Abstract
A racemic synthesis of the ABCD ring core of the ambiguines that preserves the tertiary alcohol has been accomplished in convergent synthesis in 10 synthetic steps, in an overall yield of 46% from commercially available 4-bromoindole and m-methylanisole.
Keywords: Ambiguine, Hapalindole, Tin (IV) chloride
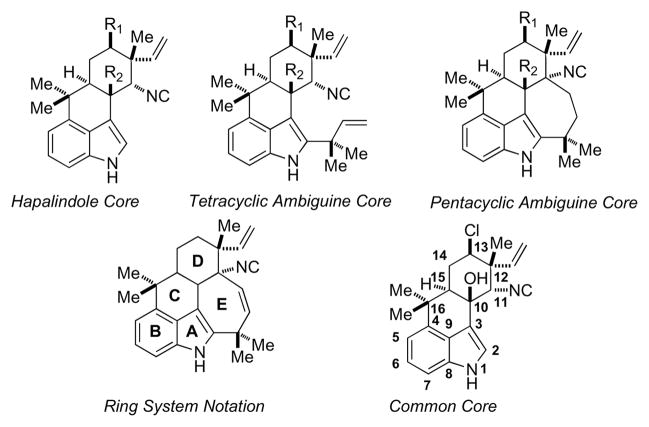
The ambiguines1,2,3 are a family of indole alkaloids that were first isolated in 1991. They are structurally related to the hapalindoles4,5 family of alkaloids (Figure 1). To date, the ambiguine family has fifteen members (Ambiguines A-O) that are broken down into two subclasses; the tetracyclic and pentacyclic systems.
Figure 1.
Hapalindole, Tetracyclic and Pentacyclic Ambiguine Cores.
The tetracyclic ambiguines are structurally the same as the hapalindoles with the exception of the presence of a reverse-prenyl group on C2 of the indole ring. Pentacyclic ambuguines contain a seven-membered ring, which contains either an epoxide, diol, vinyl cyanide, α-hydroxyl ketone or diene functionality. Carbon-13 of ring D, in both families, can either have a methylene carbon (R1) or chlorine-containing carbon. Likewise, both can either have a hydrogen at the C10 position (R2) or possess an alcohol at said carbon. The common core for both the hapalindole and ambuguine families for most of their members contains the C13 chlorine containing stereocenter as well as the C10 tertiary alcohol.
The structural complexity and densely functionalized core, has attracted considerable synthetic attention and to date ambiguine H has been conquered by total synthesis.6 We report here, our own preliminary efforts on the synthesis of these intriguing substances.
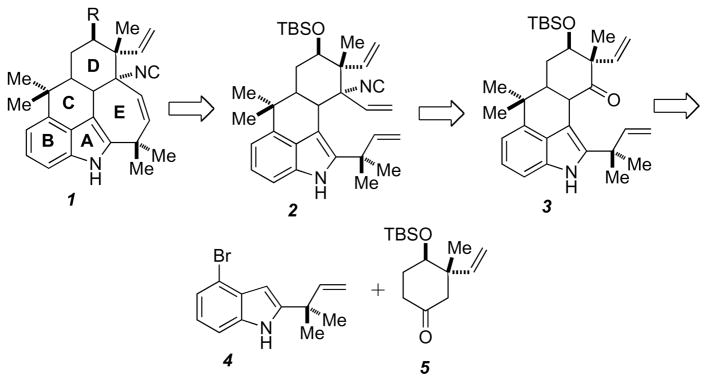
We envisioned that the pentacyclic core 1 of the ambiguines could arise from an intra-molecular RCM reaction of 2, which could be assembled through a Mannich reaction with vinyl Grignard and ammonium acetate followed by isonitrile formation from 3 (Scheme 1). Formation of the tetracyclic core 3 was anticipated to arise from the manipulation of 4 and 5. The C12 quaternary center was envisioned to arise from compound 5, which could be accessed from commercially available m-methylanisole and functionalized indole 4 from 2-bromo-6-nitrotoluene.
Scheme 1.
Retrosynthetic analysis.
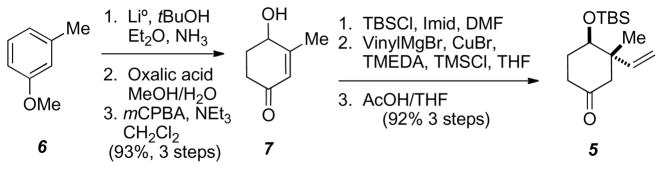
Following the protocol of Rubottom and Gruber,7 commerically available m-methylanisole was converted to 7 utilizing a Birch reduction followed by hydrolysis (Scheme 2). Secondary alcohol 7 was protected with TBSCl and imidazole, followed by treatment with vinylmagnesium bromide to afford the quaternary center substrate as the corresponding TMS-enol ether. Cleavage of the TMS-enol ether with an acetic acid/water/THF mixture afforded racemic 5 in an overall 86% yield for the six steps.
Scheme 2.
Synthesis of substrate 5.
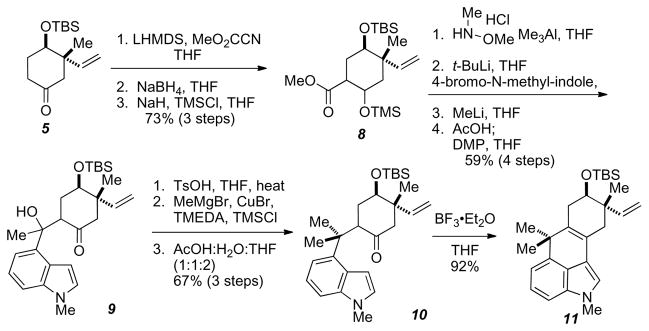
Assembly of tetracycle 11 began with treating racemic 5 with LHMDS and Mander’s reagent to give the β-ketoester (Scheme 3). Reduction of the ketone with NaBH4 followed by TMS protection of the resulting alcohol gave 6 in good yields. Treatment of 8 with N-methoxy-methylamine hydrochloride and Me3Al in THF afforded the Weinreb amide. Subjecting 4-bromo-N-methylindole to lithium-halogen exchange resulted in the corresponding 4-lithium species that was added to the Weinreb amide and worked up under acid conditions and carried on without further purification. Methyl lithium was added to the crude material at −78 °C in THF, warmed to room temperature, and worked up under acidic conditions to afford a 2.5:1 mixture of the secondary alcohol and its corresponding TMS ether. The crude mixture was treated with acetic acid for 45 min, concentrated, and taken into THF and oxidized with Dess-Martin Periodinane to afford 9 in good yields. Dehydration of 9 with TsOH gave the β-methyl, β-indole-exocyclic enone that was subjected to methyl cuprate Michael conditions to afford tricycle 10. Subjecting 10 to BF3-etherate afforded tetracycle 11 in an overall yield of 27% from 5 in 11 steps.
Scheme 3.
Weinreb amide route to tetracycle 11.
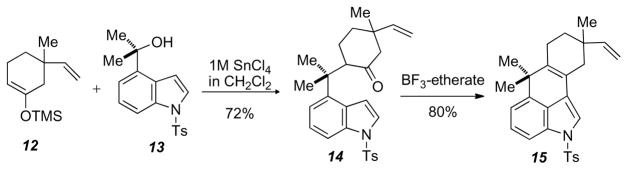
Muratake and Natsume8,9 in their total synthesis of hapalindole U employed a dilute (1M) tin (IV) chloride-mediated coupling to form 13. Treating TMS-enol ether 10 with 4-(2-hydroxy-2-propyl)-N-tosyl-indole 11 with 1 M SnCl4 at −78 °C to afforded tricyle 12 when quenched at −78 °C (Scheme 4).
Scheme 4.
Muratake and Natsume tricycle formation
Murataka’s tricycle 14 and our tricyle 11 are similar in structure, with ours being more functionalized. Both routes close the tricycle with BF3-etherate in THF to give rise to similar tetracycles. Tetracycle 15 was formed in 58% over two steps, whereas tetracycle 11 was formed in 27% over eleven steps clearly showing the advantage of the tin-mediated chemistry. It was thought that utilizing this methodology, which has been used in a variety of alkaloid syntheses,10,11,12,13 we could gain rapid access to the ABCD ring system of the ambigiunes in a more efficient manner than that extant.
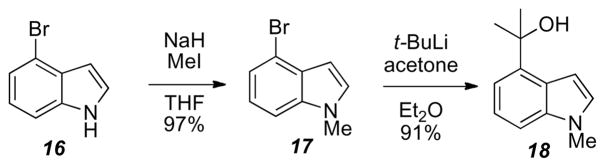
Accessing the required 4-(2-hydroxyl-2-propyl)-N-methyl-indole for the tin-mediated coupling was accomplished from 2-bromo-6-nitrotoluene under Leimgruber-Batcho14 conditions to afford 16 in high yields, (Scheme 5). N-Methylation of 16 was performed with NaH and methyl iodide in THF to afford 17. Treatment of 17 under lithium-halogen exchange conditions followed by acetone addition furnished 18 in a 91% yield.
Scheme 5.
Synthesis of indole 18.
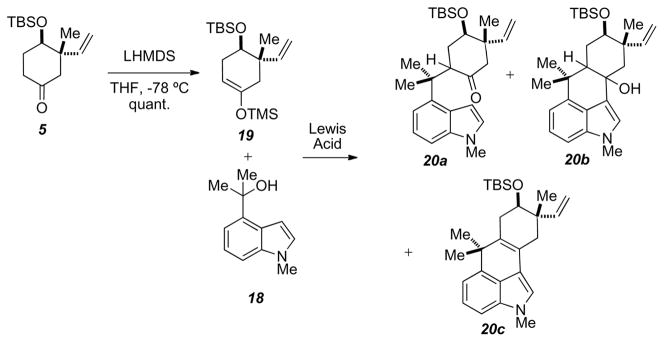
Treatment of 5 with LHMDS followed by TMSCl gave the required TMS-enol ether 19. A variety of conditions were probed to optimize the coupling of 18 to 19 (Scheme 6 and Table 1). In the event, it was observed that treating indole 18 and TMS-enol ether 19 with fuming tin (IV) chloride in DCM gave the tetracyclic species 20b in good yields. While we were anticipating the tricycle 20a, synthesis of 20b gives the carbon core along with the desired tertiary alcohol at the C10, which is present in several ambiguines.
Scheme 6.
Lewis acid-mediated coupling of 18 + 19.
Table 1.
Lewis-acid and conditions screened for Scheme 6.
| Lewis Acid | Conditions | Product |
||
|---|---|---|---|---|
| 20a | 20b | 20c | ||
| TiCl4 (fuming) | Toluene, −78 °C | 7% | X | X |
| TiCl4 (fuming) | Toluene, −44 °C | X | X | 5% |
| TiCl4 (1 M PhCH3) | Toluene, −78 °C | X | X | X |
| TiCl4 (1 M PhCH3) | Toluene, −44 °C | X | X | X |
| TiCl4 (1 M DCM) | DCM, −78 °C | 13% | X | X |
| TiCl4 (1 M DCM) | DCM, −44 °C | X | X | X |
| TiCl4 (fuming) | DCM, −78 °C | X | X | 36% |
| TiCl4 (fuming) | DCM, −44 °C | X | X | 13% |
| SnCl4 (1 M PhCH3) | Toluene, −78 °C | 15% | 24% | X |
| SnCl4 (1 M PhCH3) | Toluene, −44 °C | X | X | X |
| SnCl4 (1 M DCM) | DCM, −78 °C | 83% | X | X |
| SnCl4 (1 M DCM) | DCM, −44 °C | 54% | X | X |
| SnCl4 (fuming) | DCM, −78 °C | 5% | 61% | X |
| SnCl4 (fuming) | DCM, −44 °C | 13% | 8% | 42% |
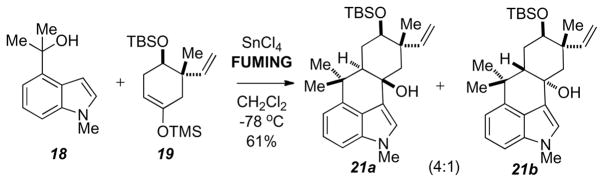
Further studies into the coupling of 18 and 19 with fuming tin (IV) chloride revealed that the reaction afforded diastereomeric tetracycles 21a and 21b (4:1 ratio) (Scheme 7), but not the dehydrated tetracycle as seen in Scheme 4. Quenching the fuming tin (IV) chloride reaction at −78 °C proved to be essential in forming the tertiary alcohol. When quenching the reaction at any temperate above −50 °C formation of the dehydrated tetracycle 20c was observed. When 18 and 19 were treated with the 1M tin (IV) chloride solution a similar tricyle as seen in Scheme 4 was formed. Subjecting this tricycle to BF3-etherate afforded the dehydrated tetracycle 20c. When performing the same procedure with either the corresponding N-Boc or N-silyl (TMS, TBS, or TIPS) protecting groups only dehydrated tetracycles were observed. Interestingly, when attempting the reaction on a substrate bearing the N-tosyl group under the same conditions, only the tricycle was obtained. Other alkyl protecting groups such as PMB or benzyl groups can be used with the current methodology, however the yields from the coupling reactions are significantly lower, 7% and 11% respectively.
Scheme 7.
Fuming SnCl4 - mediated tetracycle formation
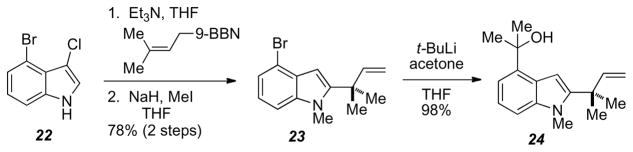
Having accessed the ABCD ring system of the ambiguines, along with the tertiary alcohol at C10, efforts were directed towards gaining access to the same ABCD system with the reverse-prenyl group on C-2 of the indole. Chlorination of the previously described 4-bromoindole with NCS in DMF afforded 22 in excellent yields. Installation of the reverse-prenyl group was accomplished using Danishefsky chemistry15 followed by N-methylation of the indole nitrogen to afford 22. Subjecting 22 to lithium-halogen exchange conditions followed by acetone gave access to 24 in 98% yield.
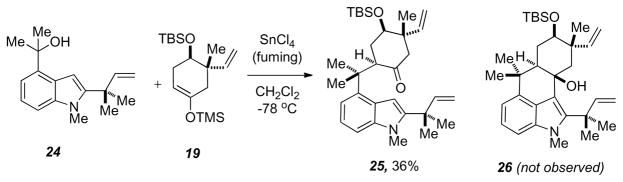
The coupling of 24 and 19 with fuming tin (IV) chloride was attempted as described previously in Table 1. Unfortunately, no tetracyclic product was observed when subjecting 24 and 19 to any of the previous coupling conditions. Furthermore, only fuming tin (IV) chloride allowed access to the tricycle 25 in 36% yield (Scheme 9). Treating tricycle 25 with BF3-etherate at room temperature for 24 hours failed to give the desired tetracycle, but rather the dehydrated tetracyclic system. Similar results were obtained when attempting the same methodology on methyl, ethyl, vinyl, and propyl C2 substituted indoles.
Scheme 9.
Attempted tetracycle formation
In summary, we have shown two methods of accessing the ABCD cores of both the hapalindoles and ambiguines. Utilizing fuming tin (IV) chloride-mediated coupling of tertiary benzylic alcohols of indoles and TMS-enol ethers we have also gained access to more functionalized tetracyclic cores. Current efforts to deploy this methodology for the concise total synthesis of several members of this family of alkaloids are under investigation.
Supplementary Material
Scheme 8.
Synthesis of indole 24.
Acknowledgments
This paper is warmly dedicated to Professor Harry H. Wasserman on the occasion of his 90th birthday. We gratefully acknowledge financial support from the National Institutes of Health Grant GM068011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Smitka TA, Bonjouklian R, Doolin L, Jones ND, Deeter JB, Yoshida WY, Prinsep MR, Moore RE, Patterson GM. J Org Chem. 1992;57:857. [Google Scholar]
- 2.Huber U, Moore RE, Patterson GM. J Nat Prod. 1998;61:1304–1306. doi: 10.1021/np9801561. [DOI] [PubMed] [Google Scholar]; Raveh A, Carmeli S. J Nat Prod. 2007;70:196. doi: 10.1021/np060495r. [DOI] [PubMed] [Google Scholar]
- 3.Mo S, Krunic A, Chlipala G, Orjala J. J Nat Prod. 2009;72:894. doi: 10.1021/np800751j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore RE, Cheuk C, Patterson GM. J Am Chem Soc. 1984;106:6456. [Google Scholar]
- 5.Moore RE, Cheuk C, Yang XQ, Patterson GM. J Org Chem. 1987;52:1036. [Google Scholar]; Moore RE, Cheuk C, Yang XQ, Patterson GM. J Org Chem. 1987;52:3773. [Google Scholar]
- 6.Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 7.Rubottom GM, Gruber JM. J Org Chem. 1977;42:1051. [Google Scholar]
- 8.Muratake H, Natsume M. Tetrahedron Lett. 1989;30:1815. [Google Scholar]
- 9.Muratake H, Natsume M. Tetrahedron. 1990;46:6331. [Google Scholar]; Muratake H, Natsume M. Tetrahedron. 1990;46:6343. [Google Scholar]; Muratake H, Kumagami H, Natsume M. Tetrahedron. 1990;46:6351. [Google Scholar]
- 10.Brown MA, Kerr MA. Tetrahedron Lett. 2001;42:983. [Google Scholar]; Kinsman AC, Kerr MA. J Am Chem Soc. 2003;125:14120. doi: 10.1021/ja036191y. [DOI] [PubMed] [Google Scholar]
- 11.MacKay JA, Bishop RL, Rawal VH. Org Lett. 2005;7:3421. doi: 10.1021/ol051043t. [DOI] [PubMed] [Google Scholar]
- 12.Brailsford JA, Lauchli R, Shea KJ. Org Lett. 2009;11:5330. doi: 10.1021/ol902173g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian X, Huters AD, Douglas CJ, Garg NK. Org Lett. 2009;11:2349. doi: 10.1021/ol9007684. [DOI] [PubMed] [Google Scholar]
- 14.Batcho AD, Leimgruber W. Org Synth. 1985;63:214. [Google Scholar]
- 15.Schkeryantz JM, Woo JC, Siliphaivanh P, Depew KM, Danishefsky SJ. J Am Chem Soc. 1999;121:11964. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.