Abstract
Bioreducible polymers, which possess mainly disulfide linkages in the polymer structures, have appeared as ideal gene delivery carriers due to the high stability in extracellular physiological condition and bioreduction-triggered release of genetic materials, as well as decreased cytotoxicity because intracellular cytosol is a reducing environment containing high level of reducing molecules such as glutathione. This review will describe the initiation and recent advances in the development of bioreducible polymers for gene delivery, which includes reducibly cross-linked PEIs, polypeptides, polyion complex micelles, and poly(amido amine)s. There have been extensive researches performed to exhibit great gene delivery efficacy but still several important issues about pharmacokinetics or safety should be answered thoroughly for further rational design of bioreducible polymers having potentials in human gene delivery systems.
Keywords: Bioreducible polymers, gene delivery, reducing environment, PEI, polypeptide, polyion complex micelle, poly(amido amine)
1. Introduction
Polymeric gene delivery carriers have attracted intense attention of researchers in gene delivery field because they possess many advantages over viral vectors, such as non-immunogenicity, no integration of exogeneous genes into host chromosomes, and convenience of manufacturing and handling [1,2]. Moreover, multiple functionalities of polymers can be tailored for specific bioactivity such as targeting ability or environment-sensitive degradation through relatively simple chemical reactions [3].
There are several biological stimuli inducing environment-sensitive change of polymer structures, for example pH, temperature, or redox potential. Bioreducible polymers, which contain characteristic disulfide linkages, can be degraded specifically in response to redox potential through thiol-disulfide exchange reaction.
Generally, most intracellular compartments are reducing while extracellular space is oxidizing [4]. Various redox-couples participate in maintaining the reducing environment inside cells for stabilization of correct protein structures, redox cycles, etc [5,6]. Glutathione (GSH, γ-glutamyl-cysteinyl-glycine) is the most abundant intracellular reducing molecule and so GSH/glutathione disulfide (GSSG) regulated by glutathione reductase, is the major cellular redox couple [7]. The high level of intracellular GSH concentration (0.5–10 mM) and GSH/GSSG ratio (30:1–100:1) are maintained, contrary to extracellular milieu (GSH: 2–20 μM) [7,8]. Therefore, bioreducible polymeric gene delivery carriers can be degraded in cytosol by thiol-disulfide exchange reaction due to the high level of GSH, maintaining the sufficient stability in physiological condition, unlike their hydrolytically degradable counterpart polymeric gene delivery carriers containing ester, amino ester, or phosphoester linkages, which are rapidly degraded upon exposing to the moistures [9–11]. Due to the biodegradation inside cells, they can also acquire additional advantages such as decreased cytotoxicity by avoiding the accumulation of cationic polymers with high molecular weights and controlled intracellular release of genetic materials leading to the enhancement of transfection efficiency. Various bioreducible polymers have been developed for gene delivery systems because the above unique features make bioreducible polymers as ideal gene delivery carriers.
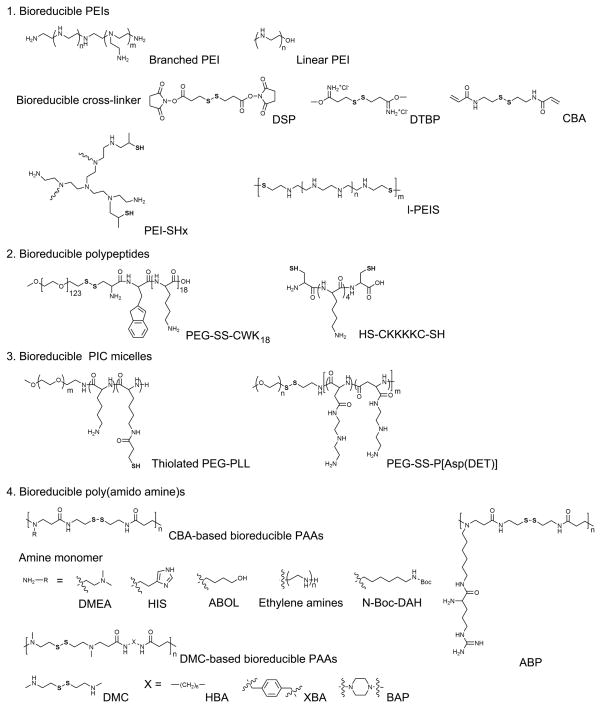
This review will focus the initiation and recent advances in the development and application of bioreducible polymers for gene delivery systems. Application of bioreducible polymers for cancer or ischemic heart disease gene therapy is notable because tumor tissues [12] or infarction sites by ischemia are highly hypoxic and reducing compared with normal tissues. Figure 1 summarizes various bioreducible polymers for gene delivery systems, cross-linkers, and monomers, which are discussed in this review.
Figure 1.
The molecular structures of various bioreducible polymers for gene delivery systems, their cross-linkers and monomers. bPEI + DSP or DTBP [14], bPEI + CBA [15], PEI-SHx [16], lPEI + DSP [19], l-PEIS [20], PEG-SS-CWK18 [28], HS-CKKKC-SH [30], thiolated PEG-PLL [38], PEG-SS-P[Asp(DET)] [42], DMEA, HIS, ABOL [45], Ethylene amines [50], N-Boc-DAH [55], ABP [59], DMC-based bioreducible PAAs [49]. PEI: polyethylenimine, DSP: dithiobis(succinimidylpropionate), DTBP: dimethyl·3,3′-dithiobispropionimidate·2HCl, CBA: cystamine bisacrylamide, l-PEIS: linear poly(ethylenimine sulfide), PAA: poly(amido amine), DMEA: N,N-dimethyl-1,3-ethylenediamine, HIS: histamine, ABOL: 4-amino-1-butanol, N-Boc-DAH: N-Boc-diaminohexane, ABP: arginine-grafted bioreducible polymer, DMC: N,N′-dimethylcystamine, HBA: N,N′-1,6-hexamethylene bisacrylamide, XBA: p-Xylylene bisacrylamide, BAP: bisacryloyl piperazine.
2. Bioreducible polyethylenemines (PEIs)
PEI is one of most efficient polymeric gene delivery carriers which can condense pDNA at low weight ratios (polymer/pDNA) due to its high charge density and induce high transgene expression by efficient endosomal escape of polyplexes through “proton sponge effect” hypothesis, which means internal amines of PEI can buffer the influx of protons into endosomes, causing mechanical swelling of PEI and increase of endosomal osmotic pressure, and finally endosomal disruption [13]. However, PEIs with high molecular weight (for example, PEI25k) has shown severe cytotoxicity aroused by accumulation of polycations with the high charge density and molecular weight. Therefore, a large variety of high molecular weight PEI syntheses by cross-linking low molecular weight PEI for enhancement of transfection efficiency and maintenance of low cytotoxicity have been studied.
Lee and coworkers showed low molecular weight PEI (800 Da) could mediate significantly improved gene delivery to Chinese hamster ovary (CHO) cells after reducible cross-linking with homobifunctional amine reactive cross-linker, dithiobis(succinimidylpropionate) (DSP) and dimethyl·3,3′-dithiobispropionimidate·2HCl (DTBP) [14]. In general, they found conjugate size to be proportional to transfection efficiency. Additionally, DTBP conjugates could exhibit higher gene expression than DSP conjugates of similar molecular weight at equal N/P ratios because DTBP cross-linker maintains net polymer charge through amidine bond formation, unlike DSP. PEI (800 Da) was also cross-linked via Michael addition of cystamine bisacrylamide (CBA) by Sun et al [15].
Another reducible cross-linking of PEI (800 Da) was performed via ring-opening reaction of methylthiirane (thiolation) followed by mild DMSO oxidation by Zhong and coworkers [16]. These cross-linked PEIs showed lower cytotoxicity and higher transfection efficiency compared with PEI25k, elucidating the importance of reductively degradable disulfide moiety in gene delivery carriers. In a similar way, branched PEIs (1.2k) were cross-linked via ring-opening of propylene sulfide and DMSO oxidation and further modified with cNGR-PEG5k-maleimide for tumor targeted gene delivery systems by Kim and coworkers [17]. Cyclic NGR (cNGRCK) shows much higher homing ability to CD13 overexpressed in neo-vasculature [18]. These cross-linked PEIs exhibited highly enhanced transfection efficiency compared to PEI (1.2k), maintaining low cytotoxicity. Cellular uptake and transfection efficiency of BPEI-SS-PEG-cNGR polyplex were reduced with free cNGR in CD13-positive cells, showing its tumor-target specificity. Additional treatment of buthionine sulfoximine (BSO) depleting intracellular GSH, reduced the transfection efficiency synergistically, implying its GSH-dependent transfection pathway.
Goepferich and coworkers reported the disulfide cross-linked low molecular weight linear PEI (MW < 4.6k) by dithiodipropionic acid or cystine linkages [19]. It was confirmed that cross-linked bioreducible PEIs showed superior transfection efficiency and low cytotoxicity compared to commercial transfection reagents such as PolyFect, SuperFect, Lipofectamine, FuGENE6, or JetPEI.
On the other hand, Lee et al. synthesized linear poly(ethylenimine sulfide) (l-PEIS) for efficient and safe gene delivery [20]. l-PEIS-6 and l-PEIS-8 displayed transfection efficiencies comparable to PEI, with marginal cytotoxicity due to the biodegradable disulfide bond. They confirmed the intracellular degradation of l-PEIS in HeLa cells via visualization using the probe-probe dequenching effect of BODIPY-FL fluorescence dye. In a following study, Koo et al. synthesized biodegradable b-PEIS (branched poly(ethylenimine sulfide)) by crosslinking l-PEIS using a crosslinker, bisepoxide [21]. The b-PEIS was readily degradable under reductive conditions (5mM GSH) with dependency on the degree of crosslinking. The b-PEIS showed highly enhanced transfection efficiency compared to l-PEIS and low cytotoxicity. Intracellular trafficking by image restoration microscopy demonstrated that b-PEIS does not accumulate in the cell interior after 48 h of transfection, suggesting whole excretion of b-PEIS due to biodegradation.
Low molecular weight PEI conjugate cholesterol was used to deliver IL-12 gene for cancer treatment and myocardium infarct respectively [22,23]. Kissel et al. reported stabilization of PEI/DNA polyplexes by cross-linking PEI with the amine reactive reducible cross-linker dithiobis(succinimidyl propionate) (DSP) [24]. They prepared PEI/DNA polyplexes by two different formulation methods, either using pre-cross-linked polymers or by cross-linking polyplexes after complexation. Only the latter method yielded nano-sized and positively charged polyplexes with HMW PEI, which showed enhanced resistance against polyanion exchange and high ionic strength and reduced interactions with major blood components like albumin and erythrocytes. Moreover, pharmacokinetic profiles of crosslinked PEI/DNA polyplexes in mice after intravenous administration showed elevated blood levels revealing improved stability in circulation, and accumulation of the polyplexes in the liver and the lungs [25]. Additional PEGylation has been performed to increase the stability of reversibly crosslinked PEI polyplexes [26]. The resulting AB-type and ABA-type copolymers (A: PEG20k or PEG30k, B: PEI25k) displayed reduced cytotoxicity and hemolytic activity, increased transfection efficiency and circulation times in blood stream. On the other hand, Ahn et al, synthesized biodegradable poly(ethylenimine) for plasmid DNA delivery using PEG succinimidyl succinate [27].
3. Bioreducible polypeptides
Similarly with synthesized polymers, several polypeptides for gene delivery systems have been developed, which have bioreducible linkages, mainly disulfide bonds formed between free thiol groups of cystein residues.
Rice and coworkers first reported that PEG-peptide (PEG-SS-Cys-Trp-Lys(18), PEG-SS-CWK18) having disulfide cross-linker displayed much enhanced gene transfer efficiency compared to PEG-peptide having non-reducible cross-linker (PEG-VS-CWK18) when complexed with plasmid DNA [28]. In a following study, low molecular weight polypeptides were developed by substitution of one to four lysine residues of CWK18 with cystein residues [29]. These polypeptides could condense plasmid DNA through ionic interactions and additionally form stable condensates after formation of cross-link structures caused by spontaneous oxidization of surficial thiols of cysteins. Theses peptide DNA condensates exhibited significant enhanced transfection efficiency in comparison with the uncross-linked condensates. They also found that a minimal peptide of four lysines and two terminal cysteins also could result in DNA condensates with similar particle size and transgene expression in HepG2 cells [30]. Furthermore, after lysines were substituted with histidines, optimal peptide of Cys-His-(Lys)(6)-His-Cys showed enough buffering capacity to enhance in vitro gene expression in the absence of chloroquine, endosomolytic agent.
Seymour and coworkers synthesized reductively degradable polycation (RPC) designed for intracellular degradation, by oxidation of terminal cysteinyl thiol groups of Cys(Lys)10Cys [31]. The complexes coated and surface-cross-linked using N-(2-hydroxypropyl)methacrylamide (PHPMA) exhibited lateral stabilization but destabilization by treatment of 2.5 mM DTT, releasing free DNA after incubation with polyanions. Moreover, enhanced transgene expression of the coated RPC complexes was observed, compared to the non-reducible PLL complexes in human retinoblast 911 cells. Several RPCs consisting of histidine and polylysine residues were also developed as gene delivery carriers by oxidation of terminal cysteinyl thiol groups [32]. A His-rich RPC vector ([Cys-His6-Lys3-His6-Cys]n) with at least 70 % histidine content could mediate efficient gene transfer without chloroquine, endosomolytic agent, which includes the efficient delivery of GFP mRNA in PC-3 cells and siRNA directed against the neurotrophin receptor p75(NTR) in rat dorsal root ganglion cell neurons. On the other hand, Oupicky and coworkers reported the synthesis of novel reducible copolypeptides (rCPP) by an oxidative copolymerization of a histidine-rich peptide (HRP, CKH3KH3KC) and a nuclear localization sequence (NLS, CGAGPK3RKVC) peptide with different relative contents [33]. rCPPs exhibited minimum toxic effects of rCPPs in human endothelial cells due to lower charge density and higher chain rigidity of the rCPPs, reductive intracellular and plasma membrane degradation. Transfection activity of rCPPs was increased with increasing content of the HRP sequence probably due to enhanced buffering capacity and no clear effect of the NLS sequence on transfection activity suggested the possibility that NLS peptides have been dissociated from the polyplexes in reducing cytosol prior to nuclear transportation of DNA.
Mok et al. reported a novel reducible peptide consisting of fusogenic peptide, KALA, with two terminal cysteine residues, self-crosslinked via disulfide linkages under mild DMSO oxidation condition [34]. The reducible crosslinked KALA (cl-KALA) exhibited more reduced cell cytotoxicity and cl-KALA/siRNA complexes displayed comparable gene silencing efficiency with PEI complexes in MDA-MB-435 cells. It is notable that cl-KALA/siRNA-PEG conjugate complexes showed superior gene inhibition in serum condition, probably because of the shielding effect of PEG on the surface.
Lo et al. also designed novel peptides incorporating 10 histidine residues (Tat-10H) and additional terminal cysteine residues in the Tat sequence, which is a cationic cell-penetrating peptide known to enhance the cellular uptake of various drugs and proteins [35]. Interestingly, 7000-fold improvement in gene transfection efficiency of Tat-10H over the original Tat peptide was observed and bis(cysteinyl) histidine-rich peptide was more effective than the Tat-10H peptide, because of enhanced stability of peptide/DNA complexes via interpeptide disulfide bonds formation by air oxidation upon binding to DNA.
More recently, another study of reducible polypeptides based on nona-arginine (D-R9), cell penetrating peptides or protein transduction domains (PTDs), has been reported by Kim and coworkers [36]. Reducible poly(oligo-D-arginine) (rPOA) was synthesized by disulfide bond formation between terminal cysteinyl-thiol groups of the Cys-(D-R9)-Cys repeating unit. Besides favorable stability and efficient in vitro DNA delivery, DNA/poly(oligo-D-arginine) polyplex exhibited higher level of gene expression than PEI, sustaining for 1 week without toxicity after intratracheal injection into mouse lung. The lungs treated with the polyplexes didn’t show any significant morphological and inflammatory changes in hematoxylin and eosin-stained histological analysis.
4. Bioreducible polyion complex (PIC) micelles
PIC micelles are self-assembling particles with a core-shell structure formed by complexation between a pair of oppositely-charged polymers having hydrophilic PEG segments [37]. They can be utilized as gene delivery vectors with high water-solubility and colloidal stability, employing negatively charged genetic materials and cationic polymers.
Kataoka and coworkers reported glutathione(GSH)-sensitive stabilization of PIC micelles with the core cross-linked through disulfide bonds, composed of antisense oligonucleotide (asODN) and thiolated PEG-PLL block copolymer [38]. The micelles showed sufficient colloidal stability due to the PEG shell and core cross-linking. Moreover, ODN entrapped in the micelles also displayed highly increased stability against nuclease, compared to that in the micelles without cross-linking. Release of ODN from the dissociated micelles at intracellular GSH concentration suggested the potential for intracellular ODN delivery. They also presented another concept of reducible PIC micelles based on PEG-asODN conjugate with disulfide linkage (PEG-SS-asODN) through the complexation with branched poly(ethylenimine) (B-PEI) [39]. These PIC micelles showed higher antisense activity than PIC micelles formulated from PEG-SS-asODN and PLL, which indicates that they are successfully escaped from the endosomal compartments by the buffering effect of the B-PEI, releasing asODN via cleavage of the disulfide linkage into the cytoplasm, responding to intracellular glutathione.
Similarly with the above study, VEGF siRNA conjugated to PEG via a disulfide linkage was complexed with PEI to polyelectrolyte complex (PEC) micelles for anti-angiogenic gene therapy [40]. An intact siRNA released from the siRNA-PEG conjugate by cleavage of the disulfide linkage was observed in reductive condition. The VEGF siRNA-PEG/PEI PEC micelles exhibited effective VEGF gene silencing effect in prostate carcinoma cells (PC-3), even superior than VEGF siRNA/PEI complexes. Park and coworkers also reported the potential of complexes between naturally occurring biomaterials, protamine and asODN-hyaluronic acid (HA) conjugates via a disulfide linkage as safe and effective nonviral carriers for nucleic acid therapeutics [41].
Recently, Takase et al. developed a block catiomer, PEG-SS-P[Asp(DET)] as a nonviral gene vector, featuring a disulfide linkage between PEG and polycation segment to trigger PEG detachment, and aspartamide with a flanking N-(2-aminoethyl)-2-aminoethyl group (Asp(DET)) acting as a buffering moiety inducing endosomal escape [42]. PEG-SS-P[Asp(DET)] and plasmid DNA (pDNA) could form stably dispersed polyplex micelles which are able to detach PEG in reductive condition. Interestingly, higher transfection efficiency of PEG-SS-P[Asp(DET)] micelles than PEG-P[Asp(DET)] micelles without disulfide linkages are much likely due to more effective endosomal escape based on the PEG detachment in endosome.
5. Bioreducible poly(amido amine)s
Poly(amido amine)s (PAAs) can be synthesize via Michael reaction of commercial amine monomers and acrylamide monomers in mild condition [43,44]. Therefore, it is convenient to construct high-throughput libraries of polymeric gene carriers for maximum efficacy by simple combination of monomers, like poly(β-amino ester) polymers [9] and there have been extensive studies about bioreducible PAAs for gene delivery systems.
Engbersen and coworkers reported a series of novel bioreducible poly(amido amine)s containing multiple disulfide linkages (SS-PAAs) as nonviral gene vectors [45]. These linear SS-PAAs were synthesized by Michael-type polyaddition of various primary amines (4-amino-1-butanol (ABOL), 5-amino-1-pentanol (APOL), N,N-dimethyl-1,3-ethylenediamine (DMEA), 2-(2-aminoethoxy) ethanol (AEEOL), 3-methoxypropylamine (MOPA), 3-morpholinopropylamine (MPA), or histamine (HIS)) with disulfide bond-containing cystamine bisacrylamide (CBA). The polymers display relative stability in physiological conditions but are rapidly degraded in reductive environment (2.5 mM DTT). SS-PAAs possess higher buffer capacities than PEI in endosomal pH range, which may contribute to the endosomal escape of the polyplexes. Some SS-PAAs also showed higher transfection efficiency in COS-7 cells than PEI with low cytotoxicity. Among them, SS-PAAs containing amino alcohol pendant groups (pAPOL, pABOL) exhibited the highest tansgene expression. Recently, pABOL polymer was also reported to show good transfection efficiency in dual bio-responsive gene delivery system using survivin-inducible plasmid DNA expressing the soluble VEGF receptor, sFlt-1 (pSURsFlt-1) by Namgung et al [46]. They observed cancer-specific expression of sFlt-1 in the mouse renal carcinoma (RENCA) cell line, which is attributed to biodegradation of pABOL polyplexes and survivin (upregulated in most cancer cells)-induced survivin promoter activation.
In a following study, Engbersen and coworkers reported the syntheses of SS-PAA random and block copolymers consisting of bioreducible CBA and amino groups with distinctly different basicity, histamine (HIS) and 3-(dimethylamino)-1-propylamine (DMPA) [47]. These copolymers showed transfection efficiencies in COS-7 cells, which increase with increasing HIS/DMPA ratio and buffer capacities. The random and block copolymers at a HIS/DMPA ratio of 70/30 were regarded to combine optimal DNA condensation ability and buffer capacity, thereby similarly displaying higher transfection efficiency than homopolymers and low cytotoxicity.
They also synthesized another series of PAAs using non-bioreducible N,N′-methylenebisacrylamide (MBA), 1,4-bis(acryloyl)piperazine (BAP), N, N′-hexamethylenebisacrylamide (HMBA), and bioreducible N, N′-cystaminebisacrylamide (CBA) as bisacrylamide monomers and trifunctional 1-(2-aminoethyl) piperazine (AEP) as a fixed amine monomer for gene delivery systems [48]. It is notable that bioreducible p(CBA-AEP) polyplexes showed significant higher transfection efficiency than those from PAAs lacking the disulfide linkage in COS-7 cells, revealing that PAA polyplexes with appropriate disulfide content have highly improved biophysical properties, yielding enhanced transgene expression with low cytotoxicity. In another study, they explored a novel route to introduce disulfide moieties in linear PAAs by synthesizing the polymers via Michael addition of reducible amine monomer, N,N′-dimethylcystamine (DMC) with various bisacrylamides [49]. DMC-based SS-PAAs showed very high buffer capacities due to the position of the disulfide bond close to tertiary nitrogen atoms in the DMC, lowering the pKa value of these basic sites. These polymers also could condense DNA into nano-scaled and positively charged polyplexes which can be degraded in reductive condition, similarly with the previously reported SS-PAAs. Interestingly, it was elucidated that the transfection efficiencies of DMC-based SS-PAAs appear to correlate with the buffer capacities in COS-7 cells.
On the other hand, Lane et al. synthesized bioreducible poly(amido ethylenimine) (SS-PAEI) polymers by using Michael addition reactions between cystamine bisacrylamide (CBA) and three different ethylene amine monomers (ethylenediamine (EDA), diethylenetriamine (DETA), or triethylenetetramine (TETA)) [50]. Contrary to previous linear SS-PAAs, SS-PAEIs were found to have branched structures due to their amine monomers containing multiple internal secondary amines still reactive with CBA, besides terminal primary amines. It is notable that poly(DETA/CBA) with the highest degree of branching showed the lowest buffering capacity. All SS-PAEIs formed polyplexes (< 200 nm, ~ 32 mV of Zeta-potentials) with pDNA, which can be degraded under reductive condition (2.5 mM DTT). Nearly 20 folds higher transfection efficiency of SS-PAEIs was observed in mammalian cell lines than PEI25k, which was maintained in the presence of 10 % serum, not like PEI25k. In accordance with the authors, it may be explained by the difference in charge density of the overall polyplexes where more densely charged polyplexes are likely to interact with anionic serum proteins, leading to aggregation and decreasing transfection efficiency. Confocal microscopy experiments using ethidium monoazide bromide (EMA)-labeled pDNA indicated that SS-PAEI polyplexes displayed more dispersed fluorescence inside cells meaning greater intracellular distribution of pDNA due to environmentally triggered release, than PEI25k which showed globular fluorescence.
Among SS-PAEIs, poly(amido ethylenediamine) (SS-PAED) was employed to study the delivery of hypoxia-inducible vascular endothelial growth factor (RTP-VEGF) plasmid [51]. The RTP promoter of the plasmid was demonstrated to regulate transcriptional activation under conditions of cellular hypoxia [52]. Declined transfection efficiency of SS-PAED with treatment of a GSH inhibitor, D,L-buthionine sulfoxamine (BSO) emphasized the importance of intracellular GSH concentration for efficient gene delivery by bioreducible polymers. SS-PAED/RTP-VEGF plasmid polyplex produced significantly higher levels of VEGF expression under hypoxic conditions compared to normoxic conditions in both primary rat cardiomyoblasts (H9C2) and rat aortic smooth muscle cells (A7R5). Moreover, in vivo VEGF gene delivery using SS-PAED examined in a rabbit myocardial infarct model, showed up to 4 fold increase of VEGF expression in the infarct region compared to SS-PAED/RTP-Luc control, suggesting the potential for the enhancement of cardiomyocyte viability and neovascular proliferation in ischemic myocardium.
Application of poly(TETA/CBA) as a delivery carrier for small interference RNA (siRNA) was reported [53]. Poly(TETA/CBA) could condense siRNA to from stable complexes under physiological condition but release siRNA completely under reductive condition (2.5 mM DTT). Poly(TETA/CBA) exhibited much higher RNAi activity against VEGF expression than linear-polyethylenimine (L-PEI, 25 kDa), when complexed with VEGF-directed siRNA n human prostate cancer cells (PC-3). Moreover, substantial dissociation and intracellular distribution of siRNA was observed with the treatment of poly(TETA/CBA)/siRNA complexes, not L-PEI/siRNA complexes by confocal microscopy.
More recently, this polymer was employed as a glucagon-like peptide 1 (GLP-1) gene delivery carrier [54]. Bioreducible poly(TETA/CBA) could perform triggered release of GLP-1 plasmid DNA having repeated binding sequence for a karyophilic protein, NFκB in cytoplasm and facilitated interaction between cytosolic NFκB and NFκB biding motif of the plasmid DNA, increasing GLP-1 expression due to the nuclear translocation activity. It should be noted that much increased GLP-1 expression and the nuclear localization of the plasmid DNA were observed after activation of intracellular NFκB by interleukin-1β (IL-1β).
In another study, Mei et al. reported a set of linear reducible poly(disulfide amines) containing pendant primary amines with different alkyl chains [55]. The polymers synthesized by Michael reaction of CBA and N-Boc protected diamines showed superior buffering capacity to branched PEI 25kDa, which may facilitate the endosomal escape of DNA. Dissociation of stable poly(disulfide amine) polyplexes was observed in the presence of 5.0 mM DTT. Transfections mediated by these poly(disulfide amine)s were diamine-chain length-dependent, in which poly(disulfide amine) with a hexaethylene spacer, poly(CBA-DAH), exhibited the highest transfection efficiency, maintaining significantly less cytotoxicity than bPEI. Contrary to this result, it is notable that increase of the alkyl spacer from ethylene to propylene between the amino units in side chains results in significantly lower transfection and increased cytotoxicity in Lin et al.’s work [56].
In a subsequent study, poly(CBA-DAH) was employed as a prostaglandin E2 (PGE2)-Fas siRNA conjugates carrier for cardiomyocyte-targeted anti-apoptosis in cardiovascular disease gene therapy [57]. The increased cellular uptake of the PGE2-siRNA/poly(CBA-DAH) polyplex was shown in rat cardiomyocytes (H9C2 cells) due to PGE2 targeting. The polyplexes also could lead to a significant increase in Fas (a key regulator of ischemia-induced apoptosis) gene silencing, displaying inhibition of cardiomyocyte apoptosis and no immune response (interferon-alpha induction). More recently, Hye Y. et al. reported the another concept of primary cardiomyocytes targeted Fas siRNA delivery using primary cardiomyocyte (PCM) specific peptide (WLSEAGPVVTVRALRGTGSW)-modified poly(CBA-DAH) [58]. The greater cellular uptake and transfection efficiency of PCM conjugated polyplexes were found in H9C2 rat cardiomyocytes than in non-cardiomyocyte NIH 3T3 cells. Fas siRNA/PCM-polymer complexes exhibited significant Fas gene silencing in rat cardiomyocytes under hypoxic conditions, resulting in inhibition of cardiomyocyte apoptosis.
According to its high transfection efficiency and low cytotoxicity, poly(CBA-DAH) was also used as a backbone polymer for arginine-grafted bioreducible polymer (ABP) [59] or guanidinylated bioreducible polymer (GBP) [60] for gene delivery systems. Arginine or guanidine is a main component of cell penetrating peptides or protein transduction domains (PTDs) playing an important role for intracellular transportation of cargo molecules [61,62]. ABP and GBP also could form nano-sized polyplexes with DNA, which can be degraded in reductive condition. The polymers exhibited greatly improved transfection efficiency compared to poly(CBA-DAH) or even bPEI25k probably due to cell penetrating functionality, maintaining minimal cytotoxicity. However, cellular uptake of ABP or GBP polyplexes didn’t show significant differences between those of poly(CBA-DAH) or bPEI25k polyplexes, irrespective of high transfection. It is notable that the discordance between above results may be explained by strong nuclear localization ability for DNA delivery of guanidine functionality, which can be supported by confocal microscopy observation of intracellular trafficking of GBP polyplexes. Recently, following studies utilizing the opposite concepts of gene therapy based on ABP delivery systems have been performed in relative hypoxic conditions. First, efficient VEGF siRNA delivery by ABP and the importance of intracellular GSH level for RNAi activity of ABP were reported in anti-angiogenesis gene therapy of tumors [63]. Second, the potential of ABP as ex vivo delivery carrier of VEGF165 plasmid DNA into primary myoblasts for improvement of infarcted myocardium function via angiogenesis and myogenesis was evaluated for therapeutic application in the treatment of ischemic heart diseases [64].
6. Conclusions
Bioreducible polymers have been extensively studied during past decades due to their attractive structural advantages. They possess unique features of high stability in extracellular physiological condition and immediate cleavage of disulfide linkages in reductive intracellular environment, reducing cytotoxicity due to the avoidance of accumulation of the high molecular weight polycations and triggering controlled delivery of genetic materials from the polyplexes. All of these make bioreducible polymers as ideal gene delivery carriers.
However, development of bioreducible polymers for gene delivery systems is at the initial step although several polymers showed great gene transfer efficacy. Especially, the pharmacokinetics and bio-safety of bioreducible polymers should be evaluated thoroughly. Additionally, detailed understanding exact mechanism and molecular biological effects of reductive cleavage of disulfide linkages (for example, place or reaction in cytosol) is also strongly required for further rational design of ideal bioreducible polymers.
Acknowledgments
This work was supported by NIH grants (HL065477-10 and CA107070-06) and Research fund (WCU 200900000000024) of the Ministry of Education, Science and Technology, Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luo D, Saltzman WM. Nat Biotechnol. 2000;18:33. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Huang L. J Control Release. 2002;78:259. doi: 10.1016/s0168-3659(01)00494-1. [DOI] [PubMed] [Google Scholar]
- 3.Jeong JH, Kim SW, Park TG. Prog Polym Sci. 2007;32:1239. [Google Scholar]
- 4.Manickam DS, Oupicky D. J Drug Target. 2006;14:519. doi: 10.1080/10611860600834409. [DOI] [PubMed] [Google Scholar]
- 5.Elias SJ, Arner AH. Eur J Biochem. 2000;267:6102. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 6.Go YM, Jones DP. Biochim Biophys Acta. 2008;1780:1273. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. J Nutr. 2004;134:489. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 8.Schafer FQ, Buettner GR. Free Radic Biol Med. 2001;30:1191. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 9.Lynn DM, Langer R. J Am Chem Soc. 2000;122:10761. [Google Scholar]
- 10.Wang J, Mao H, Leong KW. J Am Chem Soc. 2001;123:9480. doi: 10.1021/ja016062m. [DOI] [PubMed] [Google Scholar]
- 11.Lim Y-b, Kim C-h, Kim K, Kim SW, Park J-s. J Am Chem Soc. 2002;122:6524. [Google Scholar]
- 12.Kuppusamy P, Afeworki M, Shankar RA, Coffin D, Krishna MC, Hahn SM, Mitchell JB, Zweier JL. Cancer Res. 1998;58:1562. [PubMed] [Google Scholar]
- 13.Boussif O, Lezoualc‘h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr J. Proc Natl Acad Sci USA. 1995;92:7297. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosselin MA, Guo W, Lee RJ. Bioconjugate Chem. 2001;12:989. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 15.Sun YX, Zeng X, Meng QF, Zhang XZ, Cheng SX, Zhuo RX. Biomaterials. 2008;29:4356. doi: 10.1016/j.biomaterials.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 16.Peng Q, Zhong Z, Zhuo R. Bioconjugate Chem. 2008;19:499. doi: 10.1021/bc7003236. [DOI] [PubMed] [Google Scholar]
- 17.Son S, Singha K, Kim WJ. Biomaterials. 2010;31:6344. doi: 10.1016/j.biomaterials.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Buehler A, Zandvoort MAMJ, Stelt BJ, Hackeng TM, Schrans-Stassen BHGJ, Bennaghmouch A, Hofstra L, Cleutjens JPM, Duijvestijn A, Smeets MB, de Kleijn DPV, Post MJ, de Muinck ED. Arterioscler Thromb Vasc Biol. 2006;26:2681. doi: 10.1161/01.ATV.0000245807.65714.0b. [DOI] [PubMed] [Google Scholar]
- 19.Breunig M, Lungwitz U, Liebl R, Goepferich A. Proc Natl Acad Sci USA. 2007;104:14454. doi: 10.1073/pnas.0703882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Mo H, Koo H, Park JY, Cho MY, Jin GW, Park JS. Bioconjugate Chem. 2007;18:13. doi: 10.1021/bc060113t. [DOI] [PubMed] [Google Scholar]
- 21.Koo H, Jin GW, Kang H, Lee Y, Nam K, Bai CZ, Park JS. Biomaterials. 2010;31:988. doi: 10.1016/j.biomaterials.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Yockman JW, Maheshwai A, Han SO, Kim SW. J Control Release. 2003;87:177. doi: 10.1016/s0168-3659(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Rentz J, Han SO, Bull DA, Kim SW. Gene Ther. 2003;10:585. doi: 10.1038/sj.gt.3301938. [DOI] [PubMed] [Google Scholar]
- 24.Neu M, Sitterberg J, Bakowsky U, Kissel T. Biomacromolecules. 2006;7:3428. doi: 10.1021/bm060788z. [DOI] [PubMed] [Google Scholar]
- 25.Neu M, Germershaus O, Mao S, Voigt KH, Behe M, Kissel T. J Control Release. 2007;118:370. doi: 10.1016/j.jconrel.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Neu M, Germershaus O, Behe M, Kissel T. J Control Release. 2007;124:69. doi: 10.1016/j.jconrel.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Ahn CH, Chae SY, Bae YH, Kim SW. J Control Release. 2002;80:273. doi: 10.1016/s0168-3659(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 28.Kwok KY, McKenzie DL, Evers DL, Rice KG. J Pharm Sci. 1999;88:996. doi: 10.1021/js990072s. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie DL, Kwok KY, Rice KG. J Biol Chem. 2000;275:9970. doi: 10.1074/jbc.275.14.9970. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie DL, Smiley E, Kwok KY, Rice KG. Bioconjugate Chem. 2000;11:901. doi: 10.1021/bc000056i. [DOI] [PubMed] [Google Scholar]
- 31.Oupický D, Parker AL, Seymour LW. J Am Chem Soc. 2002;124:8. doi: 10.1021/ja016440n. [DOI] [PubMed] [Google Scholar]
- 32.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manickam DS, Oupický D. Bioconjugate Chem. 2006;17:1395. doi: 10.1021/bc060104k. [DOI] [PubMed] [Google Scholar]
- 34.Mok H, Park TG. Biopolymers. 2008;89:881. doi: 10.1002/bip.21032. [DOI] [PubMed] [Google Scholar]
- 35.Lo SL, Wang S. Biomaterials. 2008;29:2408. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Won YW, Kim HA, Lee M, Kim YH. Mol Ther. 2010;18:734. doi: 10.1038/mt.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada A, Kataoka K. Macromolecules. 1995;28:5294. [Google Scholar]
- 38.Kakizawa Y, Harada A, Kataoka K. Biomacromolecules. 2001;2:491. doi: 10.1021/bm000142l. [DOI] [PubMed] [Google Scholar]
- 39.Oishi M, Hayama T, Akiyama Y, Takae S, Harada A, Yamasaki Y, Nagatsugi F, Sasaki S, Nagasaki Y, Kataoka K. Biomacromolecules. 2005;6:2449. doi: 10.1021/bm050370l. [DOI] [PubMed] [Google Scholar]
- 40.Kim SH, Jeong JH, Lee SH, Kim SW, Park TG. J Control Release. 2006;116:123. doi: 10.1016/j.jconrel.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Mok H, Park JW, Park TG. Bioconjugate Chem. 2007;18:1483. doi: 10.1021/bc070111o. [DOI] [PubMed] [Google Scholar]
- 42.Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, Yamasaki Y, Koyama H, Kataoka K. J Am Chem Soc. 2008;130:6001. doi: 10.1021/ja800336v. [DOI] [PubMed] [Google Scholar]
- 43.Danusso F, Ferruti P. Polymer. 1970;11:88. [Google Scholar]
- 44.Ferruti P, Marchisio MA, Barbucci R. Polymer. 1985;26:1336. [Google Scholar]
- 45.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JFJ. Bioconjugate Chem. 2007;18:138. doi: 10.1021/bc060200l. [DOI] [PubMed] [Google Scholar]
- 46.Namgung R, Brumbach JH, Jeong JH, Yockman JW, Kim SW, Lin C, Zhong Z, Feijen J, Engbersen JFJ, Kim WJ. Biotechnol Lett. 2010;32:755. doi: 10.1007/s10529-010-0219-7. [DOI] [PubMed] [Google Scholar]
- 47.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JFJ. J Control Release. 2007;123:67. doi: 10.1016/j.jconrel.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JFJ. J Control Release. 2006;116:130. doi: 10.1016/j.jconrel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Piest M, Lin C, Mateos-Timoneda MA, Lok MC, Hennink WE, Feijen J, Engbersen JFJ. J Control Release. 2008;130:38. doi: 10.1016/j.jconrel.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 50.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong ZY, Lin C, Engbersen JFJ, Feijen J. Bioconjugate Chem. 2006;17:1233. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 51.Christensen LV, Chang CW, Yockman JW, Conners R, Jackson H, Zhong Z, Feijen J, Bull DA, Kim SW. J Control Release. 2007;118:254. doi: 10.1016/j.jconrel.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, Kalinski H, Kamer I, Rozen A, Mor O, Keshet E, Leshkowitz D, Einat P, Skaliter R, Feinstein E. Mol Cell Biol. 2002;22:2283. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong JH, Christensen LV, Yockman JW, Zhong Z, Engbersen JFJ, Kim WJ, Feijen J, Kim SW. Biomaterials. 2007;28:1912. doi: 10.1016/j.biomaterials.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 54.Jeong JH, Kim SH, Christensen LV, Feijen J, Kim SW. Bioconjugate Chem. 2010;21:296. doi: 10.1021/bc9003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Bioconjugate Chem. 2008;19:626. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin C, Blaauboer CJ, Timoneda MM, Lok MC, van Steenbergen M, Hennink WE, Zhong Z, Feijen J, Engbersen JFJ. J Control Release. 2008;126:166. doi: 10.1016/j.jconrel.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Kim SH, Jeong JH, Ou M, Yockman JW, Kim SW, Bull DA. Biomaterials. 2008;29:4439. doi: 10.1016/j.biomaterials.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nam HY, McGinn A, Kim PH, Kim SW, Bull DA. Biomaterials. 2010;31:8081. doi: 10.1016/j.biomaterials.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim T-i, Ou M, Lee M, Kim SW. Biomaterials. 2009;30:658. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim T-i, Lee M, Kim SW. Biomaterials. 2010;31:1798. doi: 10.1016/j.biomaterials.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao M, Weissleder R. Med Res Rev. 2004;24:1. doi: 10.1002/med.10056. [DOI] [PubMed] [Google Scholar]
- 62.Zorko M, Langel Ü. Adv Drug Deliv Rev. 2005;57:529. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Kim SH, Jeong JH, Kim T-i, Kim SW, Bull DA. Mol Pharm. 2009;6:718. doi: 10.1021/mp800161e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ou M, Kim T-i, Yockman JW, Borden BA, Bull DA, Kim SW. J Control Release. 2010;142:61. doi: 10.1016/j.jconrel.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]