Abstract
The normal counterparts of mantle cell lymphoma (MCL) are naïve quiescent B-cells that have not been processed through the germinal center (GC). For this reason, while lymphomas arising from GC or post-GC B-cells often exhibit plasmacytic differentiation, MCL rarely presents with plasmacytic features. Seven cases of MCL with a monotypic plasma cell (PC) population were collected from six centers and studied by immunohistochemistry, FICTION (Fluorescence immunophenotyping and Interphase Cytogenetics as a Tool for the Investigation of Neoplasms), capillary gel electrophoresis, and restriction fragment length polymorphism of immunoglobulin heavy chain analysis (RFLP/IgH) of microdissections of each of the MCL and PC populations to assess their clonal relationship. Clinical presentation was rather unusual compared to typical MCL, with two cases arising from extranodal soft-tissues of the head. All MCL cases were morphologically and immunohistochemically typical, bearing the t(11;14)(q13;q32). In all cases PC populations were clonal. In 5 of the 7 cases, the MCL and PC clones showed identical restriction fragments, indicating a common clonal origin of the neoplastic populations. The two cases with clonal diversity denoted the coexistence of two different tumors in a composite lymphoma/plasma cell neoplasm. Our findings suggest that MCL can present with a PC component that is often clonally related to the lymphoma, representing a rare but unique biological variant of this tumor.
Keywords: Mantle cell lymphoma, Plasma cells, Monotypic plasma cells, Plasmacytic differentiation, Cyclin D1
Introduction
Mantle cell lymphoma (MCL) accounts for approximately 7% of all B-cell lymphomas in North America.1,43 Its median age of diagnosis is around 60 years and patients usually present with bone marrow involvement and with advanced stage disease, the spleen and gastrointestinal tract being the most frequently involved sites. While the overall response rate to chemoimmunotherapy is usually good, relapses are frequent and the prognosis is poor, with a median overall survival of 3 to 5 years.15
MCL is characterized by a nodular and/or diffuse histologic growth pattern, and by different cytologic subtypes (classical, small cell, pleomorphic, and blastoid).5,42 The neoplastic cells typically are CD20+, CD5+, CD43+, CD23-, CD3-, CD10-, and FMC7+, and are characterized by the presence of t(11;14)(q13;q32), which results in overexpression of Cyclin D1.31,34 A small fraction of MCL may not express Cyclin D113 and be difficult to distinguish from other small B-cell lymphomas. Recently, overexpression of the nuclear transcription factor sox11 mRNA and of its nuclear protein has been reported as a specific marker for both cyclin D1-positive and negative MCL.28 Plasma cells (PCs) are usually absent in MCL or, if present in extremely small numbers, they have traditionally been regarded as part of the non-neoplastic background, being formally polyclonal.
MCL develops from naïve B-cells that reside in primary lymphoid follicles and the mantle zones of secondary follicles and still have not undergone the germinal center (GC) process.36 This process starts when antigen recognition, together with signals from surrounding T-cells, activates GC B-cells to undergo somatic hypermutation and class-switch recombination, as well as to proliferate and differentiate within the GC. The GC B cells expressing a high-affinity immunoglobulin at the end of the process will be preferentially selected for survival and will differentiate into PCs or memory B-cells.18 Thus it is not surprising that plasmacytic differentiation has been described in several B-cell lymphomas arising from B cells at the GC or post-GC developmental stage, such as diffuse large B-cell lymphoma,27 follicular lymphoma16 and marginal zone lymphoma.20 In lymphoplasmacytic lymphomas, the malignant clone shows a wide morphologic heterogeneity ranging from small B lymphocyte to PC, although little is known about its cell of origin.17 Among pre-GC deriving lymphomas, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL)9,14,47 and MCL29,46 have also been described to occasionally show plasmacytic differentiation.
In this study we report seven cases of classical MCL recruited from 6 medical centers that exhibited monotypic PC proliferation. Both the MCL cells and the PCs were analyzed with immunohistochemistry and separated by microdissection to characterize them by means of FICTION (Fluorescence immunophenotyping and Interphase Cytogenetics as a Tool for the Investigation of Neoplasms) analysis, capillary gel electrophoresis and immunoglobulin heavy chain variable region restriction fragment length polymorphism (RFLP/IgH). We found that PCs were usually clonal, and that they originated from the same neoplastic B-cell clone as MCL in the majority of cases.
Materials and Methods
Histology and immunohistochemistry
All specimens were obtained at first presentation of the lymphoma and consisted of: lymph nodes (1 case), periorbital mass (1), appendix (1), spleen (1), and nasal mass (1). In the remaining two cases the two cell populations (MCL and PCs) were analyzed on lymph node and bone marrow, respectively, due to the predominance of each population in the two different tissues. Specimens were collected at the University of Wisconsin (2 cases), the University of California San Diego School of Medicine (1), the British Columbia Cancer Agency (1), the University of Pittsburgh School of Medicine (1), the Autonomous University of Barcelona (1), and Ingham Regional Medical Center at Michigan (1). The specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, and 4 μm sections were cut and stained with hematoxylin and eosin (H&E) for histologic evaluation. Immunohistochemical stains were also performed on formalin-fixed, paraffin-embedded tissue sections. Briefly, after deparaffinization in xylene and rehydration in graded alcohols, endogenous peroxidase was blocked with 3% hydrogen peroxide. Antigen retrieval was performed in citrate buffer, pH 6.0, as appropriate for the antibodies. After rinsing in phosphate-buffered saline, antibodies against CD3, CD5, CD10, CD20, CD23, CD43, CD138, BCL2, BCL6, kappa and lambda light chains, IgG, IgM, and cyclin D1 were employed for immunohistochemical stains. These stains were performed on a Ventana ES automated immunostainer (Ventana Biotek, Tucson, AZ) using a streptavidin-biotin peroxidase detection system. Source of the antibodies was Ventana for CD3, CD10, CD43, kappa and lambda light chains (PS1, 56C6, L60 clones respectively, polyclonal kappa and lambda), Novocastra for CD5 (4C7 clone), DAKO for CD20, Bcl-2, Bcl-6 (L26, 124, PG-B6p clones respectively), the binding site for CD23 (BU38 clone), Neomarkers for Cyclin D1 (SP4 clone) and DakoCytomation for CD138 (MI15 clone).
FISH Analysis
Interphase FISH analysis was performed on formalin-fixed, paraffin-embedded tissue sections via a co-denaturation of the probe and target DNA using the HybRite system (Vysis, Inc) at 80 C for 5 minutes and then hybridization of the probe to the target DNA at 37 C overnight, followed by a post-hybridization wash (2xSSC/0.1% NP40) at 72 C for 2 minutes and a room temperature wash (2xSSC/0.1% NP40) for 1 minute and then counterstain with DAPI II (Abbott/Vysis, Inc.). For detection of the t(11;14), commercially available LSI IgH/CCND1 XT Dual-Color, Dual Fusion Translocation Probe (Abbott/Vysis, Inc.) was used. The observed fusion signals were considered to be significant if they were in greater than 8% of the cells, a cut-off determined by the study of 20 normal tissue specimens in our laboratory.
FICTION (Fluorescence immunophenotyping and Interphase Cytogenetics as a Tool for the Investigation of Neoplasms) analysis
Immunofluorescence combined with interphase FISH analysis for the t(11;14)(q13;q32) was performed as previously described with minor modifications.25,41 The staining for either immunoglobulin light chains or CD138 was performed before the interphase FISH procedure. The assay was performed on formalin-fixed, paraffin-embedded tissue sections using a fluorescein (FITC)-streptavidin-biotin detection system. Briefly, after deparaffinization and rehydration, the sections were pretreated with 10mM citrate buffer, pH 6.0, in a pressure cooker for 8 minutes, followed by boiling for 12 minutes, for antigen retrieval. The sections were then incubated with blocking buffer [50 mM Tris-HCl, pH 8.2, containing 5% bovine serum albumin (BSA), and 5% normal goat serum] at room temperature (RT) for 20 minutes. Primary antibodies (goat anti-human kappa/lambda light chain or CD138; Sigma, St Louis, MO) were then applied, and after three washes were covered with FITC-labeled, secondary antibody (rabbit anti-goat IgG; Vector Labs, Burlingame, CA). Tertiary antibody (biotinylated goat anti-FITC IgG; Vector Labs) was applied at a concentration of 20 μg/ml in 1 × PBS buffer, pH 7.4 at RT for 30 minutes. The FITC anti-FITC complexes were then detected by incubation with FITC-labeled streptavidin (Vector Labs). The sections were post-fixed in 1% paraformaldehyde at 4 C for 20 minutes, and slides were processed for hybridization with t(11;14) FISH probe and covered with Aqua Mount Medium. At least 100 cells were then evaluated by fluorescence microscopy for light chains or CD138 expression and the t(11;14) as previously described.41 The frequency of t(11;14) fusion signals in IgL or CD138 staining-positive and -negative cells was quantitated.
Immunoglobulin variable region restriction fragment length polymorphism (RFLP/IgH)
The analysis was performed as described previously.39 Briefly, genomic DNA was extracted separately from the CD138+ and CD5+ tumor cells, which were micro-dissected from the formalin-fixed, paraffin-embedded tissue sections, using a Genovison extraction kit (Qiagen, Germany), as described.10 Polymerase chain reaction (PCR) assays for IgH gene rearrangement was performed utilizing consensus FR1 and FR3 as well as J primers, as previously published.26 DNA from a normal or polyclonal B cell populations produced a bell shaped curve of amplicon products (Gaussian distribution), and was used as control. Clonal rearrangements were identified as prominent, single-sized amplification products. Examination of PCR products was carried out with the high resolution fragment length analysis (ABI 310 Genetic Analyzer, Applied Biosystems, USA).
Capillary gel electrophoresis of the PCR products
The IgH genes were amplified using a semi-nested PCR method with a primer complementary to the FR3 and two primers hybridizing the antisense JH region. The primer sequences used to amplify the IgH genes by a semi-nested PCR method were the following: 5'ACC TGA GGA GAC GGT GAC C3' for JH external, Genbank accession AY180386.1, base pairs 342-342; 5'ACC AGG GTC CCT TGG CCC CA3' for JH nested, Genbank accession AF343422, base pairs 289-308; 5'CTG TCG ACA CGG CCG TGT ATT ACT G3' for V670, Genbank accession AB067303.1, base pairs 263-287. The first round PCR amplification was performed in 50 μL total reaction containing 25 μL of 2 × GoTaq Green Master Mix (Promega), 4 μL of 10 μM of the primers V670 and JH external, 5 μL of the purified genomic DNA. The PCR conditions were predenaturing at 94 °C for 5 minutes, then 30 cycles of 94 °C for 40 seconds, 55 °C for 40 seconds, and 72 °C for 40 seconds, followed by a final extension at 72 °C for 10 minutes. The second round of PCR reaction contained 10 μL of the 1:500 dilution of the completed 1st round PCR reaction as template, 25 μL of 2 × GoTaq Green Master Mix (Promega), 4 μL of 10 μM of the primers V670 and JH nested in total 50 μL volume. The cycle conditions were the same as the first round for 25 cycles. Five μL of each completed second round PCR reactions were electrophoresed on 2% agrose gels followed by ethidium bromide staining. The gel images were captured after UV exposure. The PCR products were then gel purified and analyzed by capillary gel electrophoresis on Agilent 2100 Bioanalyzer.
Results
Clinical features
Median age of the seven patients was 74 years-old (range 62-91), and four were males. All patients except one presented with advanced stage disease and bone marrow involvement either by MCL (case 3, 4 and 8) or both clonal PCs and MCL (case 2, 6, and 7). Two cases presented with a mass arising from soft tissues of the head, and one of them had tumor limited to this site (Table 1). Clinical presentation appeared more dictated by the natural history of MCL component rather than of multiple myeloma, since bone lytic lesions and renal failure due to light chain deposition were observed only in one patient (case 2). Serum and urine electrophoresis revealed a monoclonal component in three of the patients, as reported in Table 1.
Table 1.
Clinical characteristics at presentation and survival of the seven patients
| Case-1 | Case-2 | Case-3 | Case-4 | Case-5 | Case-6 | Case-7 | |
|---|---|---|---|---|---|---|---|
| Age | 62 | 66 | 80 | 75 | 91 | 76 | 70 |
| Gender | M | M | M | F | F | F | M |
| Date of diagnosis | 03/01/2006 | 08/02/2007 | 08/27/2007 | 01/07/2003 | 11/25/2005 | 01/04/2007 | 09/10/2008 |
| Site of biopsy | Nasopharygeal mass | Left neck lymph node | Appendix | Orbital mass | Left neck lymph node | Spleen | Left axillary lymph node |
| AAS | I | IV | IV | IV | III-IV | IV | IV |
| B-symptoms | No | Yes | No | No | No | No | Yes |
| Bone marrow | Normal | Involved | Involved | Involved | ND | Involved | Involved |
| Paraprotein | No | Lambda light chain | No | No | IgM, Lambda | IgG, Lambda | No |
| Follow-up time | 55 months | 26 months | 27 months | 56 months | 12 months | 42 months | 24 months |
| Survival status | Alive and free of disease | Dead of disease | Alive with disease | Dead of disease | Dead of disease | Alive with disease | Alive and free of disease |
ND: Not done due to advanced age. AAS: Ann Arbor stage
Treatment of patients varied, including both myeloma and lymphoma directed therapies, but response to treatment and overall survival was quite poor. Although not reaching statistical significance due to the small number of patients analyzed, specific myeloma directed therapies seemed to be less effective than common immuno-chemotherapy regimens usually utilized in MCL patients. Although active in both diseases, but primarily used for treating multiple myeloma, both Velcade, Thalidomide, and Lenalidomide were ineffective or induced transitory responses in all four patients treated with these drugs. Of note, the patient presenting with a localized tumor (nasal mass, case 1) was treated with surgical resection followed by radiotherapy alone (50 Gy), and achieved a long lasting complete remission.
Histology and immunohistochemistry
All cases were reviewed by at least three hematopathologists [KHY, JTM and primary contributing pathologists], and were classified as typical MCL in terms of cytology, histology and immunohistochemistry (Figure 1-4). Histologic growth pattern was mantle-zone (2), nodular (3), diffuse (2), including one pleomorphic-blastoid cytologic subtype. The MC components from all cases had the characteristic MCL phenotype: CD20+, CD5+, CD3-, Cyclin D1+, CD23-, CD138-. Immunoglobulin heavy chain expression lacked in all cases on MCL cells, probably due to the low sensitivity of immunohistochemical staining in this setting. The PCs were all CD20- and CD138+, and exhibited cytoplasmatic kappa light restriction in two cases, lambda in four cases, while one case was indeterminate but expressed IgG heavy chain (case 7). As described in Figures 1-4, plasma cells from all cases showed typical morphology with rare mitotic figures and were focally positive for Cyclin D1 in two patients (case 2 and 6, see Table 2). In five cases sheets of PCs were observed within the neoplastic lymphoid tissue, while in two patients (case 2 and 7) clonal PCs were confined to the bone marrow together with lymphoma localization of an otherwise typical nodal MCL.
Figure 1.
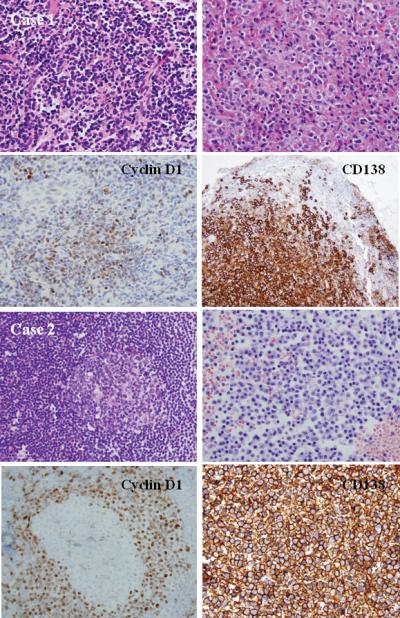
A. Histologic and phenotypic features of MCL with clonal PC differentiation (case 1): a naso-pharyngeal mass showing a diffuse pattern of atypical lymphoid cells. Clusters of PCs are present and adjacent to the lymphoid aggregates (H&E stain, upper left and right, original magnification 100X). Immunohistochemical staining for Cyclin D1 and CD138 shows the MCL and PC component, respectively (immunoperoxidase stain, lower left and right, original magnification 100X). The two clones were unrelated. This case might represent a concomitant plasma cell neoplasm or a marginal zone lymphoma and MCL in the same patient. B. Histologic and phenotypic features of MCL with clonal PC differentiation (case 2) in a lymph node showing reactive GC (upper left) with expanded mantle zone. Clusters of PCs are seen in the bone marrow biopsy (H&E stain, upper left and right, original magnification 100X). Cyclin D1 stain shows a mantle-zone growth pattern, whereas CD138 stain shows numerous positive PCs (immunoperoxidase stains, original magnification 100X). The two populations were clonally related, and both harbored the t(11;14)(q13;q32).
Figure 4.
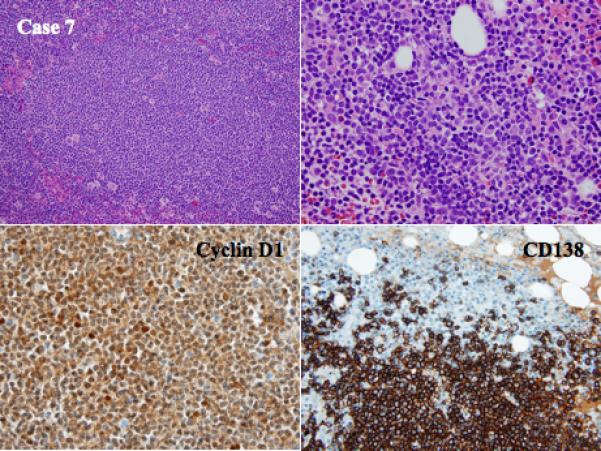
Histologic and phenotypic features of MCL with clonal PC differentiation (case 7): a combined nodular/diffuse pattern of MCL in a lymph node and bone marrow biopsy of the same patient showing an admixture of monotypic PCs and MCL aggregates (H&E stain, upper left and right, original magnification 40X and 100X). Immunohistochemical staining for Cyclin D1 and CD138 shows the MCL and PC component, respectively (immunoperoxidase stain, lower left and right, original magnification 100X). The two neoplastic populations are derived from the same clone.
Table 2.
Pathologic, Immunophenotypic, Molecular and Genetic Findings
| Case-1 | Case-2 | Case-3 | Case-4 | Case-5 | Case-6 | Case-7 | |
|---|---|---|---|---|---|---|---|
| PATHOLOGIC | |||||||
| Morphologic pattern | Diffuse | Nodular | Nodular | Nodular | Nodular | Nodular | Nodular |
| Morphologic MCL subtype | Diffuse | Mantle zone | Nodular | Mantle zone | Nodular | Nodular | Nodular |
| Plasma cell location | Adjacent to MCL cells | Admixed with MCL in the bone marrow | Between MCL nodules | Between MCL nodules | Between MCL nodules | Periarteriols and within the MCL nodules | Admixed with MCL in the bone marrow |
| Case-1 | Case-2 | Case-3 | Case-4 | Case-5 | Case-6 | Case-7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMMUNOPHENOTYPIC | MCL | PC | MCL | PC | MCL | PC | MCL | PC | MCL | PC | MCL | PC | MCL | PC |
| CD3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CD20 | + | - | + | - | + | - | + | - | + | - | + | - | + | - |
| CD5 | + | - | + | - | + | - | + | + | + | - | + | - | + | - |
| Cyclin D1 | + | - | + | + | + | - | + | - | + | - | + | + | + | - |
| CD138 | - | + | - | + | - | + | - | + | - | + | - | + | - | + |
| Kappa* | - | + | - | - | - | + | - | - | - | - | - | - | - | - |
| Lambda* | + | - | + | + | - | - | + | + | - | + | + | + | - | - |
| IgM | - | - | - | - | + | - | + | - | - | + | - | - | + | + |
| IgG | - | + | - | + | - | - | - | - | - | - | - | + | - | - |
| MOLECULAR/GENETIC ANALYSIS | ||||||||||||||
| RFLP/IgH | unrelated | related | related | related | unrelated | related | related | |||||||
| FICTION for IPX and t(11;14) | + | - | + | + | + | - | Fail | Fail | + | - | + | + | Fail | Fail |
| CGE | different | same peak | same peak | same peak | different | same peak | same peak | |||||||
All results were obtained with immunohistochemistry, and integrated by flow cytometry on case-1, case-2 and case-6.
NA: not available due to analysis failure. ND: not done. PC: plasma cell component. MCL: mantle cell lymphoma. CGE: capillary gel electrophoresis
When analyzed by immunohistochemistry (Table 2) only three cases showed identical monotypic immunoglobulin light chain expression between MCL and PCs (case 2, 4, 6), confirming that this methodic is not reliable and has low sensitivity in identifying relationships among different clones.
RFLP/IgH, capillary gel electrophoresis and FICTION analysis
The combination of these three analysis allowed us to investigate the two cell populations (MCL cells and PCs) individually in terms of clonality, relationship between clones, and cytogenetics. The rationale for the use of RFLP/IgH methodology was that each B-cell has a single productive immunoglobulin gene rearrangement that is unique in both length and sequence. According to RFLP/IgH assay results on microdissected CD138+ and CD5+ tumor cells, which are exemplified in Figure 5, all cases exhibited a clonal PC population, and in five out of seven cases (71%) the MCL cells and PCs showed identical RFLP/IgH, indicating that both populations were derived from the same neoplastic B-cell clone (also see Table 1). The remaining two cases without identical RFLP/IgH pattern (Case 1 and 5) exhibited distinct peaks by clonal PCs that differed from MCL and may represent a composite MCL and extramedullary plasmacytoma or marginal zone lymphoma with associated PC component.
Figure 5.
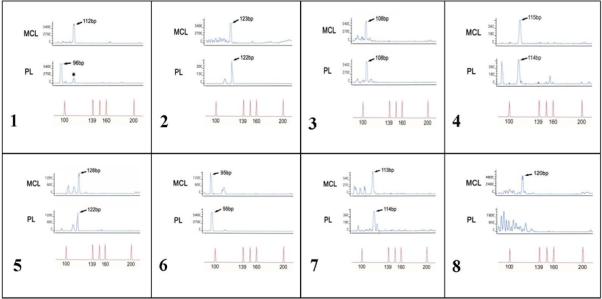
RFLP/IgH analyses on 7 cases of MCL with PC differentiation show clonal identity between the two neoplastic populations in 5 cases. In case 1 and 5, the two clones are unrelated. The case number 8 represents a control from a patient with MCL with an associated population of polyclonal PCs.
As shown in the gel images (Figure 6A), we obtained PCR products from FR3 for all of the samples except PC samples of case 4 and 6. For PC sample of case 6, where amplification of FR3 region failed, we successfully amplified FR2, which clearly showed a single band, while for the PC component of case 4 amplification failed for both regions (Figure 6A). As shown in Figure 6B, when we analyzed the FR3 products on better resolution of the amplified DNA fragments by capillary gel electrophoresis, most of the samples had single monoclonal band, while some samples contained multiple clonal bands (MCL of case 1, 2 and 3, PC of case 3 and 7). Overall, as described in Table 2, for the five patients successfully analyzed with gel electrophoresis on both populations of MCL and PC a complete concordance with the RFLP/IgH analysis was observed.
Figure 6.
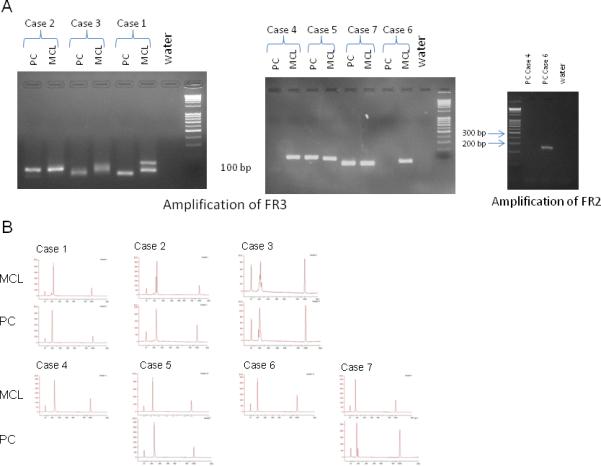
IgH-FR3 and FR2 PCR results on agarose gel (A) and capillary electrophoresis of all FR3 product (B). PC component of case 4 could not be amplified both for FR3 and FR2. PC component of case 6 was amplified for FR2 because FR3 failed and PCR product was run on capillary electrophoresis and it clearly appears to be single band on agarose gel. In case 3 the gel bands in the PC and MCL component appear different, but the peaks analysis by gel electrophoresis shows multiple related peaks (2 and 4 respectively).
By FICTION analysis we detected the characteristic t(11;14)(q13;q32) in MCL component from all five cases that were successfully studied, but also in two (Case 2 and 6) of the PC populations (Table 2, Figure 7). Both these cases were characterized by clonal identity between the two clones. In the remaining three cases (Case 4 and 7) with clonal identity the PC clone was negative for the t(11;14)(q13;q32) in case 3, while the FICTION analysis failed in case 4 and 7. Figure 7 reports the FICTION analysis of the five cases with available results.
Figure 7.
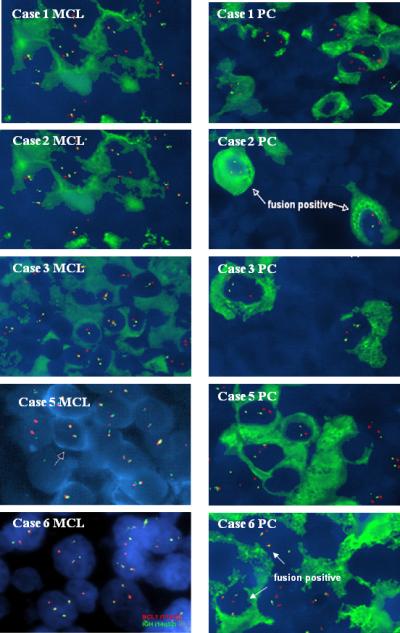
FICTION analysis for the t(11;14)(q13;q32) on MCL and PCs populations in 5 cases. In case 2 and 6 the fusion gene was present in both the MCL cells and PCs. The analysis failed in case 4 and 7. The red spots within the nuclei represent hybridization signals of the CCND1 gene on chromosome 11, whereas the green spots are hybridization signals of the IgH gene on chromosome 14. The t(11;14) fusion signals are identified as yellow or fused red-green signals, as illustrated by the arrows (FICTION, original magnification 1,000X).
Discussion
Gene expression profiling and other molecular technologies have led us to discriminate the developmental stage of the cell of origin of B-cell lymphoma subtypes.23 Since B-cell lymphomas originate from distinct steps of differentiation of normal B-cells, we would expect that some entities would be more prone to differentiate into PCs than others. Indeed, many marginal zone lymphomas and virtually all lymphoplasmacytic lymphomas have a clonal PC component.7,8,21 Others, like diffuse large B-cell lymphomas,27,35 CLL/SLL9,14,17 and follicular lymphoma16,20 may show plasmacytic differentiation, while little is known about MCL.29,46 With the current study we provide strong evidence that clonal PCs differentiation can also occur in certain cases of MCL, clearly demonstrating that the PCs and MCL cells can derive from the same B-cell clone.
In the present study, we investigated seven new cases of otherwise typical MCL that showed significant PC populations within the tumor tissue. In order to achieve robust conclusions regarding the relationships between the two neoplastic populations, we used PCR for the IGH with capillary gel electrophoresis, FICTION and RFLP/IgH assay for each microdissected MCL and PCs. The molecular analysis for the immunoglobulin heavy chain rearrangement revealed an identical clonal origin of the MCL and PC populations in five of the seven samples, indicating that these cases represent an interesting morphologic type of MCL. Results of capillary gel electrophoresis of PCR products were concordant with RFLP/IgH analysis, independently confirming our findings. As shown in Figure 5, Case 1 and Case 5 had instead no clonal relationship between PCs and MCL, and represented a concomitant plasma cell neoplasm or a nodal marginal zone lymphoma and MCL in the same patient. Composite lymphoma consisting of MCL and a clonally unrelated plasmacytoma or plasma cell myeloma have occasionally been reported.2,40,45 The distinction between MCL with a concomitant plasma cell neoplasm and a unique variant of MCL has relevant clinical significance, since prognosis and treatment of the two forms of disease would be expected to be different. A multidisciplinary approaches including detailed morphologic evaluation and a panel of immunostains, along with cytogenetic analysis for the t(11;14)(q13;q32) are needed to rule out other subtypes of B-cell lymphoid neoplasms with more frequent plasmacytic differentiation, especially when the tumor presents in unusual sites.
The present report follows two previously published cases46 describing for the first time the presence of clusters of monotypic kappa light chain expressing PCs in the center of MCL nodules and within the reactive GCs. In the previous study, the presence of the t(11;14)(q13;q32) both in the MCL cells and clonal PCs was considered compatible with a common origin from the same B-cell clone. However, the demonstration of the translocation itself in the two clones is insufficient to discriminate between MCL with plasmacytic differentiation and MCL with concurrent plasma cell neoplasm, as the t(11;14)(q13;q32) is commonly observed in nonhyperdiploid plasma cell myeloma.11 More recently, another case of MCL with evidence of clonal plasmacytic differentiation has been reported by Naushad et al.,29 using multi-parameter flow cytometry to demonstrate a close relationship between the two clones.
Monoclonal paraproteins, mostly of the IgM type, can be demonstrated in the serum of B-cell lymphoma, mostly in CLL/SLL and marginal zone lymphoma, usually at lower levels than in lymphoplasmacytic lymphoma.24 Using a sensitive serum immunoassay, monoclonal IgM and IgG were previously reported in 18% and 5% of patients with MCL, respectively.32 These findings suggest that clonal PCs can be part of MCL tumor microenvironment, and the frequency of clonal PCs in MCL may be more common than previously thought. Among our cases a monoclonal paraprotein was present in 3 of 7 (43%) patients with clonal PC population.
During normal immune response, when naïve B-cells meet an antigen with adequate affinity, they are selected and induced to proliferate either entering the GC or extra-follicular areas, where they divide further before differentiating into short-lived PCs. Therefore, plasmacytic differentiation could develop via two different pathways: GC (activated B-cells) and non-GC (naïve B-cells).30 The underlying mechanisms are better understood for the GC pathway in which environmental stimuli may induce the GC B-cell to become a PC. As GC B-cells begin to differentiate into PC, they up-regulate the transcription factor interferon regulatory factor 4 (IRF4), which initiates plasmacytic differentiation by increasing the expression of B lymphocyte-induced maturation protein 1 (BLIMP1),33 and extinguishes the GC B-cell program by repressing BCL6.38 In this scenario, growth factors (IL-6), and cell-cell interactions (CD40L) play regulatory roles in mediating transcription factor expression and lead to somatic hypermutation of their rearranged IgH genes and finally to PC differentiation.3,12,19 It is generally accepted that most biologically long-lived PCs derive from B-cells in the GCs or post-GC marginal zones. Alternatively, PCs can also arise from memory B-cells derived from GC centrocytes and can quickly differentiate into PCs through the GC-pathway upon secondary encounter with minute amounts of antigen.
In contrast, little is known regarding plasmacytic differentiation via the non-GC pathway. A proportion of naïve B-cells can undergo IgH-gene mutations outside the GCs without T-cell help and express IgG, but most of them are not switched and express IgM,37,44 as observed in CLL/SLL.6 In-vitro studies have confirmed that PCs can differentiate from naïve B-cells, but they are usually short-lived with low quantity production.30 Somatic hypermutation and class switch recombination are critical to PC differentiation through the GC, and require the presence of activation-induced cytidine deaminase (AID), which is virtually absent on naïve primary follicle, mantle zone, and extra-follicular B-cells. However, a fraction of naive B-cells can harbor mutated IgH-gene and express AID, suggesting that these cells may be able to enter the GC and differentiate into PCs.22 It is likely that MCL cells under some circumstances can enter the GC and differentiate into PCs, as outlined in two cases of MCL with clusters of clonal PCs within reactive GCs46 and as suggested by recent reports of IgH-gene mutations in a considerable subset of MCL.4 In this regard, it would be of interest to explore the correlation of IgH gene mutational status in groups of MCL cases with and without PC component, which may in part address the question of GC versus non-GC mediated PC differentiation. Further study of similar cases will be needed to better understand these processes.
In conclusion, our study clearly demonstrated the existence of MCL with related plasmacytic component. Our comprehensive analysis shows that in most MCL with clonal plasma cells, the two components are clonally related (5/7, 71%), indicating that clonal PC differentiation may occur in most of MCL in which PC component is present. However, in some cases the two clones are unrelated likely a reflection of a composite tumor, while the presence of polyclonal PCs within MCL as previously thought seems rather unusual. A molecular approach to analyse the clonal relationship between the two populations is needed to exclude a composite lymphoma and allow for appropriate treatment.
Figure 2.
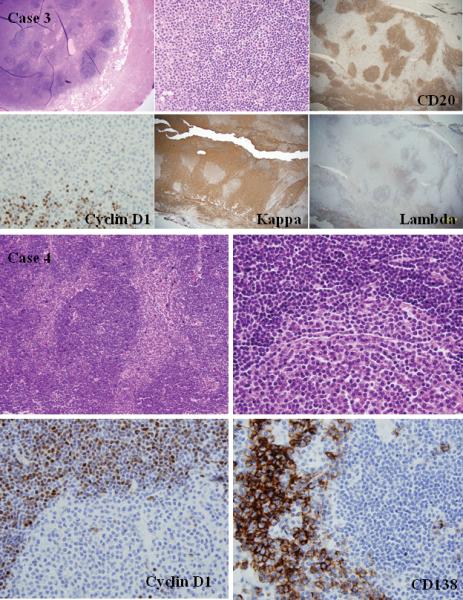
A. Histologic and phenotypic features of MCL with clonal PC differentiation (case 3): the appendix whose lumen was obliterated by sheets of PCs and nodular aggregates of mediumsized atypical lymphocytes (H&E stain, upper left and middle, original magnification 20X and 40X). Cyclin D1 and CD20 stains show a nodular growth pattern of lymphoid cells, whereas kappa stain shows numerous positive PCs between the lymphoid nodules (immunoperoxidase stains, lower left and middle, original magnification 20X and 40X). The two populations derived from the same clone, but the PCs did not show the t(11;14)(q13;q32). B. Histologic and phenotypic features of MCL with clonal PC differentiation (case 4): an orbital mass with a nodular growth pattern of atypical lymphoid cells and sheets of monotypic PCs in the internodular areas (H&E stain, upper left and right, original magnification 100X and 200X). Cyclin D1 stain shows a mantle-zone growth pattern, whereas CD138 stain shows clusters of monotypic PCs between the lymphoid nodules (immunoperoxidase stains, lower left and right, original magnification 200X). The two populations expressed lambda light chain and derived from the same clone.
Figure 3.
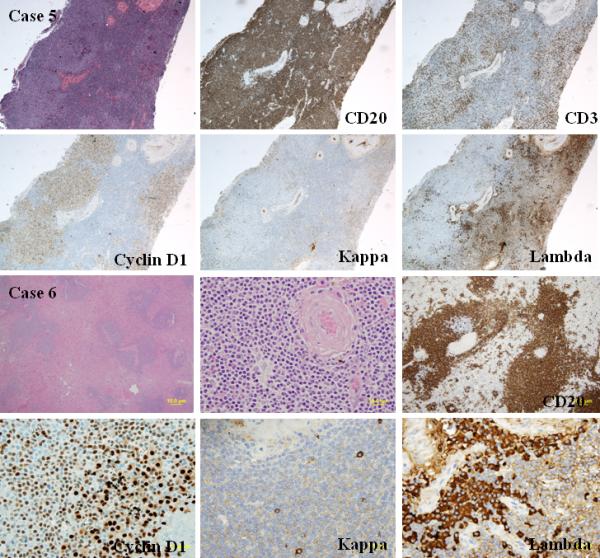
A. Histologic and phenotypic features of MCL with clonal PC differentiation (case 5): Excisional biopsy of a lymph node with pleomorphic atypical lymphoid cells and a concomitant lambda-restricted PC component (H&E stain, upper left, original magnification 20X). Cyclin D1 stain shows a diffuse pattern of lymphoid cells, whereas lambda light chain stain shows clusters of monotypic PCs between the cyclin D1 positive lymphoid aggregates (immunoperoxidase stains, lower left and right, original magnification 20X). The two populations derived from different clones, and only the MCL component harbored the t(11;14)(q13;q32). B. Histologic and phenotypic features of MCL with clonal PC differentiation (case 6): Splenic MCL with aggregates of lambda-restricted PCs around the arterioles in the context of a largely preserved splenic architecture (H&E stain, upper left and middle, original magnification 20X and 100X). Cyclin D1 stain shows a nodular pattern of CD20 positive lymphoid cells, whereas lambda light chain stain shows clusters of monotypic PCs around the arterioles (immunoperoxidase stains, lower left and right, original magnification 200X). The two neoplastic populations in the spleen derived from the same clone, and both harbored the t(11;14)(q13;q32).
Acknowledgements
CV is an honorable visiting scholar partially supported by fund from San Bortolo Hospital, Vicenza, Italy, University of Wisconsin Pathology R & D Fund and University of Wisconsin Paul Carbone Comprehensive Cancer Center Fund. KHY is supported by University of Wisconsin Paul Carbone Comprehensive Cancer Center Award, Gundersen Medical Foundation Award, University of Wisconsin Pathology R & D Fund. This study is also partially supported by NCI/NIH (P20CA103697, R01CA138688, 1RC1CA146299, and U01 CA114778).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 2.Cachia AR, Diss TC, Isaacson PG. Composite mantle-cell lymphoma and plasmacytoma. Hum Pathol. 1997;28:1291–-1295. doi: 10.1016/s0046-8177(97)90203-3. [DOI] [PubMed] [Google Scholar]
- 3.Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–-230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- 4.Camacho FI, Algara P, Rodríguez A, et al. Molecular heterogeneity in MCL defined by the use of specific VH genes and the frequency of somatic mutations. Blood. 2003;101:4042–-4046. doi: 10.1182/blood-2002-11-3456. [DOI] [PubMed] [Google Scholar]
- 5.Campo E, Raffeld M, Jaffe ES. Mantle-cell lymphoma. Semin Hematol. 1999;36:115–-127. [PubMed] [Google Scholar]
- 6.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–-815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 7.Cogliatti SB, Lennert K, Hansmann ML, et al. Monocytoid B cell lymphoma: clinical and prognostic features of 21 patients. J Clin Pathol. 1990;43:619–-625. doi: 10.1136/jcp.43.8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coupland SE, Hellmich M, Auw-Haedrich C, et al. Plasmacellular differentiation in extranodal marginal zone B cell lymphomas of the ocular adnexa: an analysis of the neoplastic plasma cell phenotype and its prognostic significance in 136 cases. Br J Ophthalmol. 2005;89:352–-359. doi: 10.1136/bjo.2004.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans HL, Polski JM, Deshpande V, et al. CD5+ true SLL/CLL with plasmacytic differentiation and an unusual 1p36 translocation: case report and review of the literature. Leuk Lymphoma. 2000;39:625–-632. doi: 10.3109/10428190009113393. [DOI] [PubMed] [Google Scholar]
- 10.Fong D, Kaiser A, Spizzo G, et al. Hodgkin's disease variant of Richter's syndrome in chronic lymphocytic leukemia patients previously treated with fludarabine. Br J Haematol. 2005;129:199–-205. doi: 10.1111/j.1365-2141.2005.05426.x. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemi. 2009;23:2210–-2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foy TM, Aruffo A, Bajorath J, et al. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–-617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 13.Fu K, Weisenburger DD, Greiner TC, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315–4321. doi: 10.1182/blood-2005-04-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George TI, Wrede JE, Bangs CD, et al. Low-grade B-Cell lymphomas with plasmacytic differentiation lack PAX5 gene rearrangements. J Mol Diagn. 2005;7:346–-351. doi: 10.1016/S1525-1578(10)60563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009;114:1469–1476. doi: 10.1182/blood-2009-02-179739. [DOI] [PubMed] [Google Scholar]
- 16.Gradowski JF, Jaffe ES, Warnke RA, et al. Follicular lymphomas with plasmacytic differentiation include two subtypes. Mod Pathol. 2010;23:71–-79. doi: 10.1038/modpathol.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutiérrez NC, Ocio EM, de Las Rivas J, et al. Gene expression profiling of B lymphocytes and plasma cells from Waldenström's macroglobulinemia: comparison with expression patterns of the same cell counterparts from chronic lymphocytic leukemia, multiple myeloma and normal individuals. Leukemia. 2007;21:541–-549. doi: 10.1038/sj.leu.2404520. [DOI] [PubMed] [Google Scholar]
- 18.Hummel M, Tamaru J, Kalvelage B, et al. Mantle cell (previously centrocytic) lymphomas express VH genes with no or very little somatic mutations like the physiologic cells of the follicle mantle. Blood. 1994;84:403–-407. [PubMed] [Google Scholar]
- 19.Kawano MM, Mihara K, Huang N, et al. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood. 1995;85:487–-494. [PubMed] [Google Scholar]
- 20.Keith TA, Cousar JB, Glick AD, et al. Plasmacytic differentiation in follicular center cell (FCC) lymphomas. Am J Clin Pathol. 1985;84:283–-290. doi: 10.1093/ajcp/84.3.283. [DOI] [PubMed] [Google Scholar]
- 21.Kojima M, Nakamura S, Motoori T, et al. Primary marginal zone B-cell lymphoma of the lymph node resembling plasmacytoma arising from a plasma cell variant of Castleman's disease. A clinicopathological and immunohistochemical study of seven patients. APMIS. 2002;110:875–-880. doi: 10.1034/j.1600-0463.2002.1101206.x. [DOI] [PubMed] [Google Scholar]
- 22.Kolar GR, Mehta D, Pelayo R, et al. A novel human B cell subpopulation representing the initial germinal center population to express AID. Blood. 2007;109:2545–-2552. doi: 10.1182/blood-2006-07-037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–-1429. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin P, Hao S, Handy BC, et al. Lymphoid neoplasms associated with IgM paraprotein: a study of 382 patients. Am J Clin Pathol. 2005;123:200–-205. [PubMed] [Google Scholar]
- 25.Martín-Subero JI, Chudoba I, Harder L, et al. Multicolor-FICTION: expanding the possibilities of combined morphologic, immunophenotypic, and genetic single cell analyses. Am J Pathol. 2002;161:413–-420. doi: 10.1016/S0002-9440(10)64197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier VS, Rufle A, Gudat F. Simultaneous evaluation of T- and B-cell clonality, t(11;14) and t(14;18), in a single reaction by a four-color multiplex polymerase chain reaction assay and automated high-resolution fragment analysis: a method for the rapid molecular diagnosis of lymphoproliferative disorders applicable to fresh frozen and formalin-fixed, paraffin-embedded tissues, blood, and bone marrow aspirates. Am J Pathol. 2001;159:2031–-2043. doi: 10.1016/S0002-9440(10)63055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montes-Moreno S, Gonzalez-Medina AR, Rodriguez Pinilla SM, et al. Aggressive large B cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B cell lymphoma with partial plasmablastic phenotype. Haematologica. 2010 doi: 10.3324/haematol.2009.016113. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozos A, Royo C, Hartmann E, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 2009;94:1555–-1562. doi: 10.3324/haematol.2009.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naushad H, Choi WW, Page CJ, et al. Mantle cell lymphoma with flow cytometric evidence of clonal plasmacytic differentiation: a case report. Cytometry B Clin Cytom. 2009;76:218–-224. doi: 10.1002/cyto.b.20463. [DOI] [PubMed] [Google Scholar]
- 30.O'Connor BP, Gleeson MW, Noelle RJ, et al. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev. 2003;194:61–-76. doi: 10.1034/j.1600-065x.2003.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pileri SA, Falini B. Mantle cell lymphoma. Haematologica. 2009;94:1488–-1492. doi: 10.3324/haematol.2009.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preud'homme JL, Gombert J, Brizard A, et al. Serum Ig abnormalities in mantle cell lymphoma. Blood. 1997;90:894–-895. [PubMed] [Google Scholar]
- 33.Shaffer AL, Lin KI, Kuo TC, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–-62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 34.Swerdlow SH, Zukerberg LR, Yang WI, et al. The morphologic spectrum of non-Hodgkin's lymphomas with BCL1/cyclin D1 gene rearrangements. Am J Surg Pathol. 1996;20:627–-640. doi: 10.1097/00000478-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Taddesse-Heath L, Meloni-Ehrig A, Scheerle J, et al. Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features. Mod Pathol. 2010;23:991–-999. doi: 10.1038/modpathol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thieblemont C, Nasser V, Felman P, et al. Small lymphocytic lymphoma, marginal zone B-cell lymphoma, and mantle cell lymphoma exhibit distinct gene-expression profiles allowing molecular diagnosis. Blood. 2005;106:4315–-4321. doi: 10.1182/blood-2003-06-2160. [DOI] [PubMed] [Google Scholar]
- 37.Toellner KM, Jenkinson WE, Taylor DR, et al. Low-level hypermutation in T cellindependent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J Exp Med. 2002;195:383–-389. doi: 10.1084/jem.20011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, et al. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173:1158–-1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 39.Wan JH, Trainor KJ, Brisco MJ, et al. Monoclonality in B cell lymphoma detected in paraffin wax embedded sections using the polymerase chain reaction. J Clin Pathol. 1990;43:888–-890. doi: 10.1136/jcp.43.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HY, Karandikar N, Payne D, et al. A 3-way collision tumor of the upper respiratory tract: a composite of 2 immunophenotypically distinct mantle cell lymphomas and a plasmacytoma. Hum Pathol. 2008;39:781–-787. doi: 10.1016/j.humpath.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Weber-Matthiesen K, Winkemann M, Muller-Hermelink A, et al. Simultaneous fluorescence immunophenotyping and interphase cytogenetics: a contribution to the characterization of tumor cells. J Histochem Cytochem. 1992;40:171–-175. doi: 10.1177/40.2.1552161. [DOI] [PubMed] [Google Scholar]
- 42.Weisenburger DD, Armitage JO. Mantle cell lymphoma - an entity comes of age. Blood. 1996;87:4483–-4494. [PubMed] [Google Scholar]
- 43.Weisenburger DD, Kim H, Rappaport H. Mantle-zone lymphoma: a follicular variant of intermediate lymphocytic lymphoma. Cancer. 1982;49:1429–1438. doi: 10.1002/1097-0142(19820401)49:7<1429::aid-cncr2820490720>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.William J, Euler C, Christensen S, et al. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–-2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi M, Ohno T, Miyata E, et al. Analysis of clonal relationship using single-cell polymerase chain reaction in a patient with concomitant mantle cell lymphoma and multiple myeloma. Int J Hematol. 2001;73:383–-385. doi: 10.1007/BF02981966. [DOI] [PubMed] [Google Scholar]
- 46.Young KH, Chan WC, Fu K, et al. Mantle cell lymphoma with plasma cell differentiation. Am J Surg Pathol. 2006;30:954–-961. doi: 10.1097/00000478-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Zupo S, Massara R, Dono M, et al. Apoptosis or plasma cell differentiation of CD38-positive B-chronic lymphocytic leukemia cells induced by cross-linking of surface IgM or IgD. Blood. 2000;95:1199–-1206. [PubMed] [Google Scholar]


