Abstract
Background
Thyroid cancer incidence has risen dramatically in the U.S. since the early 1980s. Although the prevalence of obesity has doubled during this time period, the relationship between obesity and thyroid cancer is uncertain.
Methods
We examined the association between body mass index (BMI) and thyroid cancer risk in a pooled analysis of five prospective U.S. studies, including 413,979 women and 434,953 men. Proportional hazards models with attained age as the time metric were adjusted for education, race, marital status, smoking, alcohol intake, and (where appropriate) cohort and sex.
Results
Over follow-up (mean=10.3 years), 768 women and 388 men were diagnosed with thyroid cancer. The risk of thyroid cancer was greater with increasing BMI (per 5 kg/m2: hazard ratio [HR] in women, 1.16 [95% confidence interval (CI), 1.08–1.24]; HR in men, 1.21 [95% CI, 0.97–1.49]). There was no significant heterogeneity between studies (both P>0.05). For women and men combined, the HRs for overweight (25.0–29.9 kg/m2) and obesity (≥30 kg/m2) compared to normal-weight (18.5–24.9 kg/m2) were 1.20 (95% CI, 1.04–1.38) and 1.53 (95% CI, 1.31–1.79), respectively. We found no significant effect modification by other factors, and the results did not differ significantly by histologic type. A significant positive association for BMI in young adulthood (ages 18–20) with thyroid cancer risk was also observed (per 5-kg/m2 increase: HR, 1.18 [95% CI, 1.03–1.35]).
Conclusion
BMI was positively associated with thyroid cancer risk in both men and women.
Impact
Our study provides strong evidence that obesity is an independent risk factor for thyroid cancer.
Keywords: obesity, body mass index, thyroid neoplasms, prospective studies, epidemiology
Thyroid cancer incidence in U.S. men and women has nearly tripled since 1980 (1), with the most rapid period of increase being between 1997 and 2006 (2). There is evidence that these patterns may represent a true increase, although greater diagnostic scrutiny probably also plays a role (3). Despite much interest in the reasons for the growing number of thyroid cancer diagnoses, there are few widely-recognized risk factors for thyroid cancer apart from ionizing radiation in childhood and a medical history of goiter or thyroid nodules (4), though there is some evidence of a decreased risk with smoking and alcohol consumption (4–6).
During a similar time period, the prevalence of obesity in U.S. adults has doubled, and overweight in children and adolescents has tripled (7). Excess adiposity has been implicated in the etiology of a number of cancer sites (8), but whether it may increase thyroid cancer risk has not yet been established. Of the few epidemiologic studies on this relationship, most included small numbers of incident cases and were retrospective in design, and therefore may have been influenced by differential recall and/or post-diagnostic weight change. While a positive association between obesity and thyroid cancer risk has been observed in women in several case-control (9–11) and prospective (12–15) studies, though not all (16–18), the results are less consistent in men, for whom the thyroid cancer incidence rate is lower (9–14, 16, 18–20). Furthermore, it remains unclear whether the association is modified by other factors or differs according to thyroid cancer subtypes, which are suspected to differ etiologically (4).
We conducted the first pooled analysis of prospective studies for the association between BMI and thyroid cancer risk, which was also one of the first to consider a large number of other postulated risk factors as potential effect modifiers and one of the largest prospective investigations of thyroid cancer in men to date.
METHODS
Study population
The present study combines data from prospective cohorts from the National Cancer Institute that have accrued 50 or more incident, primary thyroid cancers and have baseline height and weight data. The eligible cohorts are the NIH-AARP Diet and Health Study (NIH-AARP) (21), U.S. Radiologic Technologists Study (USRT) (22), Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) (23), Agricultural Health Study (AHS) (24), and Breast Cancer Detection and Demonstration Project (BCDDP) (25). All five cohorts are based in the U.S. The institutional review boards from the National Cancer Institute and all participating institutions approved the use of these data.
In order to be eligible for this pooling study, participants had to have responded to a baseline questionnaire, be followed for cancer incidence, have no history of cancer other than non-melanoma skin cancer at baseline, and have a diagnosis date for any cancer diagnosed during follow-up. A total of 889,026 participants met these criteria. We further excluded men and women with missing (n=37,181) or extreme (<15 kg/m2 or >50 kg/m2) BMI (n= 2,913). The final study population included 848,932 participants (434,953 men and 413,979 women).
Exposure assessment and data standardization
Participants from each cohort completed a self-administered baseline questionnaire eliciting information on general demographics (e.g., age, sex, race/ethnicity, education, marital status), lifestyle factors (e.g., cigarette smoking, alcohol intake, physical activity), and personal medical history. Anthropometric data were self-reported in all cohorts except BCDDP, in which trained medical staff measured participants' height and weight.
The level of detail on covariates differed across the cohorts. Therefore we coded height, weight, BMI, education, race, marital status, smoking status, alcohol intake, and physical activity using standardized definitions and categories. In addition, confounding by some known or potential risk factors for thyroid cancer, such as radiation exposure and a medical history of benign thyroid conditions was evaluated in the USRT cohort, which had all the relevant data.
Outcome assessment
Participants were followed from the date of completion of the baseline questionnaire to the date of any cancer diagnosis other than non-melanoma skin cancer, death, or last date of follow-up, whichever came first. Incident cancer information was obtained through different sources in each cohort: self-report (USRT, PLCO, BCDDP), cancer registry linkage (NIH-AARP, USRT, AHS, BCDDP), death certificates (USRT, PLCO, BCDDP), and/or the National Death Index (NIH-AARP, USRT, BCDDP). Participants were considered to be cases if they were diagnosed with a malignant first primary thyroid neoplasm during follow-up. Using information from medical and pathology records and cancer registry linkage, thyroid cancers were classified by papillary (8050, 8052, 8130, 8260, 8340, 8341, 8342, 8343, 8344, 8450, 8452) and follicular (8290, 8330, 8331, 8332, 8335), medullary (8345, 8346, 8510), and anaplastic (8021) histologic types according to the International Classification of Diseases for Oncology, Third Edition, morphology codes.
Statistical analysis
Study-specific hazard ratios (HR) and 95% confidence intervals (CI) for thyroid cancer were calculated using Cox proportional hazards models with attained age as the underlying time metric. BMI was modeled both continuously and categorically using indicator variables. All multivariable models were stratified by sex and adjusted for education (up to high school degree, post-high school up to college degree, post-college, missing), race (white, black, American Indian/Alaskan Native, Asian/Pacific Islander, other, missing), marital status (married/living together, divorced/separated, widowed, single/never married, missing), cigarette smoking (never, former, current, missing), and usual alcohol intake during the previous 12 months (none, <1 drink/week, 1–6 drinks/week, ≥7 drinks/week, missing). To test for log-linear trends, the median values of each BMI category were modeled continuously, and the Wald test was used to assess statistical significance. The HRs were then pooled using random effects models (26). Heterogeneity in the HRs between studies was assessed using the Q statistic and the I2 index (27).
Because there was no statistically significant heterogeneity across studies, data from all five cohorts were combined into one aggregate dataset to conduct additional analyses. BMI was modeled using higher-order (up to cubic) effects to further evaluate the shape of the association between BMI and thyroid cancer risk; the fit of these models to those using log-linear values of BMI were compared using the likelihood ratio test. To evaluate effect modification, models were stratified by categories of potential thyroid cancer risk factors including baseline age, birth cohort, education, smoking status, alcohol intake, and physical activity level; cross-product terms were included in the models and compared to models without this term using the likelihood ratio test. We also separately assessed the associations by histologic type and evaluated the differences using Mantel-Haenszel test for heterogeneity.
We conducted several sensitivity analyses. To assess the potential for confounding by ionizing radiation exposure or a medical history of benign thyroid conditions, both of which are established thyroid cancer risk factors (4), we included these factors in models of BMI and thyroid cancer risk in USRT, the only study in which these data were available. Although evidence linking physical inactivity and diabetes to thyroid cancer risk is limited (14,18,28), we additionally adjusted for physical activity level and a medical history of diabetes in a subset of the cohort without missing data to evaluate the potential for confounding or mediation, respectively, by these exposures. We also excluded the first two years of follow-up to evaluate the potential bias associated with including participants with preclinical thyroid cancer at baseline whose weight may have changed as a result of the disease.
Data on weight at young adulthood (18–20 years old) were available from the baseline questionnaire in the PLCO and AHS cohorts and from a follow-up questionnaire in the NIH-AARP cohort (collected between 1996 and 1997). We prospectively examined BMI at young adulthood in relation to thyroid cancer risk within this subset (n=521 cases).
All analyses were conducted using Stata software (version 9.2, College Station, TX). All statistical tests were two-sided and were considered statistically significant if P<0.05.
RESULTS
The mean age of subjects in the entire groups of cohorts (n=848,932) was 58 years at baseline, with 51% of the participants being male, and 20% being obese (≥30 kg/m2) (Table 1). There were different entry and exit dates for each of the cohorts within the period from 1979 to 2009, during which 1,156 participants (388 male and 768 female) were diagnosed with a first primary thyroid cancer. There were 132 thyroid cancer cases with missing information on histology. Of the 1,024 thyroid cancer cases with complete histological information, 810 (79%) were papillary, 164 (16%) were follicular, 34 (3%) were medullary, and 16 (2%) were anaplastic.
Table 1.
General characteristics of the cohorts
| Cohort | No. Participants | No. Cases | Study period | No. years of follow-up (mean [SD]) | Age at baseline (mean [SD]) | Male (%) | BMI ≥30 (%) |
|---|---|---|---|---|---|---|---|
| NIH-AARP Diet and Health Study18 | 498,957 | 583 | 1995–2006 | 9.1 (2.9) | 62.0 (5.4) | 61 | 22 |
| US Radiologic Technologists Study19 | 89,529 | 275 | 1983–2006 | 15.9 (5.3) | 40.4 (11.1) | 23 | 9 |
| Prostate, Lung, Colorectal, and Ovarian Screening Study20 | 139,131 | 168 | 1993–2009 | 9.6 (3.2) | 62.6 (5.3) | 50 | 24 |
| Agricultural Health Study21 | 67,925 | 67 | 1993–2005 | 9.9 (1.9) | 46.9 (12.6) | 58 | 21 |
| Breast Cancer Detection Demonstration Project22 | 53,390 | 63 | 1979–1999 | 15.3 (2.8) | 55.4 (8.8) | 0 | 10 |
| Total | 848,932 | 1,156 | 1979–2009 | 10.3 (4.0) | 58.2 (10.4) | 51 | 20 |
Among women, but not men, BMI was positively associated with baseline age (Table 2). Compared to their normal-weight (18.5–24.9 kg/m2) counterparts, obese (≥30 kg/m2) participants were less likely to be current smokers and have a post-high school education. The proportions of women who were white, married, and reported drinking alcohol were lower in the obese compared to normal-weight category.
Table 2.
Age-adjusted means and percentages of select baseline characteristics according to BMI category
| Body mass index, kg/m2 | ||||
|---|---|---|---|---|
| 15.0–18.5 | 18.5–24.9 | 25.0–29.9 | 30.0–49.9 | |
| Men (no. participants) | 2,101 | 125,239 | 214,697 | 92,916 |
| Body mass index, kg/m2 | 17.4 | 23.2 | 27.2 | 33.2 |
| Baseline age | 61.3 | 59.8 | 60.1 | 59.6 |
| Education (% beyond high school) | 75.0 | 76.2 | 71.7 | 67.3 |
| Smoking status | ||||
| Never | 32.4 | 35.9 | 32.5 | 29.5 |
| Former | 41.7 | 45.8 | 53.6 | 57.9 |
| Current | 22.3 | 15.3 | 10.8 | 9.2 |
| Missing | 3.6 | 2.9 | 3.1 | 3.4 |
| Alcohol intake (% non-drinker) | 27.3 | 21.6 | 20.7 | 23.6 |
| Marital status (% married) | 76.3 | 82.4 | 86.1 | 84.9 |
| Race (% white) | 89.1 | 91.6 | 92.8 | 92.5 |
| Women (no. participants) | 7,644 | 206,578 | 121,950 | 77,807 |
| Body mass index, kg/m2 | 17.7 | 22.3 | 27.2 | 34.4 |
| Baseline age | 51.1 | 54.2 | 58.7 | 59.0 |
| Education (% beyond high school) | 69.1 | 67.5 | 61.9 | 60.0 |
| Smoking status | ||||
| Never | 43.7 | 48.6 | 50.5 | 51.2 |
| Former | 24.3 | 31.2 | 32.6 | 34.8 |
| Current | 26.3 | 16.0 | 13.1 | 10.4 |
| Missing | 5.7 | 4.2 | 3.7 | 3.5 |
| Alcohol intake (% non-drinker) | 31.7 | 27.1 | 31.0 | 37.3 |
| Marital status (% married) | 56.1 | 63.6 | 63.5 | 57.6 |
| Race (% white) | 91.0 | 92.8 | 90.2 | 87.6 |
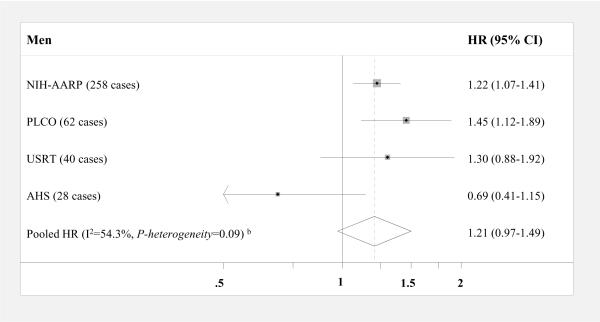
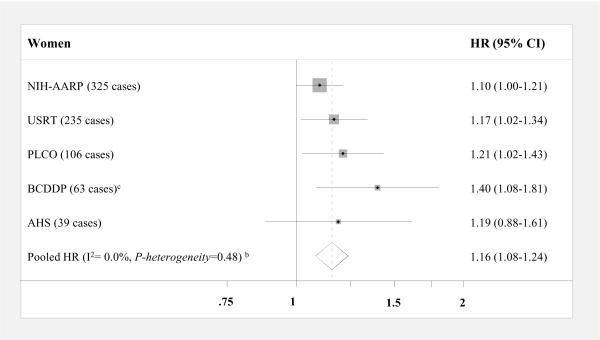
When BMI was modeled continuously (per 5 kg/m2, Figure 1), significant positive associations were observed in men from two out of four cohorts (NIH-AARP and PLCO) and, in women, four out of five cohorts (NIH-AARP, USRT, PLCO, and BCDDP). The pooled HR for thyroid cancer was 1.21 (95% CI, 0.97–1.49) in men and 1.16 in women (95% CI, 1.08–1.24); results were not significantly different by sex (P-interaction=0.34). When we excluded the first two years of follow-up, the pooled HR became slightly stronger and statistically significant in men (HR, 1.30 [95% CI, 1.15–1.47]) but remained the same for women (HR, 1.17 [95% CI, 1.08–1.27]). Despite a slightly stronger pooled HR in men compared to women, there was a non-significant inverse association among men in the AHS cohort (HR, 0.69 [95% CI, 0.41–1.15]). For approximately 31% of the AHS cohort, missing or extreme BMI values were assigned using data from a five-year follow-up questionnaire, and to a lesser extent drivers' license information on height and weight. However, exclusion of these individuals did not materially alter the HR for men in the AHS cohort, and in women, the positive association became slightly stronger.
Figure 1. Multivariable-adjusted HRsa and 95% CIs for BMI (per 5 kg/m2) and thyroid cancer risk by sex: pooled analysis.
Squares and horizontal lines correspond to the study-specific HRs and 95% CIs, respectively. The size of a square reflects the study-specific weight, and the diamond represents the pooled HR and 95% CI. The vertical dotted line represents the pooled HR. The abbreviations of the studies are same as in table 1. Abbreviations: BMI=body mass index, CI= confidence interval, HR= hazards ratio
aAdjusted for education, race, marital status, cigarette smoking, and alcohol intake
bCalculated using a random effects model
cHeight and weight were measured directly
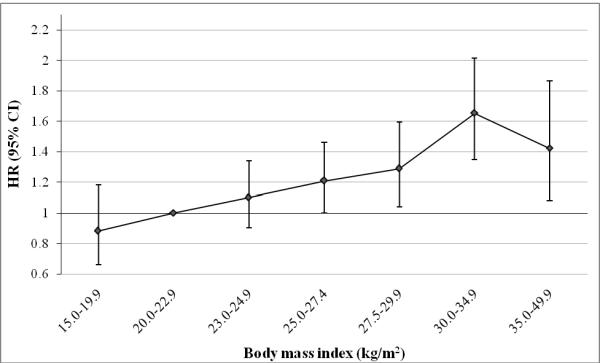
In the aggregate dataset (which combined men and women in the five cohorts), the relationship between BMI and thyroid cancer was approximately log-linear (per 5 kg/m2: HR, 1.17 [95% CI, 1.10–1.24]) (Figure 2); the inclusion of high-order terms did not significantly improve the data fit (P>0.05). We also examined the association using broader BMI categories, which correspond to the WHO criteria for normal-weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obesity (≥30 kg/m2) (29). Compared to normal-weight, the HRs for overweight and obesity were 1.20 (95% CI, 1.04–1.38) and 1.53 (95% CI, 1.31–1.79).
Figure 2. Multivariable-adjusted HRsa and 95% CIs for BMI categories and thyroid cancer risk: aggregate analysis.
Abbreviations: BMI=body mass index, CI= confidence interval, HR= hazards ratio
aAdjusted for sex, education, race, marital status, cigarette smoking, alcohol intake, and cohort
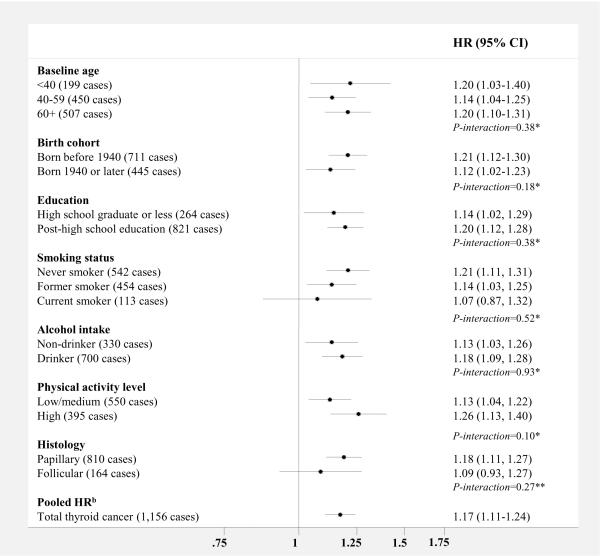
We observed no significant heterogeneity in the association between BMI (per 5 kg/m2) and thyroid cancer risk by baseline age, birth cohort, education level, smoking status, alcohol intake, or physical activity level (Figure 3). Though not significantly different, the results were stronger among never smokers (HR, 1.21 [95% CI, 1.11–1.31]) compared to former or never smokers. The results were stronger for papillary (n=810 cases) compared to follicular thyroid cancer (n=164 cases), but this difference was also not statistically significant, likely due to the smaller number of follicular tumors (P-heterogeneity=0.27). We also separately examined the associations between BMI (per 5 kg/m2) and medullary (n=34 cases, HR, 0.87 [95% CI, 0.59–1.28]) and anaplastic (n=16 cases, HR, 1.45 [95% CI, 0.95–2.22]) cancers, though the relative risk estimates were unstable due to small numbers. The differences in the results for the four histologic types were not significant (P-heterogeneity=0.23). When we restricted the results to Caucasians (n=1,075 cases), the HR was 1.19 (95% CI, 1.12–1.26).
Figure 3. Multivariable-adjusted HRsa and 95% CIs for BMI (per 5 kg/m2) and thyroid cancer risk, stratified by selected risk factors: aggregate analysis.
Abbreviations: BMI=body mass index, CI= confidence interval, HR= hazards ratio
*Test for interaction calculated using the likelihood ratio test comparing a model with a cross-product term to a model without
**Test for interaction calculated using the Mantel-Haenszel test for heterogeneity
aAdjusted for sex, education, race, marital status, cigarette smoking, alcohol intake, and cohort
bCalculated using a random effects model
Additional adjustment for physical activity level and medical history of diabetes had little influence on the results (Supplementary Table). Adjustment for other factors available only from the USRT cohort, including personal and medical exposure to radiation and medical history of benign thyroid conditions also did not change the results (Supplementary Table).
The strength of the association for BMI in young adulthood (18–20 years old) (per 5-kg/m2 increase: HR, 1.18 [95% CI, 1.03–1.35]) was very similar to that of baseline BMI (per 5-kg/m2 increase: HR, 1.17 [95% CI, 1.11–1.24]). However, mutual adjustment for baseline BMI slightly attenuated the association for young adulthood BMI (HR, 1.08 [95% CI, 0.93–1.25]), while that of baseline BMI remained similar (HR, 1.14 [95% CI, 1.04–1.25]).
We additionally examined the association between height (per 5 cm) and thyroid cancer risk in men and women, separately, and found a significant positive association in women (HR, 1.06 [95% CI, 1.00–1.12]) but no association in men (HR, 1.01 [95% CI, 0.94–1.08]). We observed significant heterogeneity between studies in men (P-heterogeneity=0.01), but the results were less heterogeneous after the exclusion of the AHS cohort (HR, 1.03 [95% CI, 0.96–1.11], P-heterogeneity=0.34).
DISCUSSION
Because thyroid cancer is a relatively rare cancer, it is difficult to investigate potential risk factors, such as obesity, within individual prospective studies. Our pooled analysis is the largest prospective study on this topic to date that has individual-level data on other key exposures, including cigarette smoking, alcohol intake, physical activity, medical history of diabetes, and included a large number of incident thyroid cancers in men, for whom the disease is less common. Overall, we observed a positive association between BMI and thyroid cancer risk, which was generally consistent across the five prospective studies.
Results from previous prospective and case-control studies on the association between obesity and thyroid cancer risk have generally been more inconsistent in men than women. A pooled analysis of 12 case-control studies from the U.S., Europe, and Asia (2,056 female and 417 male cases) found a significant, but weak, positive association between BMI and thyroid cancer risk in women (highest versus lowest tertile: RR, 1.2 [95% CI, 1.0–1.4]) but not men; however there was significant heterogeneity by study, with the positive association for women being largely driven by two U.S. studies (9). A large prospective Norwegian study (2,268 female and 778 male cases) also found a moderate positive association between BMI and thyroid cancer in women but not men, but the results were unadjusted for smoking and other factors (13). In a large prospective study of Korean men, there was a strong dose-response association after adjustment for cigarette smoking, alcohol intake, physical activity, family history of cancer, and urban versus rural residency (≥30 versus 18.4–22.9 kg/m2: RR, 2.23 [95% CI, 1.40–3.55]; P-trend <0.001]) (19); however, results were not presented for women. Although our results were consistent with those from a meta-analysis of prospective studies from the US, Europe, and Korea not included in the current analysis (12,13,17,19,20) (per 5-kg/m2 increase: pooled RR for women=1.14, 95% CI, 1.06–1.23; pooled RR for men=1.33, 95% CI, 1.04–1.70), among men, there was significant heterogeneity between studies (I2=77.4%, P-heterogeneity=0.004) (30). The inconsistent results between men and women in previous studies is likely due to smaller numbers of cases in men, possibly, to the lack of control for important covariates. Failure to adjust for smoking status, for instance, may positively bias the association between BMI and risk of thyroid cancer as current smoking is associated with lower BMI levels and has been linked to a reduced risk of thyroid cancer (4,14). When we restricted to never smokers we found that the association between BMI and thyroid cancer was slightly stronger than the association in the pooled analysis overall, which suggests that the BMI-thyroid cancer association was independent of the effect of cigarette smoking in this pooled study.
We also observed a significant positive association between BMI in young adulthood (ages 18–20) and thyroid cancer risk (HR, 1.18 [95% CI, 1.03–1.35]). This finding, as well as the lack of effect modification by baseline age on the relationship between baseline BMI and thyroid cancer risk, suggests that obesity during any stage of adulthood may predispose individuals to thyroid cancer and that obesity may have properties of both a tumor initiator and a promoter. Although no association between early adulthood BMI and thyroid cancer was observed in a pooled analysis of case-control studies (9), a recent case-control study in French Polynesia showed a significantly increased risk of thyroid cancer in both women and men with a high BMI at age 18 (11).
Several case-control and prospective studies have observed positive associations between height and thyroid cancer risk in both men and women (9, 11, 13, 16, 31). For reasons that are currently unclear, in our pooled analysis, we observed positive associations in women but not men. There may be genes or early environmental exposures, such as nutrition, that contribute both to skeletal growth and also to the risk of thyroid cancer (31). Future investigations of such factors are warranted.
There were some limitations of our study, however. The number of thyroid cancer diagnoses may have been underestimated due to the reliance on self-report in some of the studies, but given that thyroid cancer is rarely fatal this number is expected to be small. We lacked information on tumor size, which would be useful in distinguishing between tumors found incidentally with those that have potentially greater clinical significance. We would expect that small, clinically insignificant tumors were, if anything, more frequently diagnosed in thinner individuals; thus inclusion of these tumors in our case definition would have attenuated a true association between BMI and thyroid cancer. Measurement error in height and weight may have attenuated our results because all but one of the cohorts (BCDDP) had self-reported measures, which is evidenced by the slightly stronger association we observed for BCDDP compared with the other four cohorts. Even greater measurement error in the estimation of BMI in young adulthood may be expected due to the long period of time between young adulthood and age at self-report; this may partly account for the inconsistency in previous studies (9,11). We cannot rule out the possibility of residual confounding by some measured and unmeasured factors. For example, residual confounding or misclassification of physical activity may have occurred since it was defined differently across the cohorts and probably reported with some inaccuracy by the participants. Also, we only had information on exposure to ionizing radiation and history of benign thyroid diseases in the USRT cohort, although adjustment for these factors did not change the results for this particular study.
Potential biological mechanisms linking obesity and thyroid cancer risk are not well understood. Thyroid stimulating hormone (TSH), which promotes the secretion of thyroid hormones to regulate resting energy expenditure (32), may play a role in human thyroid cancer etiology. TSH has been shown to influence the growth and differentiation of thyroid cells in rodents (33), and higher concentrations have been found in thyroid surgery patients with differentiated thyroid cancer compared to patients with benign thyroid disease (34). While some cross-sectional studies among euthyroid adults have found positive associations between BMI and levels of TSH (35,36), these findings have not been confirmed in others studies (37,38). Alternatively, insulin resistance, a common metabolic perturbation in obesity, may influence thyroid tumor growth directly by binding to insulin receptors or indirectly by stimulating insulin-like growth factor, estrogen, or other hormones, such as TSH, to enhance the proliferation of thyroid cancer cells (39,40). The few studies that have examined the association of insulin resistance or diabetes with thyroid cancer risk have been inconsistent (14,28), however, and additional adjustment for diabetes history did not attenuate the positive associations between BMI and thyroid cancer risk observed in our pooled analysis. Because BMI measurements cannot distinguish fat from muscle mass (41), we had expected to observe a stronger association between BMI and thyroid cancer risk among physically-inactive participants given their presumably lower contributions of muscle to total body mass. Instead, we found that the results were slightly, though non-significantly, stronger among active versus less active participants. As some adverse metabolic risk factors, including insulin resistance, are more strongly associated with central adiposity or visceral, as opposed to subcutaneous, adipose tissue (42), future studies with data on distribution and type of adipose tissue may provide additional clues about the etiology of thyroid cancer.
This pooled analysis provides strong support that obesity is an independent risk factor for thyroid cancer in both men and women. The risk estimates we observed for thyroid cancer were consistent with previous studies and were generally stronger than those observed for some other cancers more widely recognized to be obesity-related, including pancreatic, post-menopausal breast, and colon cancer (8,30). This may have considerable public health implications in countries, like the U.S., which have experienced dramatic increases in the prevalence of overweight and obesity (7). Therefore it is plausible that the rise in the number of thyroid cancer diagnoses in the U.S. in the last three decades may have been at least partially attributable to the growing proportion of overweight and obesity in the population. Further research on the potential mechanisms underlying the BMI-thyroid cancer association may provide additional clues into the etiology of the disease and help identify more specific targets for prevention. Given the available evidence, there appears to be a particular need for prospective studies on central adiposity and obesity-related biomarkers, including markers of thyroid function (serum TSH or free T4) or insulin resistance (fasting serum glucose or insulin), with the risk of this disease.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2007. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–91. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ron E, Schneider AB. Thyroid cancer. In: Schottenfeld D, Fraumeni J, editors. Cancer Epidemiology and Prevention. 3rd ed. Oxford University Press; New York: 2006. pp. 975–994. [Google Scholar]
- 5.Meinhold CL, Park Y, Stolzenberg-Solomon RZ, Hollenbeck AR, Schatzkin A, Berrington de González A. Alcohol intake and risk of thyroid cancer in the NIH-AARP Diet and Health Study. Br J Cancer. 2009;101(9):1630–4. doi: 10.1038/sj.bjc.6605337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund. American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington, DC: 2007. [Google Scholar]
- 9.Dal Maso L, La Vecchia C, Franceschi S, Preston-Martin S, Ron E, Levi F, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control. 2000;11(2):137–44. doi: 10.1023/a:1008938520101. [DOI] [PubMed] [Google Scholar]
- 10.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guenel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am J Epidemiol. 2007;166(10):1140–9. doi: 10.1093/aje/kwm204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brindel P, Doyon F, Rachédi F, Boissin JL, Sebbag J, Shan L, et al. Anthropometric factors in differentiated thyroid cancer in French Polynesia: a case-control study. Cancer Causes Control. 2009;20(5):581–90. doi: 10.1007/s10552-008-9266-y. [DOI] [PubMed] [Google Scholar]
- 12.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6(11):863–73. [PubMed] [Google Scholar]
- 13.Engeland A, Tretli S, Akslen LA, Bjørge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95(3):366–370. doi: 10.1038/sj.bjc.6603249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, et al. Nonradiation risk factors for thyroid cancer in the U.S. Radiologic Technologists Study. Am J Epidemiol. 2010;171(2):242–252. doi: 10.1093/aje/kwp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavel-Chapelon F, Guillas G, Tondeur L, Kernaleguen C, Boutron-Ruault MC. Risk of differentiated thyroid cancer in relation to adult weight, height and body shape over life: the French E3N cohort. Int J Cancer. 2010;126(12):2984–90. doi: 10.1002/ijc.25066. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Matsuo K, Hasegawa Y, Hiraki A, Kawase T, Tanaka H, et al. Anthropometric factors at age 20 years and risk of thyroid cancer. Cancer Causes Control. 2008;19(10):1233–42. doi: 10.1007/s10552-008-9194-x. [DOI] [PubMed] [Google Scholar]
- 17.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93(9):1062–7. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitzmann MF, Brenner A, Moore SC, Koebnick C, Park Y, Hollenbeck A, et al. Prospective study of body mass index, physical activity, and thyroid cancer. Int J Cancer. 2010;126(12):2947–56. doi: 10.1002/ijc.24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23(21):4742–54. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 20.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17(7):901–9.2. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 21.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 22.Boice JD, Jr, Mandel JS, Doody MM, Yoder RC, McGowan R. A health survey of radiologic technologists. Cancer. 1992;69(2):586–98. doi: 10.1002/1097-0142(19920115)69:2<586::aid-cncr2820690251>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team Design of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 24.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104(4):362–9. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calton BA, Stolzenberg-Solomon RZ, Moore SC, Schatzkin A, Schairer C, Albanes D, et al. A prospective study of physical activity and the risk of pancreatic cancer among women (United States) BMC Cancer. 2008;8:63. doi: 10.1186/1471-2407-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 28.Rapp K, Schroeder J, Klenk J, Ulmer H, Concin H, Diem G, et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945–52. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 29.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 30.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 31.Sung J, Song YM, Lawlor DA, Smith GD, Ebrahim S. Height and site-specific cancer risk: A cohort study of a Korean adult population. Am J Epidemiol. 2009;170(1):53–64. doi: 10.1093/aje/kwp088. [DOI] [PubMed] [Google Scholar]
- 32.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–4. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 33.Hill RN, Crisp TM, Hurley PM, Rosenthal SL, Singh DV. Risk assessment of thyroid follicular cell tumors. Environ Health Perspect. 1998;106:447–57. doi: 10.1289/ehp.98106447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16(4):1251–60. doi: 10.1677/ERC-09-0036. [DOI] [PubMed] [Google Scholar]
- 35.Fox CS, Pencina MJ, D'Agostino RB, Murabito JM, Seely EW, Pearce EN, et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168:587–92. doi: 10.1001/archinte.168.6.587. [DOI] [PubMed] [Google Scholar]
- 36.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol (Oxf) 2005;62:487–91. doi: 10.1111/j.1365-2265.2005.02247.x. [DOI] [PubMed] [Google Scholar]
- 37.Figueroa B, Velez H, Irizarry-Ramirez M. Association of thyroid-stimulating hormone levels and body mass index in overweight Hispanics in Puerto Rico. Ethn Dis. 2008;18:S2–S4. [PubMed] [Google Scholar]
- 38.Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol (Oxf) 2006;64:125–8. doi: 10.1111/j.1365-2265.2006.02433.x. [DOI] [PubMed] [Google Scholar]
- 39.Hard GC. Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environ Health Perspect. 1998;106:427–36. doi: 10.1289/ehp.106-1533202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hursting SD, Lashinger LM, Wheatley KW, Rogers CJ, Colbert LH, Nunez NP, et al. Reducing the weight of cancer: mechanistic targets for breaking the obesitycarcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659–69. doi: 10.1016/j.beem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141–7. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 42.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Supp 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.