Abstract
Optimization of thienopyrimidinone derivatives as FGFR1 kinase inhibitors is being pursued. The present results confirm predictions of computational modeling that an aryl subtituent can be introduced at the 2-position in strucure 3. The substituent is anticipated to project deeper into the binding site and provide opportunities for enhanced activity and selectivity. The most potent analog reported here, 13, has a 4-hydroxyphenyl substituent and yields an IC50 of 6 μM for inhibition of phosphorylation by FGFR1 kinase. It was also found that the western anisole-containing substituent in 3 can be replaced by a propionic acid group with no loss in potency and with potentially significant gains in pharmacologically relevant properties.
Keywords: FGFR1 Kinase Inhibitors, Structure-Based Inhibitor Design, Thienopyrimidinones, ATP Competitive Small Molecule Inhibitors
Fibroblast growth factors (FGF) play an important role in many biological processes including cell proliferation, migration, differentiation, survival, morphogenesis, and angiogenesis.1 The diverse biological effects of FGFs are mediated by cell surface receptors (FGFR1-4) with intrinsic protein tyrosine kinase activity.2 FGFR is activated by binding of FGF to its extracellular domain, resulting in receptor dimerization and autophosphorylation of specific tyrosine residues in the cytoplasmic domain. The activated FGFR interacts with downstream intracellular proteins to initiate signal transduction, such as in the MEPK and PKB/Akt pathways.3 Abnormal FGF signaling either through gain or loss of function by mutations in FGF or FGFR has been implicated in cancer and other disorders, including skeletal, cardiovascular, immunological, and neurological diseases.1, 4 Therefore, targeted inhibition of FGFR kinases with ATP-competitive small molecule inhibitors has become an attractive therapeutic strategy. Several classes of such inhibitors of have been reported, including indolinones,5 pyrido[2,3-d]pyrimidines,6 napthyridines,7 triazines,8 indenes,9 quinolines,10 and thioindoles.11 However, only a limited number of FGFR inhibitors have entered clinical trials.12-17 Furthermore, since the human genome encodes at least 518 protein kinases and nearly all kinase inhibitors target the well-conserved ATP binding site,18 development of selective kinase inhibitors continues to be a significant challenge.
In the course of studies to identify new FGFR1 kinase inhibitors, we discovered two inhibitors 1 and 2, a benzylidene derivative of pseudothiohydantoin and a thienopyrimidinone derivative, by structure-based virtual screening followed by initial lead optimization.19 1 and 2 inhibit phosphorylation by FGFR1 kinase with IC50 values of 23 and 1.9 μM, respectively. The pseudothiohydantoin 1 was also found to be an inhibitor of EGFR, Src, and InsR kinases with IC50 values of 10-56 μM. The thienopyrimidinone 2 was found to inhibit EGFR and Src kinases with IC50 values of 2.4 and 1.9 μM, while it did not inhibited InsR. Thus, little selectivity was observed with these compounds. In an effort to develop more potent and selective FGFR1 kinase inhibitors, the thienopyrimidinones were chosen for further optimization.
A structure from docking calculations19 for the 21-μM inhibitor 3 complexed with FGFR1 kinase is illustrated in Figure 2A. Formation of two hydrogen bonds between the amide fragment in the pyrimidinone ring of 3 and Ala564 in the hinge region is anticipated. The tricyclic core of thienopyrimidinone 3 is spatially limited to the ATP binding site, and the western end of the inhibitor is solvent exposed. Exploration eastward, deeper into the active site, is expected to be potentially more fruitful, especially for selectivity owing to variation among kinases in this area. In the eastern end of 3 (see Figures 1 and 2A), position 1 is solvent exposed and substitutions here should have limited effect on modulating activity. However, the de novo ligand-design program BOMB20,21 (Biomolecular and Organic Molecule Builder) was used to consider introduction of substituents at positions 2-4 on the ligand in the binding site; the utilized protein coordinates came from the structure of the complex with a nicotinic acid derivative, which we previously reported (PDB ID:3JS2).19,22 Substituents larger than methyl cannot be introduced at positions 3 and 4 as they would clash with the protein backbone and likely cause disruption of the critical hydrogen bonds between the tricyclic core and the hinge region. However, extension at the 2-position with monocyclic and possibly fused bicyclic aryl substituents emerged as feasible from the model building. The computed structure of the complex with 4 (Figure 2B) shows that it appears possible to insert a phenyl group between Lys514 and Ile545 with potential benefits of cation-π interations with Lys514 and hydrophobic contacts with Ile545, Val561 and Ala640. Importantly, this is achieved while retaining the position of the tricyclic core and the hydrogen bonds with the hinge region. The present study summarizes initial experimental results that have been obtained to test this prediction. Successful introduction of a phenyl group would open up exploration of a wide range of substituted analogs and other aryl substituents.
Figure 2.
Computed structures of FGFR1 kinase with 3 (A) and 4 (B). Selected residues of FGFR1 kinase are shown; carbon atoms of the inhibitors are colored green. Hydrogen bonds are highlighted with black lines
Figure 1.
Chemical structure of 1, 2, and 3
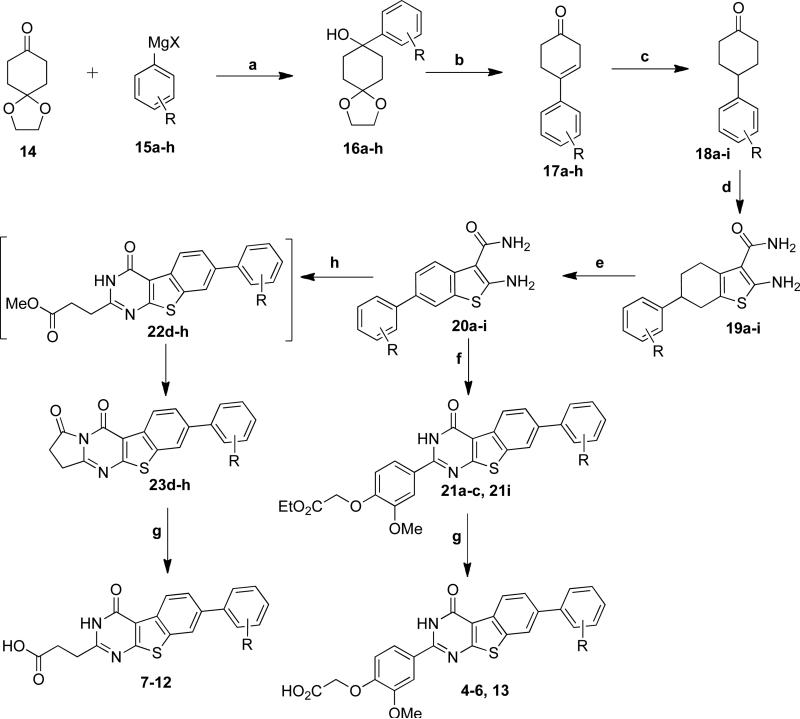
To pursue this notion, syntheses of compounds 4-13 with a phenyl group at the 2-position on the core of 3 were performed as summarized in Scheme 1. On the western end, 4-6 and 13 retain the substituted anisole ring as previously reported, e.g., in 2.19 However, 7-12 incorporate a smaller alternative, propionic acid, that was found to not diminish activity (vide infra). Preparation of 5-12 commenced with ketal 14 and the syntheses of 4 and 13 began with commercially available cyclohexanones 18a and 18i. Grignard reaction of ketal 14 with phenyl magnesium halides 15a-h produced hydroxy ketals 16a-h. Simultaneous removal of the protecting group and dehydration with TFA provided 17a-h, which after hydrogenation and Gewald reaction with 2-cyanoacetamide yielded 2-aminothiophenes 19a-i. p-Choranil oxidation of 19a-i delivered the benzothiophenes 20a-i. Condensation of 20a-c and 20i with aldehyde 24 (see Scheme 1 footnote) resulted in esters 21a-c and 21i, which were hydrolyzed to give 4-6, and 13. Condensation of 20d-h with aldehyde 25 afforded 23d-h via intermediate formation of 22d-h. Hydrolysis of esters 23d-h produced 7-12. The structures of 4-13 were validated through NMR and high-resolution mass spectrometry,23 and their purities were typically >95% by HPLC. The ALPHAScreen assay was performed using purified FGFR1 kinase domain and a biotinylated peptide substrate, as described previously.19 The assay results are summarized in Table 1.
Scheme 1. aReagents and conditions.
(a) RMgX, THF, 10 °C-rt, 3h, 70-95% (b) TFA, DCM, rt, 5h, 84%-quant (c) 10% Pd/C, EtOAc, rt, 16h, 73-83% (d) 2-Cyanoacetamide, S, Piperidine, EtOH, 50 °C, 4h, 42-50% (e) p-Chloranil, 1,4-Dioxane, 90 °C, 5h, 56-65% (f) Ethyl 2-(4-formyl-2-methoxyphenoxy)acetate (24), Conc. HCl, n-BuOH, reflux, 2h, 57-80% (g) 5N NaOH, EtOH, reflux, 2h, 60%-Quant. (h) Methyl-4-oxobutanone (25), Conc. HCl, n-BuOH, 80 °C, 8h, 48-67%
Table 1.
Inhibitory Activities of FGFR1 Kinase by 4-13
 | ||||||
|---|---|---|---|---|---|---|
| Compd | R | R1 | R2 | R3 | R4 | IC50 (μM)a |
| 4 | a | H | H | H | H | 75 |
| 5 | a | H | OMe | H | H | na |
| 6 | a | H | OMe | H | OMe | 28 |
| 7 | b | H | OMe | H | OMe | 21 |
| 8 | b | H | H | Me | H | 26 |
| 9 | b | H | Me | H | H | na |
| 10 | b | Me | H | H | H | 43 |
| 11 | b | H | H | Cl | H | 18 |
| 12 | b | H | Cl | H | H | na |
| 13 | a | H | H | OH | H | 6 |
na indicates not active in the assay.
The parent phenyl-containing analog 4 did show activity, 75 μM, though at a diminished level from 2 and 3. Addition of a single methoxy group in the meta position, 5, was found to lead to inactivity, while 3,5-dimethoxy substitution restored activity for 6 and 7 to about the level for 3. Comparison of the results for 6 and 7 also confirms the point above that the two options for the westernmost part of the inhibitors are equally viable. Potential benefits of the change to the smaller propionic acid side chain are a predicted 100-fold enhancement in aqueous solubility in going from 6 to 7 and lowering of the computed log Po/w for 6 of 4.7 to 3.4 for 7.24 Mono substitution in the meta position was again found to lead to inactivity for the methyl and chloro analogs 9 and 12. The one case with an ortho substituent, 10, showed a roughly two-fold gain in activity over 4. However, the methyl-scan represented by 8-10 revealed that para-substitution of the phenyl ring may be the most fruitful with the IC50 for 8 (26 μM) showing a three-fold improvement over the unsubstituted 4. Additional gain was found for the para-chloro analog 11 at 18 μM, and finally the para-hydroxy analog 13 yielded an additional three-fold boost to 6 μM. Further examination of analogs with ortho and/or para substituents is indicated.
In summary, as predicted by the computational modeling, aryl extension at the 2-position on the tricyclic core of theinopyrimidinone 3 was shown to be possible. It was also found that the anisole-based western susbstituent could be replaced by the smaller propionic acid alternative with no loss of activity and with concomitant expected enhancement of pharmacologically relevant properties. The most potent analog reported here, the hydroxyphenyl analog 13, yields inhibitory activity of 6 μM for FGFR1 kinase. Though the activity is similar to that of 2 (1.9 μM), the present results provide a platform for further elaboration of analogs to probe beyond the ATP binding site in search of enhanced interactions and selectivity. Such studies are underway with continued emphasis on the synergies between computational modeling, synthesis, and crystallography.
Acknowledgments
Gratitude is expressed to the National Institutes of Health (GM032136, AR051448, AR051886, P50-AR054086) and to Yale University (YSM0061AM) for support. This paper is dedicated with great affection to Prof. Harry H. Wasserman, friend and Yale colleague, on the occasion of his 90th birthday.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As affect service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Beenken A, Mohammadi M. Nat. Rev. Drug Discov. 2009;8:235. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaye M, Schlessinger J, Dionne CA. Biochim. Biophys. Acta. 1992;1135:185. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- 3.Eswarakumar VP, Lax I, Schlessinger J. Cytokine Growth Factor Rev. 2005;16:139. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Turner N, Grose R. Nat. Rev. Cancer. 2010;10:116. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Science. 1997;276:955. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- 6.a Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR. EMBO J. 1998;17:5896. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hamby JM, Connolly CJC, Schroeder MC, Winters RT, Showalter HDH, Panek RL, Major TC, Olsewski B, Ryan MJ, Dahring T, Lu GH, Keiser J, Amar A, Shen C, Kraker AJ, Slintak V, Nelson JM, Fry DW, Bradford L, Hallak H, Doherty AM. J. Med. Chem. 1997;40:2296. doi: 10.1021/jm970367n. [DOI] [PubMed] [Google Scholar]
- 7.Thompson AM, Connolly CJC, Hamby JM, Boushelle S, Hartl BG, Amar AM, Kraker AJ, Driscoll DL, Steinkampf RW, Patmore SJ, Vincent PW, Roberts BJ, Elliott WL, Klohs W, Leopold WR, Showalter HDH, Denny WA. J. Med. Chem. 2000;43:4200. doi: 10.1021/jm000161d. [DOI] [PubMed] [Google Scholar]
- 8.Cai Z-W, Zhang Y, Qian L, Barbosa S, Wei D, Zheng X, Wu L, Fan J, Shi Z, Wautlet BS, Mortillo S, Jeyaseelan R, Kukral DW, Kamath A, Marathe P, D'Arienzo C, Derbin G, Barrish JC, Robl JA, Hunt JT, Lombardo LJ, Fargnoli J, Bhide RS. J. Med. Chem. 2008;51:1976. doi: 10.1021/jm7013309. [DOI] [PubMed] [Google Scholar]
- 9.Barvian MR, Panek RL, Lu GH, Kraker AJ, Amar A, Hartl B, Hamby JM, Showalter HDH. Bioorg. Med. Chem. Lett. 1997;7:2903. [Google Scholar]
- 10.Shimizu T, Fujiwara Y, Osawa T, Sakai T, Kubo Kazao, Kubo Kinya, Nishitoba T, Kimura K, Senga T, Murooka H, Iwai A, Fukushima K, Yoshino T, Miwa A. Bioorg. Med. Chem. Lett. 2004;14:875. doi: 10.1016/j.bmcl.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Fry DW, Nelson JM. Anti-Cancer Drug Des. 1995;10:607. [PubMed] [Google Scholar]
- 12.Koziczak M, Holbro T, Hynes NE. Oncogene. 2004;23:3501. doi: 10.1038/sj.onc.1207331. [DOI] [PubMed] [Google Scholar]
- 13.Sarker D, Molife R, Evans TRJ, Hardie M, Marriott C, Butzberger-Zimmerli P, Morrison R, Braendle E, Heise C, Louie S, Aziz N, Garzon F, Michelson G, Judson IR, Jadayel D, Fox JA, de Bono JS. Clin. Cancer Res. 2008;14:2075. doi: 10.1158/1078-0432.CCR-07-1466. [DOI] [PubMed] [Google Scholar]
- 14.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, Rettig WJ. Cancer Res. 2008;68:4774. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Lee, Benjamin H, Williams IR, Kutok JL, Mitsiades CS, Duclos N, Cohen S, Adelsperger J, Okabe R, Coburn A, Moore S, Huntly BJP, Fabbro D, Anderson KC, Griffin JD, Gillilnad DG. Oncogene. 2005;24:8259. doi: 10.1038/sj.onc.1208989. [DOI] [PubMed] [Google Scholar]
- 16.Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. Int. J. Cancer. 2008;122:664. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 17.Machida S, Saga Y, Takei Y, Mizuno I, Takayama T, Kohno T, Konno R, Ohwada M, Suzuki M. Intl. J. Cancer. 2005;114:224. doi: 10.1002/ijc.20751. [DOI] [PubMed] [Google Scholar]
- 18.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 19.Ravindranathan KP, Mandiyan V, Ekkati AR, Bae JH, Schlessinger J, Jorgensen WL. J. Med. Chem. 2010;53:1662. doi: 10.1021/jm901386e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen WL. Science. 2004;303:1813. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen WL. Acc. Chem. Res. 2009;42:724. doi: 10.1021/ar800236t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae JH, Ravindranathan KP, Mandiyan V, Ekkati AR, Schlessinger J, Jorgensen WL. Protein Data Bank ID 3JS2. [DOI] [PMC free article] [PubMed]
- 23.Spectral data for representative compounds: 2-(4-(7-(3,5-dimethoxyphenyl)-4-oxo-3,4-dihydrobenzo[4,5]thieno[2,3-d]pyrimidin-2-yl)-2-methoxyphenoxy)acetic acid (6) 1H NMR (500 MHz, DMSO-d6) δ 12.94 (s, 1H), 8.50 (d, J = 8.3, 1H), 8.42 (d, J = 1.4 Hz, 1H), 7.90 – 7.82 (m, 3H), 7.03 (d, J = 9.3 Hz, 1H), 6.92 (d, J = 2.2 Hz, 2H), 6.53 (t, J = 2.2 Hz, 1H), 4.81 (s, 2H), 3.92 (s, 3H), 3.84 (s, 6H); 13C NMR (126 MHz, DMSO-d6) δ 169.8, 168.3, 166.9, 160.9, 158.5, 154.4, 150.4, 148.6, 141.7, 137.8, 135.5, 133.0, 124.8, 124.1, 123.8, 121.4, 120.9, 115.1, 112.5, 111.3, 104.9, 99.7, 64.7, 55.7, 55.3; HRMS (ESI-TOF) calcd for C27H23N2O7S [M+H]+ : 519.1226, found: 519.1221. 3-(7-(3,5-dimethoxyphenyl)-4-oxo-3,4-dihydrobenzo[4,5]thieno[2,3-d]pyrimidin-2-yl)propanoic acid (7) 1H NMR (500 MHz, DMSO-d6) δ 12.82 (s, 1H), 12.29 (s, 1H), 8.46 (d, J = 8.3 Hz, 1H), 8.40 (d, J = 1.4 Hz, 1H), 7.85 (dd, J = 1.7, 8.4 Hz, 1H), 6.91 (d, J = 2.2 Hz, 2H), 6.52 (t, J = 2.2 Hz, 1H), 3.83 (s, 6H), 2.96 (t, J = 6.9 Hz, 2H), 2.78 (t, J = 7.0 Hz, 2H); 13C NMR (126 MHz, DMSO-d6) δ 173.4, 166.6, 160.9, 159.7, 158.0, 141.7, 137.7, 135.2, 132.9, 124.8, 123.7, 120.9, 115.3, 104.9, 99.7, 55.3, 29.8, 28.9; HRMS (ESI-TOF) calcd for C21H19N2O5S [M+H]+ : 411.1015, found: 411.1014.
- 24.Results from QikProp v. 3.0. Schrödinger Inc.; New York, NY: 2006. [Google Scholar]