analyzed by FACS. Left, Gate for CD4+CD8+ DP and CD4 SP thymocytes. Right, SHP-1 expression on DP and CD4 SP thymocytes. B, Endogenous CD4 SP thymocytes from K14/A βb or control Aβb +/− (WT) mice (left) or AND CD4 SP cells purified five days after intrathymic transfer to H2-DM−/−, K14/Aβb, or H2-DM+/− (WT) mice (right) were stained for intracellular SHP-1 and analyzed by flow cytometry. Data represent three experiments.
Developmental tuning is not complete until late during medullary residency
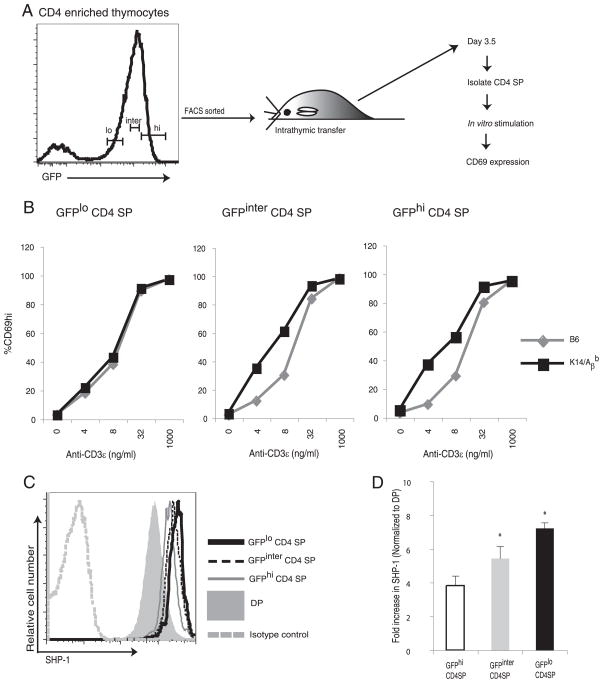
Maturing CD4 SP thymocytes spend ~1–2 wk in the thymic medulla (23, 31). Thus, SP thymocytes are a heterogeneous population of immature, intermediate, and fully mature CD4 cells. We therefore asked whether tuning occurs throughout thymic medullary residency or at a particular point during medullary residency. Hogquist and colleagues have recently shown that, as SP thymocytes in RAG2/GFP reporter mice (16) mature, they slowly lose expression of GFP (31). CD4 SP thymocytes can be divided into GFPhigh immature, GFPinter intermediately mature (where “inter” denotes “intermediate”), and GFPlow mature populations (31). As shown in the experimental outline in Fig. 7A, thymocytes from RAG2/GFP reporter mice were enriched for CD4 by CD8 depletion. CD4-enriched cell populations were then FACS sorted into GFPhigh, GFPinter, and GFPlow populations. The sorted cells were transferred separately into K14/Aβb or B6 thymi. We hypothesized that SP cells that were susceptible to tuning would have different reactivities following maturation in class II-sufficient and -deficient medullae. In contrast, thymocytes that have undergone developmental tuning before transfer would respond similarly following transfer into the different medullary environments. Three and half days later, CD4 SP thymocytes were enriched from the transferred thymi and stimulated in vitro with increasing doses of anti-CD3ε Ab and Aβb −/− APCs. Results are shown in Fig. 7B. GFPhigh and GFPinter CD4 SP thymocytes that matured in K14/Aβb thymi without MHC II interactions remain hyperactive at lower doses of anti-CD3 stimulations. However, GFPlow mature populations recovered from the K14/Aβb thymi were less reactive to TCR stimulation, and their TCR reactivity matches that of GFPlow cells matured in the WT thymi. Thus, the most mature GFPlow thymocytes have undergone tuning and the less mature SP thymocytes must be the cells susceptible to tuning.
FIGURE 7.
Developmental tuning occurs during the late maturation stages of CD4 SP thymocytes. A and B, As outlined in the experimental design (A), CD4-enriched thymocytes from RAG2/GFP reporter mice were FACS sorted into GFPhigh (GFPhi), GFPinter, and GFPlow (GFPlo) populations. Sorted cells were intrathymically injected into separate K14/Aβb or B6 mice. Whole thymocytes were recovered 3.5 days after transfer and stimulated in vitro with different doses of anti-CD3ε Ab and Aβb −/− APCs for 16 h and assayed for CD69 expression (B) by flow cytometry. Representative data of two independent experiments. C, SHP-1 expressions on DP, GFPhigh, GFPinter, and GFPlow CD4 SP thymocytes. The thymocytes from RAG2/GFP reporter mice were stained for SHP-1, CD4, and CD8 and analyzed by FACS. Data represent three experiments. D, The relative SHP-1 RNA expression levels in GFPhigh, GFPinter, and GFPlow CD4 SP thymocytes compared with that of DP thymocytes. *, p < 0.05 between GFPhigh to GFPinter and GFPinter to GFPlow populations.
We then asked whether changes in SHP-1 expression correlate developmentally with susceptibility to tuning. We again used RAG2/GFP reporter mice to permit analysis of CD4 SP thymocytes of different ages. Fig. 7C shows that the up-regulation of the SHP-1 level in maturing CD4 SP thymocytes parallels maturation. Thus, the highest level of SHP-1 expression occurs in the GFPlow mature CD4 SP thymocytes (Fig. 7C). As shown in Fig. 7D, the maturational change in SHP-1 expression is regulated transcriptionally. Thus, TCR-MHC II-mediated interactions that occur during the early stages of CD4 SP medullary residency up-regulate SHP-1 expression to increase negative regulation of the TCR signaling machinery.
Discussion
We have examined the mechanisms that regulate the reduction in TCR responsiveness accompanying the maturation of CD4 SP thymocytes. We previously showed that developmental tuning is an active process requiring the expression of MHC II on thymic stroma. In this study, we report that decreases in TCR reactivity during medullary maturation require interactions between the self-peptide-MHC II expressed on mTECs and thymic DCs with the TCR, but not with CD4. Tuning is associated with inhibition of ERK activation and up-regulation of SHP-1 phosphatase levels, resulting in enhanced negative regulation of TCR signaling.
Tuning can be initiated by MHC II expression limited to either mTECs or thymic DCs; however, the effect mediated by mTECs is complete whereas DCs are less efficient. Why are MHC II-positive mTECs more efficient in mediating tuning than thymic DCs? MHC II expression on thymic DCs in the CD11c/Aβb mouse is lower than that of control Aβb +/− DCs (supplementary Fig. 1) (15). The TCR threshold may be different when a T cell selected on WT peptide-MHC II molecules encounters medullary stroma expressing less class II. However, a fraction of CD4 SP thymocytes were not tuned (Fig. 2C) in WT → Aβb −/− BMCs wherein thymic DCs express the WT level of MHC II. Thus, even with WT levels of MHC II, thymic DCs were not able to tune the whole maturing CD4 SP thymocytes when mTECs were MHC II negative. Multiple studies have demonstrated that peptide-MHC complexes can be transferred from mTECs to thymic DCs in vivo, whereas DC to epithelial cell transfer does not occur (32–34). Indeed, this transfer may contribute to negative selection of thymocytes specific for peripheral tissue Ags expressed by mTECs. If thymic DCs participate in tuning via peptide-MHC II complexes acquired from mTECs, then tuning in CD11c/A βb mice and WT → Aβb −/− BMCs in which mTECs are MHC II-negative would be reduced. It is interesting to note that the MHC II expression levels on mTECs are lower than those of thymic DCs (supplemental Fig. 1). Therefore, in a WT thymus wherein both mTECs and thymic DCs are MHC II positive, DC acquisition of peptide-MHC II complexes from mTECs may, in fact, enhance overall tuning efficiency. Our data indicate the possibility of a functional collaboration between mTECs and thymic DCs in tuning the maturing SP thymocytes and the induction of central tolerance. However, at present we do not know whether the CD4 SP thymocyte populations tuned by mTCEs and thymic DCs are the same or different. Finally, the autoimmune regulator AIRE regulates the expression of a wide array of tissue restricted Ags (35–38) in mTECs that may enhance their efficiency.
Developmental tuning requires the expression of H2-DM and, presumably, peptide-dependent TCR-MHC II interactions. In contrast, tuning does not require interactions between nonpolymorphic CD4 and MHC II. This is surprising, as TCR signaling in mature T cells depends on the CD4 coreceptor (39, 40). Similarly, the change in responsiveness of CD8+ T cells that occurs with maturation is mediated, in part, by increased sialylation of the CD8 coreceptor and decreased CD8-MHC class I interactions (41–43). Moreover, we previously proposed that tuning leads to an increased dependence on CD4 engagement for TCR signaling (5). However, MHC class II-dependent reorganization of proximal signaling during tuning is regulated by the TCR, not by CD4. In fact, we observed increased tyrosine phosphorylation of Lck, the key signaling molecule associated with proximal signaling (44) when there is no TCR-peptide-MHC II interaction in the thymic medulla (T. L. Stephen and T. M. Laufer, unpublished data). Even though productive MHC II-CD4 interactions are not required for developmental tuning, the coreceptor may still participate in reorganization of the proximal signaling by peptide-MHC II-TCR interactions. Evaluation of tuning in CD4-deficient SP thymocytes may provide more information about the requirements for a CD4 molecule in developmental tuning with the caveat that altered distribution of CD4-associated molecules such as Lck in CD4-deficient cells will make it difficult to interpret the results.
We previously proposed that MHC II-dependent postselection tuning is controlled in part by regulation of the subcellular localization of Lck; the association of Lck with the CD4 coreceptor in mature SP thymocytes increases the dependency on CD4 ligation for TCR signaling (5). Now, we report that MHC II-dependent tuning also regulates the expression of the inhibitory tyrosine phosphatase SHP-1. The negative regulation of TCR signaling by SHP-1 is probably mediated via dephosphorylation of the activation tyrosine of Lck, Y394 (44, 45). Although, SHP-1-deficient mature T cells are hyperactive, the absence of SHP-1 does not alter the basal phosphorylation of Lck (29). Taken together, these data suggest that SHP-1 may have more access to CD4-associated Lck present in mature T cells than to the CD4 nonassociated “free” Lck present in immature SP thymocytes (5). It will be important to examine this possibility by using biochemical and ultrastructural analyses. Nonetheless, the changes in both SHP-1 expression and Lck localization result in increasing the TCR threshold in mature peripheral T cells.
Germain and colleagues have proposed a “seesaw” model of TCR regulation in which SHP-1 and ERK determine the response to high vs low affinity ligands. Weakly binding ligands induce recruitment of SHP-1, which inactivates Lck. Strong agonist ligands activate ERK, which protects Lck from SHP-1 binding during TCR signaling (11, 46–48). In agreement with this model, our data shows that peptide-MHC II signals to T cells during medullary residency are associated with increased SHP-1 phosphatase levels and decreased ERK activation by weak ligands. Together, these changes contribute to the increased TCR signaling threshold of mature CD4+ T cells.
Our results suggest that tuning has been completed between the intermediate (GFPinter) and late (GFPlow) stages of medullary residency. These experiments also suggest that interactions of immature SP thymocytes with peptide-MHC II result in nonreversible changes in the TCR signaling properties, as mature GFPlow cells transferred into MHC II-deficient thymi did not alter their responsiveness. Identification of the T cell developmental stage in which tuning occurs will guide future experiments to the biochemical changes that prevent T cell autoreactivity.
This study is a first step toward unraveling the complex cellular interactions during thymic medullary residency that set the TCR response to ligands. We identified the importance of TCR interactions with peptide-MHC II on thymic medullary stroma in establishing regulatory mechanisms to maturing thymocytes. The establishment of such regulatory check points increases the T cell threshold for ligands in such a way that self-Ags cannot activate the TCR in mature T cells and thereby avoid autoreactivity in the healthy immune system.
Supplementary Material
Acknowledgments
We thank Gregory F. Wu for critical reading of the manuscript and Eric J. Allenspach and A. Bhandoola for discussions.
Footnotes
This work was supported by National Institutes of Health Grant R01 AI068819 (to T.M.L.) and an Arthritis Foundation postdoctoral fellowship (to T.L.S.).
Abbreviations used in this paper: SP, single positive; APL, altered peptide ligand; BM, bone marrow; BMC, BM chimera; DC, dendritic cell; DP, double positive; inter, intermediate; MHC II, MHC class II; mTEC, medullary thymic epithelial cell; MUT, mutant; PCC, pigeon cytochrome c; ppERK, diphosphorylated ERK; SHP-1, Src homology region 2 domain-containing phosphatase 1; Tg, transgenic; WT, wild type.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Berg LJ, Pullen AM, Fazekas de St Groth B, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 2.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 3.Laufer TM, Fan L, Glimcher LH. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo. J Immunol. 1999;162:5078–5084. [PubMed] [Google Scholar]
- 4.Siggs OM, Makaroff LE, Liston A. The why and how of thymocyte negative selection. Curr Opin Immunol. 2006;18:175–183. doi: 10.1016/j.coi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Eck SC, Zhu P, Pepper M, Bensinger SJ, Freedman BD, Laufer TM. Developmental alterations in thymocyte sensitivity are actively regulated by MHC class II expression in the thymic medulla. J Immunol. 2006;176:2229–2237. doi: 10.4049/jimmunol.176.4.2229. [DOI] [PubMed] [Google Scholar]
- 6.Grossman Z, Singer A. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. Proc Natl Acad Sci USA. 1996;93:14747–14752. doi: 10.1073/pnas.93.25.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogquist KA, Starr TK, Jameson SC. Receptor sensitivity: when T cells lose their sense of self. Curr Biol. 2003;13:R239–R241. doi: 10.1016/s0960-9822(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 8.Grossman Z, Paul WE. Autoreactivity, dynamic tuning and selectivity. Curr Opin Immunol. 2001;13:687–698. doi: 10.1016/s0952-7915(01)00280-1. [DOI] [PubMed] [Google Scholar]
- 9.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 11.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 12.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 13.Martin WD, Hicks GG, Mendiratta SK, Leva HI, Ruley HE, Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 14.Riberdy JM, Mostaghel E, Doyle C. Disruption of the CD4-major histocompatibility complex class II interaction blocks the development of CD4+ T cells in vivo. Proc Natl Acad Sci USA. 1998;95:4493–4498. doi: 10.1073/pnas.95.8.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemos MP, Fan L, Lo D, Laufer TM. CD8α+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol. 2003;171:5077–5084. doi: 10.4049/jimmunol.171.10.5077. [DOI] [PubMed] [Google Scholar]
- 16.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 17.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 18.Feinerman O, Veiga J, Dorfman JR, Germain RN, Altan-Bonnet G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science. 2008;321:1081–1084. doi: 10.1126/science.1158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 20.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering: requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 21.Cosulich ME, Rubartelli A, Risso A, Cozzolino F, Bargellesi A. Functional characterization of an antigen involved in an early step of T-cell activation. Proc Natl Acad Sci USA. 1987;84:4205–4209. doi: 10.1073/pnas.84.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 23.Egerton M, Scollay R, Shortman K. Kinetics of mature T-cell development in the thymus. Proc Natl Acad Sci USA. 1990;87:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, Ploegh H, Benoist C, Mathis D. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 25.Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 26.Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 27.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 28.Pani G, Fischer KD, Mlinaric-Rascan I, Siminovitch KA. Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J Exp Med. 1996;184:839–852. doi: 10.1084/jem.184.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz U, Ravichandran KS, Burakoff SJ, Neel BG. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc Natl Acad Sci USA. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasserman HA, Beal CD, Zhang Y, Jiang N, Zhu C, Evavold BD. MHC variant peptide-mediated anergy of encephalitogenic T cells requires SHP-1. J Immunol. 2008;181:6843–6849. doi: 10.4049/jimmunol.181.10.6843. [DOI] [PubMed] [Google Scholar]
- 31.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millet V, Naquet P, Guinamard RR. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur J Immunol. 2008;38:1257–1263. doi: 10.1002/eji.200737982. [DOI] [PubMed] [Google Scholar]
- 33.Humblet C, Rudensky A, Kyewski B. Presentation and intercellular transfer of self antigen within the thymic microenvironment: expression of the E α peptide-I-Ab complex by isolated thymic stromal cells. Int Immunol. 1994;6:1949–1958. doi: 10.1093/intimm/6.12.1949. [DOI] [PubMed] [Google Scholar]
- 34.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 36.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 37.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 39.Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 40.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 41.Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, Jameson SC. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–1061. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 42.Starr TK, Daniels MA, Lucido MM, Jameson SC, Hogquist KA. Thymocyte sensitivity and supramolecular activation cluster formation are developmentally regulated: a partial role for sialylation. J Immunol. 2003;171:4512–4520. doi: 10.4049/jimmunol.171.9.4512. [DOI] [PubMed] [Google Scholar]
- 43.Gil D, Schrum AG, Daniels MA, Palmer E. A role for CD8 in the developmental tuning of antigen recognition and CD3 conformational change. J Immunol. 2008;180:3900–3909. doi: 10.4049/jimmunol.180.6.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 45.Chiang GG, Sefton BM. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 2001;276:23173–23178. doi: 10.1074/jbc.M101219200. [DOI] [PubMed] [Google Scholar]
- 46.Mueller DL. Tuning the immune system: competing positive and negative feedback loops. Nat Immunol. 2003;4:210–211. doi: 10.1038/ni0303-210. [DOI] [PubMed] [Google Scholar]
- 47.Stefanova I, Dorfman JR, Tsukamoto M, Germain RN. On the role of self-recognition in T cell responses to foreign antigen. Immunol Rev. 2003;191:97–106. doi: 10.1034/j.1600-065x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 48.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.