Abstract
Background
Insulin-like growth factor-1 may be involved in regulation of blood pressure through multiple pathways; however, the prospective association between plasma insulin-like growth factor-1level and risk of hypertension has never been explored.
Methods
We prospectively examined the association between plasma insulin-like growth factor-1 level and the risk of incident hypertension among 2046 women without a history of hypertension or diabetes. Cox proportional hazards regression models were used to adjust for potential confounders.
Results
We identified 181 incident cases of hypertension during 4-years of follow-up. After adjusting for plasma insulin-like growth factor binding protein-3 level and other potential confounders, women in the top tertile of insulin-like growth factor-1 had decreased risk of incident hypertension (relative risk 0.56, 95% confidence interval 0.35–0.91) compared with women in the bottom tertile. After further adjusting for C-peptide level and C-reactive protein level in subsets of participants who also had those markers measured, the association between insulin-like growth factor -1 and risk of incident hypertension remained robust.
Conclusions
Higher circulating insulin-like growth factor-1 level is associated with a decreased risk of incident hypertension among non-diabetic women.
Introduction
Insulin-like growth factor-1 (IGF-1) is a polypeptide synthesized and released from multiple tissues1; 75% to 85% of circulating IGF-1 is bound to the most abundant serum IGF binding protein (IGFBP), IGFBP-32. The central role of IGFBP-3 is to regulate IGF-1 bioavailability, and IGFBP-3 also competitively inhibits IGF-1 action at the cellular level2.
In vitro and in vivo experiments indicate that IGF-1 decreases vascular resistance, mediated partly by stimulation of nitric oxide synthesis in endothelial and vascular smooth muscle cells3. Other vasodilatory mechanisms of IGF-1may be inhibition of intracellular Ca2+ influx, and stimulation of the Na+, K+-ATPase3. Murine knockouts of liver-derived IGF-1, the major fraction of circulating IGF-1, show impaired endothelial-dependent vasodilatation, increased endothelin-1 production, and increased systolic blood pressure4. In humans, cross-sectional studies demonstrate an inverse relation between circulating IGF-1 levels and prevalent hypertension, both in diabetic and non-diabetic populations5–10. Additional cross-sectional data7 suggest that the inverse association between IGF-1 levels and the metabolic syndrome is restricted to those with adequate 25-hydroxyvitamin D (25[OH] D) levels, possibly because of the metabolic interaction between the IGF-1 and vitamin D axes7, 11–12. No published study has prospectively investigated whether plasma IGF-1 levels are associated with the risk of developing hypertension. Therefore, we prospectively investigated the association between plasma IGF-1 level and the risk of incident hypertension among 2046 non-hypertensive, non-diabetic women from the Nurses’ Health Study (NHS).
Methods
Source Population
The NHS cohort was assembled in 1976, when 121 700 female nurses aged 30 to 55 years returned a mailed questionnaire. Participants are followed via biennial questionnaires that gather updated information on health-related behaviors and medical events. From 1989 to 1990, 32 826 consenting women provided blood samples returned with a cold pack by overnight mail; 97% of samples were received within 24 hours of collection. All blood samples were stored in liquid nitrogen (−130 °C or less) until laboratory analysis. This study was approved by the institutional review board at Brigham and Women’s Hospital. Receipt of each questionnaire implied participant’s consent.
Study Population
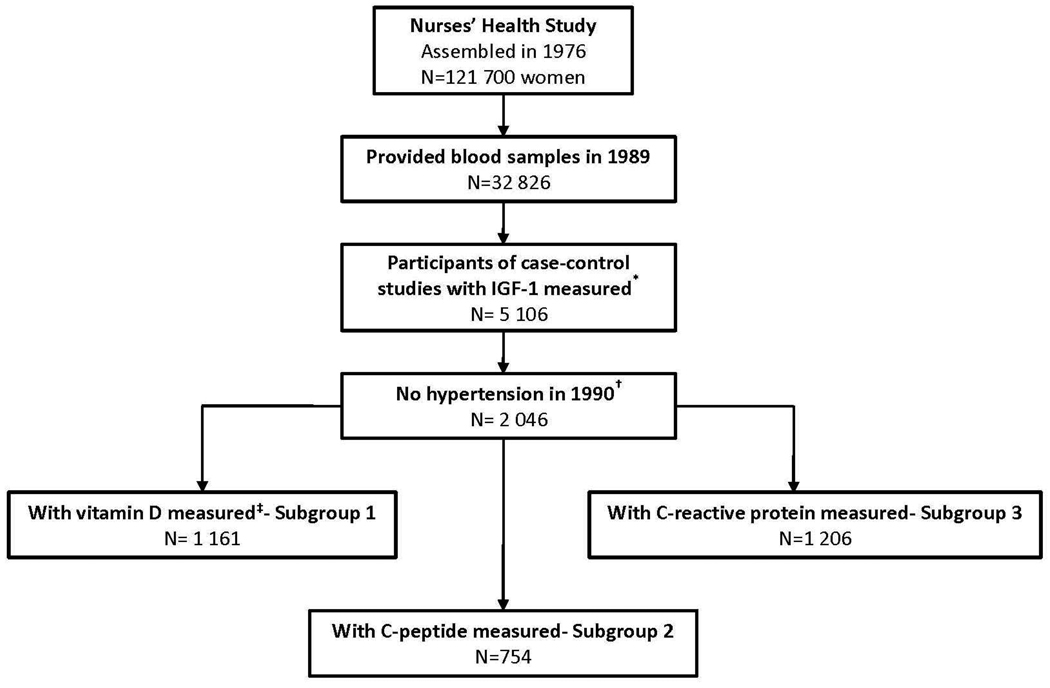
Assembly of the study population for the present analysis is depicted in Figure 1. We identified previous nested case-control studies13–16 (studies of breast cancer, colorectal cancer, colorectal adenoma, and ovarian cancer) from the NHS in which plasma IGF-1 levels were measured. To minimize potential bias, the participants who had IGF-1 levels measured specifically because they developed cancer were excluded from our analyses. Women were also excluded if, at the time that blood was collected, they had: 1) a history of hypertension and/or use of blood pressure-lowering medications; 2) a history of diabetes; or 3) a prior history of cancer (except for non-melanoma skin cancer). After these exclusions, 2 046 women were included in our primary analysis.
Figure 1.
Study population for analysis of plasma insulin-like growth factor-1levels and risk of incident hypertension.
* Nested case-control studies are described elsewhere13–16.
† Participants were also excluded if they reported use of antihypertensive medication, history of diabetes or history of cancer (except for non-melanoma skin cancer) at baseline.
‡ Including measurements of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D. IGF-1, insulin-like growth factor-1
Assessment of IGF-1 and IGFBP-3 levels
IGF-1 and IGFBP-3 levels were assayed by enzyme-linked immunosorbent assay with reagents from Diagnostic Systems Laboratory (Webster, TX). All analyses were performed in the same laboratory, with intra-assay coefficients of variation for IGF-1 and IGFBP-3 ranging from 2% to 10%.
Assessment of Vitamin D, C-peptide and C-reactive protein levels
Plasma levels of 25(OH) D and 1, 25-dihydroxyvitamin D (1, 25(OH) 2D) were measured on 1 161 out of these 2 046 women (Subgroup-1, Figure 1) as described in detail previously17–19. Among another subset of participants (n=754, Subgroup-2, Figure 1), plasma C-peptide levels were assayed using enzyme-linked immunosorbent assay with reagents from Diagnostic Systems Laboratory (Webster, TX). C-reactive protein (CRP) levels were measured among 1206 participants (Subgroup-3, Figure 1) via a high-sensitivity latex-enhanced immunonephelometric assay on a BN II analyzer (Dade Behring, Newark, DE). Subgroup-1 was used to analyze whether the association between IGF-1 and the risk of hypertension varied by 25(OH) D and 1, 25(OH) 2D levels, as suggested by some cross-sectional data7. Because hyperinsulinemia may be an intermediate pathway of the IGF-1 – hypertension association, we used Subgroup-2 as an attempt to account for insulin secretion (insulin level was not available). Since inflammation plays an important role in the pathogenesis of hypertension20, we adjusted for CRP levels in Subgroup-3 as a marker of inflammation.
Assessment of Other Covariates
Body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), physical activity (metabolic equivalent tasks), smoking status, menopausal status and use of postmenopausal hormones were ascertained by questionnaire at baseline. Intake of sodium was ascertained from a food frequency questionnaire in 1990, and was adjusted for total energy intake by the residual method 21. Details about the blood collection time and fasting status at the time of blood collection were reported on the questionnaire that accompanied the blood sample.
Assessment of Hypertension
The baseline and biennial follow-up questionnaires asked participants to report whether a clinician had made a new diagnosis of hypertension during the preceding 2 years. Self-reported hypertension was previously validated in the NHS22. In a subset of women who reported hypertension (n=51), medical record review confirmed a documented systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg in 100% of participants22. Among another subset of women without prior self-reported hypertension (n=161), only 6.8% of them had recorded blood pressure ≥ 140/90 mmHg, and none of them had blood pressure ≥ 160/95 mmHg22. Furthermore, 12 years after baseline (2002), the participants in NHS who became hypertension cases were more likely to develop stroke (2.3% vs. 0.9%) and diabetes (8.1% vs. 3.2%) compared to those participants who did not become hypertension cases. A participant was considered to have prevalent hypertension if she reported this diagnosis on any questionnaire up to and including the 1990 questionnaire (the final year of blood collection), and therefore was excluded from the present study. Incident cases included individuals who first reported hypertension on questionnaires after 1990.
Statistical Analyses
Person-time was truncated at the date of hypertension diagnosis, at the first date of anti-hypertensive medication initiation, at the date of diabetes diagnosis, at the date of cancer diagnosis (except for non-melanoma skin cancer), at the date of death, or at the end of the study, whichever came first.
Plasma IGF-1 levels were analyzed in batch-specific tertiles (due to laboratory variation over time), using the lowest tertile as the reference group. The relation between IGF-1 and other covariates at baseline (in 1990) were analyzed using the Kruskall-Wallis test for continuous variables and the Mantel-Hanzel χ2 test of trend for categorical variables.
The association between IGF-1 and incident hypertension was analyzed using Cox proportional hazards regression models to estimate relative risks (RRs) and 95% confidence intervals (CIs). Because IGF-1 and IGFBP-3 levels are positively correlated but have opposing effects biologically2, levels of IGFBP-3 (in batch-specific tertiles) were included in regression models to observe the independent associations with IGF-1. Furthermore, multivariable models were constructed to adjust for other potential confounding variables that have been previously associated with incident hypertension, including: age (continuous), BMI (4 categories), current smoking (yes/no), family history of hypertension (yes/no), menopausal status (premenopausal/postmenopausal), postmenopausal hormone use (yes/no), physical activity (continuous), fasting status (< 8 hours vs. ≥ 8 hours since last meal), and intake of sodium (continuous). Primary analyses were carried out during 4 years of follow-up because the correlation coefficient between IGF-1 and IGFBP-3 levels from blood collections 2–3 years apart was reasonable (0.86 for IGF-1 and 0.82 for IGFBP-3)23, and thus misclassification of IGF-1 levels was probably minimal. We also examined and report results during 6 years and 8 years of follow-up, although we do not have data for the consistency of IGF-1 and IGFBP-3 levels over this duration.
We performed secondary analyses in three subgroups, depending upon whether vitamin D levels, C-peptide levels or CRP levels were also measured in addition to IGF-1 levels. First, we investigated whether the association between IGF-1 and the risk of hypertension varied by 25(OH)D and 1,25(OH) 2D levels (Subgroup-1; N=1161). We performed stratified analyses using the median levels of 25(OH) D and 1,25(OH) 2D, which were 28.1 ng/mL and 33.0 pg/mL, respectively; appropriate interaction terms were generated to test whether interactions were statistically significant. Using Subgroup-2 (N=754) and Subgroup-3 (N=1206), we analyzed whether the association between IGF-1and hypertension was attenuated after adjusting for C-peptide level or CRP levels which were both modeled as continuous variables.
All P values are 2-tailed. Statistical tests were performed using SAS version 9.1 for UNIX statistical software package (SAS Institute Inc, Cary, NC).
Results
During the 4 years (7664 person-years) of follow-up, 181 incident cases of physician-diagnosed hypertension were reported. Participant characteristics are presented in Table 1. Age was significantly inversely associated with plasma IGF-1 levels. IGFBP-3 levels were positively associated with IGF-1 after adjusting for age, with a partial correlation coefficient of 0.46 (P<0.001).
Table 1.
Baseline characteristics by tertiles of insulin-like growth factor-1*
| Variables | Tertile 1 | Tertile 2 | Tertile 3 | P value |
|---|---|---|---|---|
| Number of participants | 635 | 699 | 712 | |
| Age (years) | 58 (52–64) | 56 (50–63) | 53 (48–61) | <0.001 |
| Body mass index (kg/m2) | 24.0 (21.6–27.1) | 23.7 (21.9–26.4) | 23.9 (22.1–26.3) | 0.34 |
| Physical activity (METs/w) | 9.1 (3.4–20.3) | 10.4 (4.2–22.1) | 10.4 (4.0–21.5) | 0.03 |
| Family history of hypertension (%) | 43.5 | 45.2 | 40.5 | 0.22 |
| Current smoker (%) | 14.8 | 16.4 | 14.3 | 0.52 |
| Postmenopausal (%) | 79.7 | 79.0 | 76.0 | 0.23 |
| Postmenopausal hormone use (%) † | 58.9 | 38.4 | 23.6 | <0.001 |
| Sodium intake (mg/day) | 1812 (1619–1998) | 1809 (1622–2042) | 1822 (1641–2038) | 0.40 |
| IGF-1 (ng/mL) | 115.7 (95.7–136.2) | 161.5 (146.0–181.6) | 229.0 (201.3–275.1) | <0.001 |
| IGFBP-3 (ng/mL) | 3659 (3140–4250) | 4155 (3567–4680) | 4686 (4102–5308) | <0.001 |
| 25(OH)D (ng/mL) ‡ | 28.0 (21.4–35.3) | 28.1 (20.7–35.0) | 28.2 (21.9–34.8) | 0.90 |
| 1,25(OH) 2D (pg/mL) ‡ | 34.1 (29.0–39.4) | 32.0 (27.7–37.6) | 33.1 (29.1–38.6) | 0.001 |
| C-peptide (ng/mL) § | 1.2 (0.8–1.9) | 1.4 (0.9–2.1) | 1.5 (1.1–2.2) | 0.001 |
| C-reactive protein (mg/L) ‖ | 2.1 (1.0–4.3) | 1.4 (0.7–2.9) | 1.0 (0.5–1.9) | <0.001 |
Except for age, data are presented as age-adjusted median (inter-quartile range) or percentages.
Data are percentage among post-menopausal women.
Results from Subgroup-1 (n=1 161);
Results from Subgroup-2 (n=754);
Results from Subgroup-3 (n=1206).
MET, metabolic equivalent; IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH) 2D, 1,25-dihydroxyvitamin D.
Plasma IGF-1 level was inversely associated with the risk of incident hypertension in age-adjusted and multivariable analyses (Table 2). After adjusting for age and BMI, the top tertile of IGF-1 was associated with a 38% reduced risk (RR=0.62, 95% CI 0.41–0.93) of developing hypertension. After further adjusting for IGFBP-3 level, the RR for the top tertile was 0.54 (95% CI 0.34–0.87). Adjusting for additional covariates did not materially attenuate the association. Besides, we have information on previous oral contraceptives use on 1984, which is 6 years prior to our baseline year. The percentage of previous use was 50.4% among participants of our study who became hypertensive cases and 44.0% among those who did not (P=0.09). Further adjusting for previous oral contraceptives did not change the RRs markedly (the point estimates and the CIs remained the same).
Table 2.
Plasma IGF-1 level and risk of incident hypertension among 2 046 women in the primary analysis.
| Plasma IGF-1 levels | |||
|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |
| Person years | 2332 | 2614 | 2717 |
| Number of cases | 73 | 62 | 46 |
| Age- adjusted relative risk | 1.00 (reference) | 0.83 (0.57–1.21) | 0.61 (0.40–0.91) |
| Age, BMI and IGFBP-3 adjusted relative risk | 1.00 (reference) | 0.84 (0.57–1.24) | 0.54 (0.34–0.87) |
| Model a* | 1.00 (reference) | 0.80 (0.54–1.19) | 0.56 (0.35–0.91) |
Data are presented as relative risk (95% confidence interval)
Model a was adjusted for age, body mass index, physical activity, family history of hypertension, current smoking, menopausal status, postmenopausal hormone use, fasting status, and intake of sodium.
When we extended the analyses to 6 years, there were 11 382 person-years of follow-up and 296 incident cases. The multivariable adjusted RR for top tertile of IGF-1 was attenuated to 0.71 (95% CI 0.49–1.03). Further extension of the analyses to 8 years resulted in additional attenuation; the multivariable adjusted RR for top tertile of IGF-1 was 0.83 (95% CI 0.61–1.13).
We analyzed Subgroup-1 to determine whether the association between IGF-1 and hypertension varied by vitamin D level. The multivariable RR for the highest compared to lowest tertile of IGF-1 levels was 0.49 (95% CI 0.16–1.52) among women whose 25(OH)D level was <28.1 ng/mL, and was 0.27 (95% CI 0.07–0.95) among women whose 25(OH)D level was ≥28.1 ng/mL. The P value for interaction was 0.76. Similarly, there was no statistically significant difference in the RRs among women whose 1,25(OH) 2D was < 33.0 pg/mL compared to women whose level was ≥ 33.0 pg/mL (0.72 [95% CI 0.23–2.26] and 0.26 [95% CI 0.08–0.86] for the top tertile, respectively; P value for interaction=0.77).
We analyzed Subgroup-2 to determine whether the association between IGF-1 and hypertension persisted after controlling for C-peptide levels (as a marker of insulin secretion). Among these 754 women, 73 incident hypertension cases were reported during 2811 person-years of follow-up. The age, BMI and IGFBP-3 adjusted RR for the middle and top tertiles were 0.73 (95% CI 0.36–1.46) and 0.24 (95% CI 0.09–0.64), respectively. Further adjusting for plasma C-peptide levels did not change the RRs substantially (0.73 [95% CI 0.36–1.48] and 0.23 [95% CI 0.08–0.62] for the middle and top tertiles, respectively.
We analyzed Subgroup-3 to determine whether CRP level confounded the association between IGF-1 and hypertension. During 4597 person-years of follow-up, 112 incident hypertension cases were reported. The age, BMI and IGFBP-3 adjusted RR for the top tertile of IGF-1 were similar before and after adjusting for CRP level (from 0.32 [95% CI 0.16–0.64] to 0.32 [95% CI 0.16–0.65]).
Discussion
We report the first prospective study to examine the relation between plasma IGF-1 level and the risk of incident hypertension. We found that plasma IGF-1 levels were inversely associated with the risk of incident hypertension, and the association remained robust after adjusting for C-peptide and CRP levels.
Experimental studies support a relation between IGF-1 and blood pressure. In vitro and in vivo experiments indicate that IGF-1 decreases vascular resistance 3. The possible mechanisms include: stimulation of nitric oxide synthesis by endothelial and vascular smooth muscle cells, reduction of Ca2+ influx into vascular smooth muscle cells, and stimulation of Na+, K+-ATPase pumps thereby attenuating vascular contractility 3. Impaired IGF-1 vasorelaxant properties and IGF-1 signaling were observed in spontaneously hypertensive rats24. In contrast, IGF-1 may have other biological functions, such as inotropic and growth effects on the heart and endothelium25 and induction of vascular smooth muscle cell migration and proliferation26; these effects may be involved in the promotion, rather than prevention, of hypertension. Nevertheless, the overall blood pressure effect of lower IGF-1 seems to be induction of hypertension. When liver-specific IGF-1 is knocked out 4, mice develop endothelial dysfunction, increased peripheral vascular resistance with higher expression of endothelin-1, and increased systolic blood pressure.
An inverse correlation between circulating IGF-1 level and blood pressure was observed in the majority of published cross-sectional studies, including studies among type 1 and type 2 diabetic patients6, 9, patients with borderline hypertension10, as well as studies of middle aged and elderly general populations5, 7–8. However, several cross-sectional studies showed no association between IGF-1 and blood pressure27–28, while others indicated that plasma IGF-1 levels were actually elevated among hypertensive patients, especially those with left ventricular hypertrophy29–30. This contradiction may possibly be explained by the effect of established high blood pressure on IGF-1 production. For example, in the Goldblatt hypertensive rat model, IGF-1 messenger RNA in the left ventricle was increased, and ventricular wall stress was the most likely stimulating factor for IGF-1 synthesis31. Therefore, the finding that IGF-1 levels were elevated among hypertensive individuals with left ventricular hypertrophy may be indicative of increased synthesis as a result of high pressure load and wall stress. 32. After antihypertensive treatment, IGF-1 among hypertensive patients decreased, particularly among those in whom left ventricular hypertrophy regressed29. Studies that examine the prospective association between IGF-1 and incident hypertension, such as the current study, would be less likely to be influenced by that kind of reverse causation bias.
One other longitudinal study reported the relation between IGF-1 and blood pressure 33. That study analyzed young normotensive men (aged 20–34 years) with average baseline blood pressures of 112–114/69–71 mmHg 33. In multivariable analyses, baseline IGF-1 levels were not associated with the 8-year change in either 544 black men or 747 white men. However, it was not clear whether any substantial blood pressure change occurred among this population of healthy young men. Furthermore, since IGF-1 levels decrease with advancing age1, and since the underlying mechanisms linking IGF-1 with blood pressure may well vary with age 34, that study of young men may not be directly comparable with our study of middle-aged women.
Another possible mechanism for the inverse association between IGF-1 level and hypertension is via the hyperinsulinemic profile, since low IGF-1 levels have been associated with decreased insulin sensitivity9 and the metabolic syndrome7. However, in our own analysis of non-diabetic participants as well as in a separate cross-sectional study35, the relation between IGF-1 and hypertension persisted after further adjusting for C-peptide levels or insulin levels, suggesting that insulin resistance may not explain the association. Furthermore, it has been reported that CRP level is negatively correlated with IGF-1 level36, therefore systematic inflammation could be involved in the association between IGF-1 and hypertension. In our analyses, adding CRP to multivariable models did not attenuate the association, suggesting that other mechanisms explaining the IGF-1-hypertension link might exist.
IGF-1 may exert some effects through changes in vitamin D activation, since IGF-1 stimulates renal 1-α-hydroxylase activity in vivo, promoting the synthesis of 1,25(OH)2D 7, 11. Previous studies indicate that 1,25(OH)2D may inhibit renin expression37 and growth of cultured vascular smooth muscle cells 38, and therefore may be associated with a reduced risk of hypertension. Furthermore, ten functional vitamin D response elements, most of which are known to actively respond to 1,25(OH)2D, are present in human IGFBP gene promoter regions12, suggesting interrelations between the IGF-1 and vitamin D axes. Indeed, a cross-sectional analysis of IGF-1 and the metabolic syndrome documented effect modification by vitamin D status7. In our analysis, the association between IGF-1 and the risk of incident hypertension appeared more prominent among participants with higher levels of 25(OH) D and 1,25(OH) 2D, which is consistent with the previous study about metabolic syndrome7. However, the interactions were not statistically significant in our analysis.
A previous study suggested that administration of oral estrogen in healthy postmenopausal women suppresses hepatic IGF-1 production39, which is consistent with our findings of lower IGF-1 levels in women using post-menopausal hormones. However, available data suggest that estrogen replacement has little effect on systemic blood pressure40. Therefore, postmenopausal hormone use should not confound of the association between IGF-1 level and risk of hypertension. We confirmed the lack of confounding by postmenopausal hormone use by including it in our multivariable models, and finding that it did not substantially alter the effect estimate for IGF-1 and did not change the significance of IGF-1.
In our subgroup analyses, the RRs were larger and the CIs were wider than in the primary analysis. This apparent discrepancy may simply be due to the smaller sample size and smaller number of cases, leading to more unstable estimates. Yet the RR estimates from the secondary analyses were largely overlapping with the CIs from the primary analysis, so the results are internally consistent.
Our study has limitations that deserve mention. First, our study population was derived from pre-existing case-control studies. Although we only included controls from those studies (with the exception for case-control studies of colorectal adenoma) to avoid bias, the generalizability of the results from the present study might be limited. As our study was entirely female and mostly white, the results may also not be generalizable to non-whites or men. Second, blood pressure was not directly measured and hypertension was self-reported. However, all of the participants were registered nurses, and hypertension reporting by these nurses is highly accurate 22. Even if a few truly hypertensive individuals were misclassified as being non-hypertensive on questionnaires, such misclassification would tend to diminish the magnitude of the RR. Therefore, our finding may indeed be an underestimate of the true association. Third, we only had a single measurement of plasma IGF-1. Because levels may fluctuate over time, longer periods of follow-up may result in more random misclassification. This would also tend to diminish the magnitude of the RR, as we indeed observed, thereby underestimating the true association. Fourth, we lacked information on renal function, and decreased IGF-1 expression has been observed in uremic rats41. Yet since all participants in our study were free from hypertension and diabetes at baseline, it is unlikely that many women had impaired renal function. Indeed, other studies have indicated a very low prevalence of renal dysfunction in this cohort42–43. Finally, since our study was observational, the possibility of residual confounding exists.
In conclusion, our prospective analysis suggests that circulating IGF-1 level is inversely associated with the risk of incident hypertension among non-diabetic women. Our findings may increase the understanding of hypertension pathogenesis in humans, and should be confirmed in other cohorts.
Acknowledgements
None
Funding Sources This study was funded by the American Heart Association grant 0535401T, NIH grants CA87969 and HL079929, and the Beijing NOVA program from the Beijing Municipal Science and Technology Commission. Additionally this work has been made possible through the International Society of Nephrology-funded Fellowship (L.Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None
Reference
- 1.Ohlsson C, Mohan S, Sjogren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009 Aug;30(5):494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer. 2005 Jul;41(11):1515–1527. doi: 10.1016/j.ejca.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Sowers JR. Insulin and insulin-like growth factor in normal and pathological cardiovascular physiology. Hypertension. 1997 Mar;29(3):691–699. doi: 10.1161/01.hyp.29.3.691. [DOI] [PubMed] [Google Scholar]
- 4.Tivesten A, Bollano E, Andersson I, et al. Liver-derived insulin-like growth factor-I is involved in the regulation of blood pressure in mice. Endocrinology. 2002 Nov;143(11):4235–4242. doi: 10.1210/en.2002-220524. [DOI] [PubMed] [Google Scholar]
- 5.Hunt KJ, Lukanova A, Rinaldi S, et al. A potential inverse association between insulin-like growth factor I and hypertension in a cross-sectional study. Ann Epidemiol. 2006 Jul;16(7):563–571. doi: 10.1016/j.annepidem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Capoluongo E, Pitocco D, Lulli P, et al. Inverse correlation between serum free IGF-I and IGFBP-3 levels and blood pressure in patients affected with type 1 diabetes. Cytokine. 2006 Jun;34(5–6):303–311. doi: 10.1016/j.cyto.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008 Feb;57(2):298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 8.Paolisso G, Tagliamonte MR, Rizzo MR, et al. Mean arterial blood pressure and serum levels of the molar ratio of insulin-like growth factor-1 to its binding protein-3 in healthy centenarians. J Hypertens. 1999 Jan;17(1):67–73. doi: 10.1097/00004872-199917010-00011. [DOI] [PubMed] [Google Scholar]
- 9.Sesti G, Sciacqua A, Cardellini M, et al. Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care. 2005 Jan;28(1):120–125. doi: 10.2337/diacare.28.1.120. [DOI] [PubMed] [Google Scholar]
- 10.Lemne C, Brismar K. Insulin-like growth factor binding protein-1 as a marker of the metabolic syndrome--a study in borderline hypertension. Blood Press. 1998 May;7(2):89–95. [PubMed] [Google Scholar]
- 11.Gomez JM. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 2006 Apr;7(2):125–132. doi: 10.2174/138920106776597621. [DOI] [PubMed] [Google Scholar]
- 12.Matilainen M, Malinen M, Saavalainen K, Carlberg C. Regulation of multiple insulin-like growth factor binding protein genes by 1alpha,25-dihydroxyvitamin D3. Nucleic Acids Res. 2005;33(17):5521–5532. doi: 10.1093/nar/gki872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schernhammer ES, Holly JM, Pollak MN, Hankinson SE. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005 Mar;14(3):699–704. doi: 10.1158/1055-9965.EPI-04-0561. [DOI] [PubMed] [Google Scholar]
- 14.Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005 Apr;14(4):850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Pollak M, Platz EA, et al. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and the risk of colorectal adenoma and cancer in the Nurses' Health Study. Growth Horm IGF Res. 2000 Apr;10 Suppl A:S30–S31. doi: 10.1016/s1096-6374(00)90014-5. [DOI] [PubMed] [Google Scholar]
- 16.Tworoger SS, Lee IM, Buring JE, Pollak MN, Hankinson SE. Insulin-like growth factors and ovarian cancer risk: a nested case-control study in three cohorts. Cancer Epidemiol Biomarkers Prev. 2007 Aug;16(8):1691–1695. doi: 10.1158/1055-9965.EPI-07-0319. [DOI] [PubMed] [Google Scholar]
- 17.Bertone-Johnson ER, Chen WY, Holick MF, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005 Aug;14(8):1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 18.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004 Apr;15(3):255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 19.Platz EA, Hankinson SE, Hollis BW, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and adenomatous polyps of the distal colorectum. Cancer Epidemiol Biomarkers Prev. 2000 Oct;9(10):1059–1065. [PubMed] [Google Scholar]
- 20.Grundy SM. Inflammation, hypertension, and the metabolic syndrome. JAMA. 2003 Dec 10;290(22):3000–3002. doi: 10.1001/jama.290.22.3000. [DOI] [PubMed] [Google Scholar]
- 21.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986 Jul;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 22.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986 May;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 23.Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN. Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006 May;15(5):972–978. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
- 24.Vecchione C, Colella S, Fratta L, et al. Impaired insulin-like growth factor I vasorelaxant effects in hypertension. Hypertension. 2001 Jun;37(6):1480–1485. doi: 10.1161/01.hyp.37.6.1480. [DOI] [PubMed] [Google Scholar]
- 25.Galderisi M, Vitale G, Lupoli G, et al. Inverse association between free insulin-like growth factor-1 and isovolumic relaxation in arterial systemic hypertension. Hypertension. 2001 Oct;38(4):840–845. doi: 10.1161/hy1001.091776. [DOI] [PubMed] [Google Scholar]
- 26.Jones JI, Prevette T, Gockerman A, Clemmons DR. Ligand occupancy of the alpha-V-beta3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2482–2487. doi: 10.1073/pnas.93.6.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total IGF-I, free IGF-I, and IGFB-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol. 1998 Feb;18(2):277–282. doi: 10.1161/01.atv.18.2.277. [DOI] [PubMed] [Google Scholar]
- 28.Laviades C, Mayor G, Diez J. Elevated circulating levels of insulin-like growth factor I in essential hypertensive patients with left ventricular hypertrophy. Arch Mal Coeur Vaiss. 1991 Aug;84(8):1039–1041. [PubMed] [Google Scholar]
- 29.Diez J, Laviades C. Insulin-like growth factor-1 and cardiac mass in essential hypertension: comparative effects of captopril, lisinopril and quinapril. J Hypertens Suppl. 1994 Jul;12(4):S31–S36. [PubMed] [Google Scholar]
- 30.Diez J, Ruilope LM, Rodicio JL. Insulin response to oral glucose in essential hypertensives with increased circulating levels of insulin growth factor I. J Hypertens Suppl. 1991 Dec;9(6):S174–S175. [PubMed] [Google Scholar]
- 31.Wahlander H, Isgaard J, Jennische E, Friberg P. Left ventricular insulin-like growth factor I increases in early renal hypertension. Hypertension. 1992 Jan;19(1):25–32. doi: 10.1161/01.hyp.19.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Andronico G, Mangano MT, Nardi E, Mule G, Piazza G, Cerasola G. Insulin-like growth factor 1 and sodium-lithium countertransport in essential hypertension and in hypertensive left ventricular hypertrophy. J Hypertens. 1993 Oct;11(10):1097–1101. doi: 10.1097/00004872-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Colangelo LA, Liu K, Gapstur SM. Insulin-like growth factor-1, insulin-like growth factor binding protein-3, and cardiovascular disease risk factors in young black men and white men: the CARDIA Male Hormone Study. Am J Epidemiol. 2004 Oct 15;160(8):750–757. doi: 10.1093/aje/kwh289. [DOI] [PubMed] [Google Scholar]
- 34.Ren J, Jefferson L, Sowers JR, Brown RA. Influence of age on contractile response to insulin-like growth factor 1 in ventricular myocytes from spontaneously hypertensive rats. Hypertension. 1999 Dec;34(6):1215–1222. doi: 10.1161/01.hyp.34.6.1215. [DOI] [PubMed] [Google Scholar]
- 35.Colao A, Di Somma C, Cascella T, et al. Relationships between serum IGF1 levels, blood pressure, and glucose tolerance: an observational, exploratory study in 404 subjects. Eur J Endocrinol. 2008 Oct;159(4):389–397. doi: 10.1530/EJE-08-0201. [DOI] [PubMed] [Google Scholar]
- 36.Efstratiadis G, Tsiaousis G, Athyros VG, et al. Total serum insulin-like growth factor-1 and C-reactive protein in metabolic syndrome with or without diabetes. Angiology. 2006 May–Jun;57(3):303–311. doi: 10.1177/000331970605700306. [DOI] [PubMed] [Google Scholar]
- 37.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002 Jul;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989 Jun;13(6 Pt 2):954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 39.Jorgensen JO, Christensen JJ, Vestergaard E, Fisker S, Ovesen P, Christiansen JS. Sex steroids and the growth hormone/insulin-like growth factor-I axis in adults. Horm Res. 2005;64 Suppl 2:37–40. doi: 10.1159/000087752. [DOI] [PubMed] [Google Scholar]
- 40.Prime DD, Brosnihan KB, Herrington DM. Effects of hormone therapy on blood pressure and the renin-angiotensin system in postmenopausal women. Minerva Cardioangiol. 2007 Aug;55(4):477–485. [PubMed] [Google Scholar]
- 41.Chan W, Valerie KC, Chan JC. Expression of insulin-like growth factor-1 in uremic rats: growth hormone resistance and nutritional intake. Kidney Int. 1993 Apr;43(4):790–795. doi: 10.1038/ki.1993.112. [DOI] [PubMed] [Google Scholar]
- 42.Knight EL, Rimm EB, Pai JK, et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol. 2004 Jul;15(7):1897–1903. doi: 10.1097/01.asn.0000128966.55133.69. [DOI] [PubMed] [Google Scholar]
- 43.Knight EL, Stampfer MJ, Rimm EB, Hankinson SE, Curhan GC. Moderate alcohol intake and renal function decline in women: a prospective study. Nephrol Dial Transplant. 2003 Aug;18(8):1549–1554. doi: 10.1093/ndt/gfg228. [DOI] [PubMed] [Google Scholar]