Abstract
Even though VEGF-B is a homologue of the potent angiogenic factor VEGF, its angiogenic activities have been controversial. Intrigued by findings that VEGF-B may also affect neuronal cells, we assessed the neuroprotective and vasculoprotective effects of VEGF-B in the skin, in which vessels and nerves are functionally intertwined. Although VEGF-B and its FLT1 receptor were prominently expressed in dorsal root ganglion (DRG) neurons innervating the hindlimb skin, they were not essential for nerve function or vascularization of the skin. However, primary DRG cultures lacking VEGF-B or FLT1 exhibited increased neuronal stress and were more susceptible to paclitaxel-induced cell death. Concomitantly, mice lacking VEGF-B or a functional FLT1 developed more retrograde degeneration of sensory neurons in a model of distal neuropathy. On the other hand, the addition of the VEGF-B isoform, VEGF-B186, to DRG cultures antagonized neuronal stress, maintained the mitochondrial membrane potential and stimulated neuronal survival. Mice overexpressing VEGF-B186 or FLT1 selectively in neurons were protected against the distal neuropathy, whereas exogenous VEGF-B186, either delivered by gene transfer or as a recombinant factor, was protective by directly affecting sensory neurons and not the surrounding vasculature. Overall, this indicates that VEGF-B, instead of acting as an angiogenic factor, exerts direct neuroprotective effects through FLT1. These findings also suggest a clinically relevant role for VEGF-B in preventing distal neuropathies.—Dhondt, J., Peeraer, E., Verheyen, A., Nuydens, R., Buysschaert, I., Poesen, K., Van Geyte, K., Beerens, M., Shibuya, M., Haigh, J. J., Meert, T., Carmeliet, P., Lambrechts, D. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons.
Keywords: neurovascular link, distal neuropathy
Given its high sequence homology to the other VEGF family members, VEGF-B has traditionally been considered a prototypical angiogenic factor. Functional analyses related to the angiogenic activities of VEGF-B have led, however, to inconsistent results (1–4), raising the question of whether this molecule perhaps has other, more relevant, biological properties. Recent evidence indeed suggests that VEGF-B also regulates fatty acid transporter expression in endothelial cells and thereby determines fatty acid accumulation in peripheral tissues, such as muscle, heart, and brown adipose tissue (5). Others have shown that VEGF-B also affects the central nervous system: in a culture model of Parkinson's disease, VEGF-B was able to improve neuronal survival (6), and in rodent models of motor neuron degeneration and experimental nerve injury, VEGF-B exerted potent protective effects (7, 8). Overall, this suggests that VEGF-B exerts dual effects, affecting both endothelial cells and neurons.
In recent years, it has become increasingly evident that the vascular and nervous systems have many developmental features in common, but only recent evidence suggests that both systems also share pathways for their survival and function (9). A molecule particularly renowned for its dual “neurovascular” activities is the vascular endothelial growth factor (VEGF). In the adult nervous system, VEGF enhances vascular perfusion and ensures endothelial survival, stimulates the release of neurogenic signals from endothelial cells, promotes the survival of multiple types of adult neurons, and protects them against a variety of stress conditions (10). Other examples of prototypical angiogenic factors with dual neurovascular properties include the transforming growth factor-β-1 (TGFβ1; ref. 11) and angiopoietin-1 (12). The discovery that some of these factors are also required for adult neuronal survival is of tremendous interest, since such pleiotropic effects provide attractive opportunities for the treatment of neurodegenerative diseases (11).
The neuronal and vascular activities of these factors are mostly studied independently from each other. To better characterize and assess whether these factors preferentially affect the vascular or nervous system or both, their effects need to be assessed simultaneously in both systems. The sensory nervous system, where the vascular and peripheral nervous systems are intimately associated and functionally intertwined (13), thus represents an ideal model to investigate the neurovascular link. In the case of VEGF, for instance, systemic gene therapy with VEGF restored nerve function in animal models of ischemic and diabetic neuropathy (14, 15) and inhibited pathological changes in rodents with paclitaxel- or cisplatin-induced neuropathies (16). Although the effect of VEGF in these models was initially attributed to its effects on the vasculature, subsequent studies indicated that VEGF also exerts direct protective effects on dorsal root ganglion (DRG) neurons (17). In vivo data later revealed that VEGF also has neuroprotective activities in these models, thus confirming its dual activity.
Given the recent evidence that VEGF-B affects both vessels and neurons, we aimed to better characterize the neurovascular properties of VEGF-B. For reasons mentioned above, we focused on the sensory nervous system. Furthermore, since sensory nerve function can easily be assessed at the functional level in the dermis of the hind paw, the plantar dermis of the hind paw was studied. To carefully assess the neuronal effects of VEGF-B, mice overexpressing VEGF-B or its receptor FLT1 specifically in postnatal neurons were generated, and the effects on development and maintenance of sensory innervation and vascularization were studied under normal conditions, as well as in a model of retrograde degeneration of sensory nerves.
MATERIALS AND METHODS
Animals
Wild-type (WT) rats from a Sprague-Dawley background were used for in vitro experiments and immunohistochemistry; mice were in a FvB background unless specified otherwise. VEGF-B−/− mice were bred in a pure C57/BL6 and FLT1-TK−/− mice in a 50% FvB/C57BL6 background. Transgenic stoplox/loxVEGF-B186 and stoplox/loxFLT1 mice (in a 75% C57BL6 and 25% 129SvJ background) were generated in house, as reported previously (18, 19) and bred to homozygosity. Thy1.2:Cre mice were generated in house by cloning the cre transgene downstream of the mouse Thy1.2 expression cassette and by microinjecting the linearized construct into FvB mouse zygotes. Founders were identified using PCR and expression of the transgene by RT-PCR. stoplox/loxVEGF-B186 or stoplox/loxFLT1 mice, and Thy1.2:Cre mice were subsequently intercrossed, producing VEGF-B186/+ and VEGF-B186/WT, and FLT1+ and FLT1WT offspring, respectively. The local ethical committee, Proefdierencentrum Leuven, approved all experiments.
Subplantar paclitaxel model
Paclitaxel (Taxol; 6 mg/ml in Cremaphor EL solution; Bristol Myers Squibb, New York, NY, USA) was formulated in saline (60 or 75 μg paclitaxel, 10 μl/injection) and injected by the subplantar route into the left hind paw for 4 consecutive days. Injections were given under the skin between the second and third toe and by gently sliding the needle toward the heel. Recombinant mouse VEGF-B186 (10 μg; R&D Systems, Minneapolis, MN, USA) was also delivered by subplantar injection (50 μl vol), 1 h prior to paclitaxel. All animals were pinpricked before they received subplantar paclitaxel injections.
Behavioral analysis
Pinprick test
All animals were allowed to habituate for 30 min in individual testing chambers on a metal mesh floor. A needle was gently applied to the plantar surface of the hind paw, and ≥30 s was allowed between applications. A response to the needle prick (scored as 0) was defined as lifting, shaking, or licking the hind paw. No response was scored as 1. Three scores of each hind paw were taken, and a cumulative score was calculated as the measure of the paclitaxel-induced neuropathy (a score of 0 was a normal response, and a score of 3 was complete irresponsiveness to the pinprick).
Von Frey test
Gradually increased pressure was applied with a mechanical probe (Senselab; Somedic, Hörby, Sweden) perpendicularly into the midplantar surface. The stimulus was continued until the hind paw was withdrawn.
Hargreave's text (paw-flick test)
Radiant heat from underneath (Plantar test; IITC Inc. Life Science, Woodland Hills, CA, USA) was administered to the plantar surface of the hind paw. The heat stimulus was given until the animal withdrew its paw or until the cutoff time (20 s) was reached.
Hot plate test
Mice were placed on a metal surface (maintained at 53±0.3°C), and response latency to the heat stimulus was measured. Mice remained on the plate until they responded with a lick, shake/flutter, or lift of the hind paw, or until the cutoff time (30 s) was reached.
Isolation of DRG neurons
DRGs (all levels, unless explicitly specified) were enzymatically dissociated by incubating them with 0.5% collagenase followed by 0.25% trypsin. Subsequently, ganglia were mechanically dissociated into single cells. To purify the cultures, the cell suspension was placed in dishes coated with FCS. The DRG neurons were then collected and plated into poly-l-lysine-coated 96-well plates or coverslips (10 μg/ml) in neurobasal medium with B27 supplement. In all experiments, neurons were cultured for ≥24 h after the addition of paclitaxel. To examine the neuroprotective effect of VEGF-B186, different concentrations were added 4 h before paclitaxel. For most experiments, 10 nM paclitaxel was used, unless specified differently. The monoclonal rat-anti-mouse antibody MF1 against mouse FLT1 was kindly provided by Dr. D. Hicklin (ImClone Systems, Branchburg, NJ, USA).
Immunohistochemistry for ATF3
DRG neurons were fixed for 10 min using 0.5% Triton X-100 and 0.5% glutaraldehyde dissolved in PHEM buffer. Subsequently, the cells were washed in PHEM buffer, after which 0.5% Triton X-100 was added for 30 min. After washing, DRG neurons were incubated for 10 min with 1 mg/ml NaBH4 dissolved in PHEM. Subsequently, primary antibodies, preincubated in PBS containing 0.1% BSA and 5% goat serum, were added. Primary antibodies were a polyclonal anti-ATF3 antibody (1:800; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a monoclonal anti-neurofilament antibody (SMI32, 1:1000; Covance, Princeton, NJ, USA). The cells were washed and incubated with secondary antibodies (Alexa Fluor 555 goat-anti-rabbit-IgG and Alexa Fluor 488 goat-anti-mouse-IgG, 1:200; Invitrogen, Carlsbad, CA, USA) for 2 h. DRG neurons were distinguished from other nonneuronal cells by their SMI32+ signal. The percentage of ATF3+ neurons was determined using fluorescence microscopy. All data shown were derived from experiments where ≥100 neurons/well were counted. For survival experiments, surviving neurons were recognized on the basis of their typical shape, the integrity of the membrane, and the formation of vesicles.
Histology
Unfixed L4–L5 DRGs were embedded in tissue-freezing medium (Jung, Nussloch, Germany), and 10-μm serial sections were stained. DRGs were used in immunohistochemical stainings with antibodies against VEGF-B (AF-590, 1:20; R&D Systems), FLT1 (sc-316 and sc-9029, 1:50; Santa Cruz Biotechnology), CD31 (557355, 1:500; BD Pharmingen, San Jose, CA, USA), GFAP (G3893, 1:400; Sigma Aldrich, St. Louis, MO, USA), PGP9.5 (AB1761, 1:1000; Chemicon, Billerica, MA, USA) or NEUN (AB9337, 1:1000; Chemicon). All tissues were subsequently incubated with fluorescently conjugated secondary antibodies (Alexa 488 or 546; Invitrogen) or with biotin-labeled IgGs followed by amplification with the signal amplification system (streptavidin-HRP-Cy3/fluorescein; Perkin Elmer, Waltham, MA, USA) for VEGF-B and FLT1.
To stain the epidermis of the hindlimb skin, epidermal and dermal layers were dissociated according to a procedure adapted from Tschachler et al. (20). Briefly, skin tissue was incubated with 2.4 U/ml dispase. After 45 min at 37°C, the epidermis was removed from the underlying dermis. Whole preparations were then fixed for 2 h with Zamboni's fixative (0.2% saturated picric acid, 4% paraformaldehyde and 0.1 M phosphate buffer; pH 7.4). Tissues were incubated with antibodies directed against CD31 or PGP9.5 and subsequently with biotin-labeled secondary IgGs, followed by amplification with the signal amplification system (Perkin Elmer). Blood vessel density and axonal density were assessed by quantification of CD31+ and PGP9.5+ area/total area using a LSM510 confocal microscope with KS300 image analysis software (Zeiss, Oberkochen, Germany).
Mitochondrial membrane potential (Ψm) measurements
Mouse DRG neurons were cultured overnight in 96-well plates. The next day, 4 h after preincubation with VEGF-B186 or PBS, neurons were loaded with 2 μM JC1 (Invitrogen) in PBS (containing Mg2+, Ca2+, and glucose; 1 mg/ml) for 30 min at 37°C. In healthy cells, JC1 is taken up by the mitochondria. When a critical concentration is reached, J aggregates, which are fluorescent red, are formed. Loss of Ψm causes the dye to diffuse to the cytoplasm, where it exists in a monomeric, green fluorescent, form. After washing, Ψm was measured with a confocal microscope (13 images in a 15-s interval): paclitaxel (10 μM, diluted in PBS), or PBS was added after 60 s and FCCP (10 μM), as a positive control for mitochondrial depolarization, after an additional 90 s. Red to green ratios were calculated and normalized to PBS-treated cells.
RNA extraction, cDNA preparation, and quantitative RT-PCR
The RNeasy kit (Qiagen, Hilden, Germany) was used for RNA extraction from primary DRG cultures, whereas TRIzol (Invitrogen) was used for RNA extraction from tissues, followed by purification with the RNeasy cleanup protocol. Approximately 1 μg of RNA was transcribed to cDNA using the QuantiTect reverse transcription kit (Qiagen). Gene expression was analyzed using the 7500 Fast Real-Time PCR system and normalized to β-actin expression levels. Sequences of home-designed primers and probes and TaqMan gene expression assay IDs (Applied Biosystems, Carlsbad, CA, USA) were as follows: β-actin: Mm00607939_s1; Atf3: Mm00476033_m1; Flt1: Mm01210866_m1; Vegfb: forward TGCCATGGATAGACGTTTATGC, reverse TGCTCAGAGGCACCACCAC, probe CGTGCCACATGCCAGCCCA; Vegfb186: forward TCTGTTCCGGGCTGGGA, reverse AGTGGGATGGATGATGTCAGCT, probe TCTACCCCGGGAGCATCCTCCC; Vegfb167: forward GCCAATGTGAATGCGACCAA, reverse TGCAAGGCGGGCAGA, probe TGTGAAGCCAGACAGCCCCAGGATC; Plgf: forward TTCAGTCCGTCCTGTGTCCTT, reverse GCACACAGTGCAGACCTTCA, probe ACCACAGCAGCCACTACAGCGACTCA; Gfap: forward GGAGGTCCGCTTCCTGGAA, reverse GGCTCGAAGCTGGTTCAGTT, probe AGCTGCCAGCGCCTTGTTTTGCT; Cd31 (Pecam1): forward TACTGCAGGCATCGGCAAA, reverse GCATTTCGCACACCTGGAT, probe TGGTCAAGAGAAGCGGCCTGGTACC; cre: forward TGGAAAATGCTTCAGTCCGTTT, reverse CATTTCCGGTTATTCAACTTGCA, probe CATGCCGCCCACGACCGG; eGFP: forward CGAAGGAGACGGGATCTCCT, reverse AGGCGGCATTCTCAGCG, probe CCGCTGGCACTCGTCAATATCGAAA.
Statistics
All data are shown as means ± se. To calculate differences between groups, unpaired Student's t tests were performed, considering equal variances. Curves of the paclitaxel-induced neuropathy experiments show means ± se, and overall differences between the curves were assessed by calculating the area under the curve (AUC) for every mouse and the statistical difference between these AUC values using a standard Student's t test. A χ2 test was used to calculate statistical differences on individual days.
RESULTS
Expression of VEGF-B in the sensory nervous system
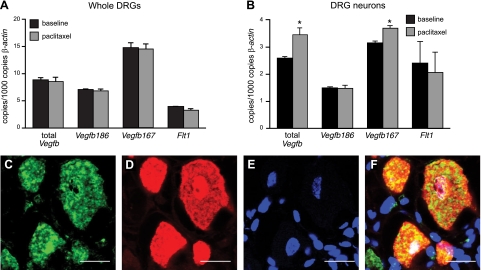
We first assessed whether VEGF-B, under normal conditions, is implicated in the sensory nervous system that innervates the hind paw. For VEGF-B to be involved, it should be expressed in DRGs descending from the lumbar spinal cord at the L4 to L5 level. When analyzing cDNA isolated from freshly dissected L4 and L5 DRGs, Vegfb expression was clearly detectable (Fig. 1A). Obvious Vegfb expression was also detected in cDNA isolated from the corresponding primary neuronal DRG cultures (Fig. 1B). The expression of VEGF-B in DRG neurons was confirmed using double-immunohistochemistry for VEGF-B and the neuronal marker NEUN on whole DRGs (Fig. 1C–F) and on fixed primary DRG neurons (Supplemental Fig. S1A–C). VEGF-B was, however, not detectable in blood vessels or glial cells, as revealed by double immunohistochemistry for astrocyte and vessel-specific markers (GFAP and CD105, respectively). We also failed to detect clear expression of VEGF-B in whole-mount skin preparations of the hind paw, indicating that VEGF-B expression in the paw was below detection levels.
Figure 1.
VEGF-B is expressed in the sensory nervous system. A, B) Real-time PCR measurements for Vegfb and Flt1 on cDNA preparations from whole L4–L5 DRGs and primary cultures of L4–L5 DRG neurons show that Vegfb and Flt1 are expressed in whole DRGs (A) and DRG neurons (B). No differences between normal and paclitaxel-challenged paws were noticed in whole DRGs (n=4–5/group; P=NS), whereas total Vegfb and Vegfb167 levels were slightly increased in primary DRG neurons treated with paclitaxel (n=4/group; P<0.05). Expression levels are represented relative to those of β-actin. C–F) VEGF-B expression is detected on DRG neurons, as revealed by double immunostaining (F) on cryounfixed sections for endogenous VEGF-B (C), the neuronal marker NEUN (D), and the nuclear dye DAPI (E). Scale bars = 20 μm.
VEGF-B is dispensable for sensory nerve function
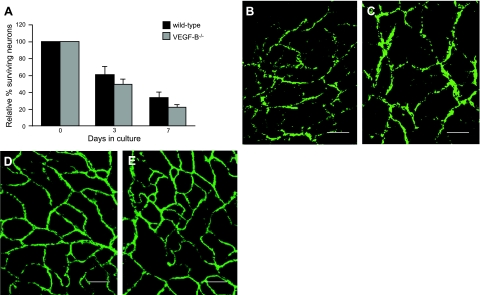
We then analyzed whether VEGF-B regulates sensory neuron homeostasis. Despite prominent expression of VEGF-B in adult DRG neurons, primary neurons isolated from VEGF-B−/− mice exhibited a normal survival (percentage of surviving DRG neurons on d 3: 61.2±9.5% in WT vs. 50.1±6.1% in VEGF-B−/− cultures; P=NS, Fig. 2A). VEGF-B was also dispensable for sensory neuron survival during development, since the density of sensory axons in the dermis from VEGF-B−/− hind paws did not differ from that of WT mice (axonal densities after PGP9.5 staining: 12.9±3.3% in WT vs. 12.6±3.6% in VEGF-B−/− mice; P=NS; Fig. 2B, C). Consistent with previous reports, we confirmed that VEGF-B−/− mice were healthy, fertile, and displayed normal longevity. Additional analyses also revealed that VEGF-B−/− mice had no sensory abnormalities when tested in a spectrum of functional tests, such as the von Frey aesthesiometer for mechanic pressure, the paw flick, and hot plate test for thermal sensitivity (Supplemental Fig. S1D–F). Overall, this indicates that endogenous VEGF-B produced by sensory neurons is not critical for their survival or function.
Figure 2.
VEGF-B is dispensable for the sensory nervous system and vasculature. A) Number of surviving primary DRG neurons isolated from WT and VEGF-B−/− mice is not different after 0, 3, and 7 d in culture (n=7 wells from 3 mice/genotype; P=NS). B, C) Whole-mount preparations of the hind-paw dermis from WT (B) and VEGF-B−/− (C) mice stained for the pan-axonal PGP9.5 marker reveal that the density of innervating sensory nerve axons does not differ (n=3–4/group; P=NS). D, E) Staining for the endothelial CD31 marker reveals that there are no differences in the vascular density of the paws of WT (D) and VEGF-B−/− (E) mice in baseline conditions (n=5–6/group; P=NS). Scale bars = 50 μm.
VEGF-B deficiency does not affect the vasculature in the hind paw
Since the nervous and vascular system in the hindlimb skin are intimately associated and functionally interacting, we also analyzed the vasculature in the dermis of VEGF-B−/− hind paws. Tail vein injection of a fluorescent dextran (i.e., Oregon green 488 dextran, 70 kDa), followed by microscopic imaging of the hind-paw vasculature, revealed that the vessels were perfused to a similar degree (perfused vessel area: 7.50±0.56% in VEGF-B−/− vs. 9.46±1.02% in WT mice; branching points of perfused vessels: 28.45±2.04 in VEGF-B−/− vs. 30.40±1.42 in WT mice; P=NS). This was confirmed using whole-mount immunostaining for the vessel marker CD31 (CD31+ area: 12.18±1.26% in VEGF-B−/− vs. 12.09±1.12% in WT mice; P=NS; Fig. 2D, E). Overall, this indicates that VEGF-B is dispensable for the development and function of the sensory nervous and vascular system, at least under healthy conditions.
VEGF-B-deficient DRGs display more neuronal stress
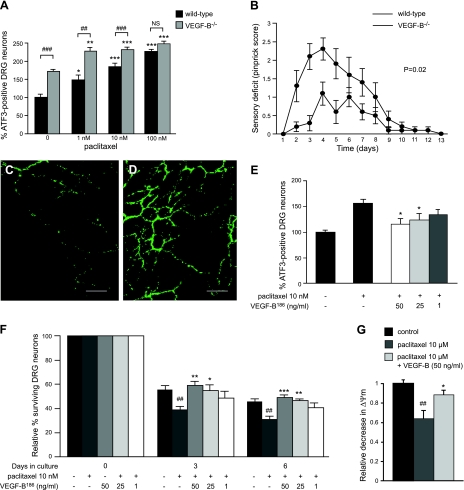
Because it is possible that deficiency for VEGF-B increases neuronal stress without affecting the survival or function of DRG neurons, we generated primary DRG cultures from WT and VEGF-B−/− mice and quantified, as a measure of neuronal stress, the expression of the activating transcription factor 3 (ATF3; refs. 21, 22). When primary DRG cultures isolated from VEGF-B−/− mice were cultured under standard conditions, significantly more ATF3-immunopositive (ATF3+) neurons were detected than in WT cultures (P<0.001; Fig. 3A).
Figure 3.
VEGF-B deficiency increases susceptibility to neuronal stress. A) Percentage of ATF3+ DRG neurons is 73% higher in DRG cultures lacking VEGF-B compared to WT DRG neurons under normal conditions. When exposed to different concentrations of paclitaxel (i.e., 1, 10, and 100 nM), WT cultures exhibit maximal ATF3 levels with 100 nM paclitaxel. In contrast, VEGF-B−/− cultures already exhibit a maximal ATF3 response when exposed to 1 nM paclitaxel (n=6 wells from 3 mice/genotype). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ##P < 0.01, ###P < 0.001 vs. WT. B) Mice lacking VEGF-B develop a more pronounced sensory deficit in a model of retrograde degeneration of sensory nerves compared to WT mice (n=10/group; P=0.02). Mean ± se pinprick scores are shown to visualize the sensory deficit in time. C, D) Quantification of PGP9.5+ axons in skin of hind paws from VEGF-B−/− mice (C) reveals fewer axons on the first day after paclitaxel injections compared to WT paws (D; n=4–8/group; P=0.023). Scale bars = 50 μm. E) Addition of 3 different VEGF-B186 concentrations (1, 25, and 50 ng/ml VEGF-B186) dose-dependently protects primary DRG neurons from neuronal stress induced by 10 nM paclitaxel (n=6 wells from 3 rats/condition). *P < 0.05 vs. control. F) VEGF-B186 protects DRG cultures from cell death induced by 1 μM paclitaxel (n=6 wells from 3 rats/condition). *P < 0.05, **P < 0.01, ***P < 0.001 vs. 1 μM paclitaxel; ##P < 0.01 vs. control. G) VEGF-B186 protects DRG neurons from paclitaxel-induced loss of Ψm: 10 μM paclitaxel reduces Ψm compared to PBS administration (n=5/group). This decrease was counteracted by pretreatment with 50 ng/ml VEGF-B186 (n=6/group). *P < 0.05 vs. 1 μM paclitaxel; #P < 0.05 vs. control.
In the next set of experiments, we assessed whether primary DRG neurons from VEGF-B−/− mice were also more susceptible to neuronal stress when challenged with paclitaxel. Low concentrations of paclitaxel (i.e., 1 to 100 nM) were used, as such concentrations do not affect the survival of primary DRG neurons (Supplemental Fig. S2A). The number of ATF3+ cells increased gradually with increasing paclitaxel concentrations, reaching a maximal level of 226.0 ± 6.9% on exposure to 100 nM paclitaxel (Fig. 3A). In contrast, ATF3 levels already reached a maximum when VEGF-B−/− DRGs were exposed to 1 nM paclitaxel (227.0±11.2% in VEGF-B−/− vs. 148.3±13.2% in WT DRGs receiving 1 nM paclitaxel; P<0.01). The addition of higher concentrations of paclitaxel to VEGF-B−/− cultures (i.e., 10 or 100 nM) did not affect survival of DRG neurons (Supplemental Fig. S2B). Overall, this demonstrates that VEGF-B deficiency renders primary DRG neurons more susceptible to neuronal stress cultivated under both baseline and stressed conditions.
Paclitaxel affects vessels and nerves in the hind paw
To additionally investigate the activities of VEGF-B under stressed conditions, we used an in vivo model of paclitaxel-induced sensory nerve degeneration in mice. In this model, cumulative injections of paclitaxel were given by the subplantar route into the left hind paw during 4 consecutive days, leading to retrograde degeneration of intraepidermal nerve fibers and the development of a sensory deficit, from which the mice gradually recovered once paclitaxel injections were arrested (23). To reliably and functionally assess this sensory deficit, mice were subjected to the pinprick test. The plantar surface of the hind paw was punctured with a sharp pin, and the withdrawal response of the paw was monitored: a response to the pinprick was scored as 0, no response as 1. When quantifying the extent to which axons are affected in this model using mice expressing the yellow-fluorescent protein (YFP) under control of the neuronal Thy1.2 promoter, the degree of retrograde degeneration after paclitaxel exposure, as assessed by quantifying YFP+ axons, correlated strongly with the pinprick score (Supplemental Fig. S2C–F), indicating that it was a reliable readout of the model.
Since VEGF-B can affect both the vascular and nervous system, we first characterized the effect of paclitaxel on vessels and axons in the hind paw. On the first day after the fourth paclitaxel injection, vessels were perfused to a smaller extent (perfused vessel area: 9.9±2.1% vs. 4.6±0.7% in saline- vs. paclitaxel-injected paws; P<0.05) and fewer branching points of perfused vessels were detectable (27.2±2.9 vs. 7.7±0.8 in saline- vs. paclitaxel-injected paws; P<0.05). Quantification of the sensory nerves further revealed that significantly less PGP9.5+ axons was detectable in the hind paw after paclitaxel (PGP9.5+ area: 9.7±2.2 vs. 6.5±0.6% in saline- vs. paclitaxel-injected paws; P<0.01). At the level of the DRGs innervating the paws (i.e., L4–L5), we failed to detect neuronal cell death using the Fluoro-Jade marker after paclitaxel. A 10-fold increase in ATF3 (but not GFAP) expression level was, however, detectable in DRGs (Supplemental Fig. S3A), indicating that the neurons in these DRGs suffered from increased neuronal stress, similar to the primary DRG cultures challenged with low concentrations of paclitaxel (i.e., 10 nM).
VEGF-B−/− mice are more susceptible to retrograde degeneration of nerves
We then challenged mice lacking VEGF-B with paclitaxel in the left hind paw. A low dose of paclitaxel (i.e., 60 μg) was chosen because it fails to induce a complete sensory deficit in WT mice and allows aggravated sensory phenotypes to be more easily detected. Using the pinprick test as a readout, VEGF-B−/− mice developed a more pronounced sensory deficit than their corresponding WT littermates (P=0.02; Fig. 3B). For instance, on the first day after the fourth paclitaxel injection, 70% of the WT mice responded, compared to only 30% of the VEGF-B−/− mice (P<0.05 by χ2 analysis). Similar results were obtained when VEGF-B−/− mice were challenged with a high dose of paclitaxel (i.e., 75 μg; Supplemental Fig. S3B). When analyzing the number of PGP9.5+ axons in the hind paw on the first day after the fourth (low-dose) paclitaxel injection, fewer sensory axons were present in VEGF-B−/− mice (P=0.023; Fig. 3C, D). In contrast, the perfused vessel area did not differ between WT and VEGF-B−/− mice (2.8±0.4 and 3.0±0.6%; P=NS). In addition, we could not detect differences in the number of vascular branching points (12.1±1.2 in VEGF-B−/− vs. 9.4±1.6 in WT paws; P=NS). Thus, deficiency for VEGF-B specifically aggravates the distal degeneration of sensory nerve endings induced by paclitaxel.
VEGF-B is protective for DRG neurons
Next, we analyzed whether the aggravated phenotypes in these mice might be attributable to reduced neuroprotective activities of VEGF-B. To test this hypothesis, primary DRG cultures were isolated, and the potency of recombinant mouse VEGF-B to protect DRG cultures from neuronal stress and cell death was investigated. The more diffusible VEGF-B186 isoform was used for these experiments, as the shorter VEGF-B167 isoform might be trapped in the extracellular matrix via its heparin-binding domain. We first investigated whether VEGF-B186 was capable of reducing neuronal stress induced by paclitaxel in primary DRG cultures. Neuronal stress induced by low paclitaxel concentrations (10 nM) was, indeed, reduced by VEGF-B186 in a dose-responsive fashion (r2=0.99 after a sigmoidal fit; Fig. 3E). For instance, 50 ng/ml VEGF-B186 reduced ATF3 immunoreactivity from 156.4 ± 3.1 to 115.3 ± 4.6% (P<0.05).
In a second set of experiments, we tested whether VEGF-B186 was capable of protecting DRG neurons against paclitaxel-induced cell death. Primary DRG cultures were challenged with a high paclitaxel dose (1 μM) known to induce cell death of DRG neurons. After 6 d, untreated cultures contained 45.4 ± 2.7% surviving neurons, and cultures treated with paclitaxel alone or cultures receiving paclitaxel and 50 ng/ml VEGF-B186 contained 30.7 ± 3.1% (P<0.001 vs. untreated cultures) and 49.0 ± 2.5% neurons (P<0.001 vs. paclitaxel-treated cultures; Fig. 3F).
We next also assessed whether VEGF-B counteracts the paclitaxel-induced decrease in the mitochondrial membrane potential Ψm, which is believed to be the earliest event in the process of cell death (24). We applied the cationic dye JC1 to DRG cultures and used two-color ratiometric confocal microscopy to monitor changes in Ψm (ΔΨm). In healthy cells with a high Ψm, JC1 is taken up by the mitochondria and forms J aggregates that exhibit red fluorescence. However, as Ψm drops, JC1 diffuses to the cytoplasm, where it exists in a monomeric green fluorescent form. As expected, paclitaxel administration (10 μM) caused a 36% reduction in Ψm compared to PBS administration (P<0.05; Fig. 3G). This decrease was counteracted by pretreatment with VEGF-B, as neurons incubated with 50 ng/ml VEGF-B186 exhibited a higher Ψm (P<0.05; Fig. 3G). Overall, these data clearly indicate that VEGF-B prevents paclitaxel-induced cell death and exerts potent neuroprotective effects in DRG neurons.
Expression of FLT1 in the sensory nervous system
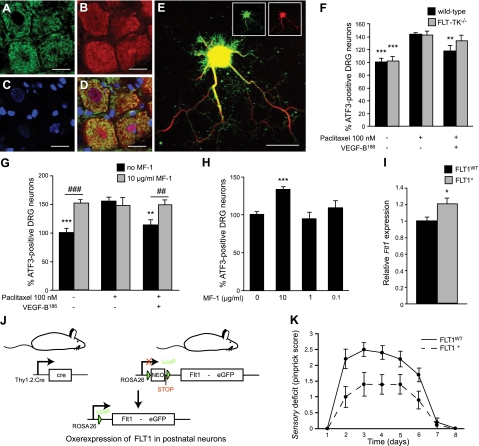
We then analyzed whether the selective receptor for VEGF-B, i.e., FLT1, mediates the neuroprotective effects of VEGF-B. For FLT1 to exert these effects, it should be expressed on DRG neurons. Analysis of Flt1 transcript levels revealed that Flt1 was indeed expressed in whole L4–L5 DRG cultures, as well as in primary neuronal cultures from corresponding DRGs (Fig. 1A, B). Double immunohistochemistry for FLT1 on L4–L5 DRG sections, using two different antibodies directed against the C- or N-terminal extracellular domain of FLT1 and the pan-neuronal marker NEUN, also revealed that FLT1 was expressed in DRG neurons (Fig. 4A–D). Weak expression of FLT1 was also seen in blood vessels from DRGs, as revealed by double immunohistochemistry for FLT1 and the vessel-specific marker CD31 (not shown). On whole-mount preparations of the hind-paw skin, immunohistochemistry revealed FLT1 expression on sensory nerve endings (Supplemental Fig. S4A). Expression of FLT1 (and VEGF-B) was also detectable on cultured primary DRG neurons and axons after double-immunostaining with antibodies against FLT1 (or VEGF-B) and 2H3 neurofilament (Fig. 4E for FLT1, Supplemental Fig. S1A–C for VEGF-B). RT-PCR analysis on whole DRGs or primary DRG cultures revealed that the Flt1 expression levels did not change after exposure to paclitaxel (Fig. 1A, B). Moreover, in whole DRGs, the expression of Plgf, another FLT1-selective ligand, was unaltered after treatment with paclitaxel (Supplemental Fig. S4B). On the other hand, expression of total Vegfb and Vegfb167, but not that of Vegfb186, was increased after paclitaxel in primary DRG cultures (Fig. 1B). Overall, these data indicate that the molecular players necessary for VEGF-B to exert direct neuroprotective effects are present in the sensory nervous system.
Figure 4.
FLT1 is a functional VEGF-B receptor in the nervous system. A–D) FLT1 expression is detected on DRG neurons, as revealed by double immunostaining (D) on cryounfixed sections for endogenous Flt1 (sc-9029 antibody; A) and the neuronal marker NEUN (B). Nuclear dye DAPI (C) colocalizes with NEUN and FLT1 staining. E) FLT1 expression on DRG neurites was confirmed in vitro using double immunostaining for FLT1 (sc-316, green) and the neurofilament antibody 2H3 (red). F) Neuroprotective effects of VEGF-B186 on DRG neurons depend on FLT1 tyrosine-kinase activity: recombinant VEGF-B186 (50 ng/ml) is capable of reducing ATF3 immunoreactivity in primary DRG neurons from WT mice (P<0.01), but not in primary DRG cultures isolated from FLT-TK−/− mice (n=12 wells from 3 mice/genotype; P=NS). **P < 0.01, ***P < 0.001 vs. 100 nM paclitaxel. G) MF1, a neutralizing FLT1 antibody, inhibits the neuroprotective activity of VEGF-B186 on primary DRG neurons: VEGF-B186 reduces 100 nM paclitaxel-induced ATF3 immunoreactivity from 154.9 ± 7.7 to 113.3 ± 9.5%, whereas the addition of MF1 blocks this effect by increasing ATF3 immunoreactivity again to 148.7 ± 9.0% even in the presence of 50 ng/ml VEGF-B186 (n=6 wells from 3 rats). **P < 0.01, ***P < 0.001 vs. 100 nM paclitaxel; #P < 0.05, ###P < 0.001 as indicated. H) Addition of 10 μg/ml FLT1-neutralizing antibody MF1 increases neuronal stress in primary DRG cultures. ATF3 immunoreactivity: 133.1 ± 4.4 vs. 100.0 ± 4.0% in untreated cultures. Lower doses of MF1 did not exhibit an effect. ***P < 0.001 vs. control. I) Strategy for the generation of a transgenic mouse line, in which FLT1 is selectively overexpressed in postnatal neurons. The Flt1 cDNA is cloned downstream of a floxed STOP sequence and upstream of an IRES sequence followed by an enhanced green fluorescent protein (eGFP) marker. Transgenic mice expressing this construct under control of the endogenous ROSA promoter are subsequently generated (stoplox/loxFLT1 mice). In the absence of Cre, ROSA promoter activity is blocked by an in-frame STOP sequence resulting in no overexpression of Flt1 (FLT1WT mice). In the presence of the Cre protein, which is expressed in postnatal neurons under control of the Thy1.2 promoter, the STOP sequence is excised, resulting in increased expression of Flt1 (FLT1+ mice). *P < 0.05. J) Flt1 expression levels are 20% higher in whole DRGs from FLT1+ mice compared to FLT1WT mice (n=8–10/group; P<0.05). K) Neuronal overexpression of FLT1 protects mice from developing a severe sensory deficit (n=10/group; P<0.01). Mean ± se pinprick scores are shown to visualize the sensory deficit in time. Scale bars = 20 μm.
VEGF-B exerts its neuroprotective effects through FLT1
To investigate whether the neuroprotective effects of VEGF-B186 are mediated through FLT1, we used mice expressing a tyrosine-kinase dead FLT1 (FLT-TK−/− mice). Expression levels of Vegfb in FLT-TK−/− mice were comparable to those in WT mice (Supplemental Fig. S4C). Primary DRG cultures from WT and FLT-TK−/− mice were prepared, and the capacity of VEGF-B186 to reduce paclitaxel-induced ATF3 immunoreactivity in both genotypes was compared. As expected, ATF3 immunoreactivity in WT cultures exposed to paclitaxel was reduced by the addition of VEGF-B186: 143 ± 3.1% without VEGF-B186 vs. 117.2 ± 6.7% with 50 ng/ml VEGF-B186 (P<0.01; Fig. 4F). By contrast, VEGF-B186 failed to protect FLT-TK−/− DRG cultures from paclitaxel-induced neuronal stress: 142.2 ± 6.3% without VEGF-B186 vs. 133.9 ± 6.0% with 50 ng/ml VEGF-B186 (P=NS; Fig. 4F). Moreover, coadministration of a neutralizing FLT1 antibody (MF1, ImClone) abolished the direct neuroprotective effects of VEGF-B186 on WT DRG cultures (Fig. 4G). Intriguingly, as observed for VEGF-B−/− DRG cultures, the addition of this FLT1-neutralizing antibody also increased neuronal stress in WT DRG cultures grown under normal conditions (i.e., without paclitaxel; Fig. 4H). Likewise, as observed for VEGF-B−/− mice challenged by the subplantar route with paclitaxel, FLT-TK−/− mice developed a more severe sensory deficit than WT mice when challenged with a high dose of paclitaxel (P=0.018). Overall, these findings suggest that the FLT1 receptor, indeed, transmits the neuroprotective signals from VEGF-B on sensory neurons.
Neuronal overexpression of FLT1 or VEGF-B186 protects sensory nerves
To explore whether FLT1 expressed on DRG neurons and the sensory nerves can, indeed, protect against the retrograde degeneration of sensory nerves, transgenic mice specifically overexpressing FLT1 in neurons were generated (Fig. 4I). First, stoplox/loxFLT1 knock-in mice were generated by inserting a floxed STOP sequence upstream of the Flt1 cDNA into the ROSA26 locus of embryonic stem cells (25). To subsequently drive FLT1 expression from the ubiquitously active ROSA26 locus, we crossed mice expressing this construct (i.e., stoplox/loxFLT1) with mice expressing the Cre recombinase. In particular, since we wanted to overexpress FLT1 in neurons, stoplox/loxFLT1 mice were intercrossed with mice expressing Cre postnatally under control of the neuronal Thy1.2 promoter (i.e., Thy1.2:Cre mice). To achieve overexpression of FLT1 within physiological ranges, heterozygous stoplox/wtFLT1 mice expressing the Flt1 cDNA from only one ROSA locus were used (i.e., stoplox/wtFLT1xThy1.2:Cre+ mice, further called FLT1+ mice), as well as littermate control mice, in which the STOP sequence was not excised, and which therefore expressed normal levels of FLT1 (i.e., stoplox/wtFLT1xThy1.2:Cre−; hereafter referred to as FLT1WT mice). As expected, FLT1+ mice appeared healthy and fertile, and expressed 20% more Flt1 in whole DRGs compared to FLT1WT mice (P<0.05; Fig. 4J). In contrast, expression levels of Vegfb were not altered (Supplemental Fig. S4D). To subsequently assess the protective role of FLT1 overexpression in neurons, paclitaxel was administered into the hind paw of FLT1+ and FLT1WT littermates. A high dose of paclitaxel was chosen, because it induces a sensory deficit in almost all WT mice and is, therefore, suitable to study the protective effects of FLT1. Neuronal overexpression of FLT1 significantly protected against the paclitaxel-induced neuropathy (P<0.005; Fig. 4K). For instance, 4 d after the fourth paclitaxel injection, 78% of the FLT1+ mice reacted to a pinprick stimulus, whereas only 20% of the FLT1WT mice were still responsive (P=0.025; χ2). Thus, overexpression of FLT1 in sensory neurons directly protects the sensory nerves from a distal neuropathy.
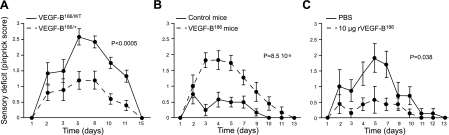
In a next step, we assessed whether neuronal overexpression of VEGF-B186 was also capable of protecting against paclitaxel-induced neuropathies in vivo. To test this hypothesis, we generated VEGF-B186/+ and VEGF-B186/WT mice, as described for the FLT1+ mice. RT-PCR analysis on whole DRG and primary neuronal DRG cultures from VEGF-B186/+ mice confirmed that Vegfb186 was 1.4- and 3.8-fold overexpressed compared to VEGF-B186/WT mice (Supplemental Fig. S4E, F). VEGF-B186/+ mice were healthy and fertile, and exhibited a normal pattern of sensory axons in their hind paw, as revealed by whole-mount PGP9.5 immunostaining. The VEGF-B186/+ mice also exhibited a normal vasculature, as the percentage of CD31+ area in the skin of the hind paw did not differ, thereby confirming our previous findings that VEGF-B is dispensable for normal sensory nerve function. Neuronal overexpression of VEGF-B186 significantly protected against a high dose of paclitaxel in the hind paw (P<0.0005; Fig. 5A).
Figure 5.
Exogeneous VEGF-B is neuroprotective without affecting the vasculature. A) Mice overexpressing VEGF-B186 (VEGF-B186/+ mice) develop a less pronounced retrograde degeneration in a model of paclitaxel-induced distal neuropathy compared to VEGF-B186/WT mice (n=10–12/group; P<0.0005). B) Mice receiving gene delivery of a DNA plasmid containing the Vegfb186 cDNA sequence under control of the rabbit β-actin promoter (VEGF-B186 mice) develop almost no sensory deficit compared to mice to which an empty plasmid was delivered (n=11–12/group; P=8.5×10−5). C) Mice receiving 10 μg recombinant VEGF-B186 develop a less pronounced sensory deficit compared to PBS-injected control mice (n=7/group; P=0.038). Mean ± se pinprick scores are shown to visualize the sensory deficit in time.
Exogeneous VEGF-B prevents retrograde degeneration of sensory nerves
Since neuronal overexpression of FLT1 or VEGF-B186 protected against the retrograde degeneration of sensory nerves, we assessed whether exogeneous delivery of recombinant VEGF-B186 has similar effects. Electric pulse-mediated gene delivery of a DNA plasmid in the hind paw was used to locally deliver Vegfb186. After having confirmed that a plasmid containing the Vegfb186 cDNA under control of the rabbit β-actin promoter (mVEGF-B186-pcDNA3) was capable of driving VEGF-B186 expression when transiently transfected in HEK293 cells (not shown), we assessed whether gene delivery of 15 μg of this plasmid in the hind paw also resulted in increased VEGF-B186 expression. We found that VEGF-B186 expression levels measured at 4 wk after the delivery were still increased by 73% (327 pg/ml VEGF-B186 vs. 189 pg/ml in the paws of control mice; P=0.046). As expected, gene delivery of VEGF-B186 was protective in the paclitaxel-induced neuropathy model and was even able to completely alleviate the neuropathic effects of paclitaxel (P=8.5×10−5 vs. mice to which an empty control plasmid was given; Fig. 5B). One day after the fourth paclitaxel injection, 100% of the mice receiving VEGF-B186 gene delivery still responded to a pinprick challenge, whereas only 30% of the mice receiving an empty plasmid responded (P<0.001; χ2). Thus, local increase of VEGF-B186 levels achieved by gene therapy prevented the retrograde degeneration of sensory axons.
Exogeneous VEGF-B is neuroprotective without affecting the vasculature
In a final set of experiments, we assessed whether mice receiving 10 μg VEGF-B186 protein by the subplantar route were also protected against a distal neuropathy. When monitoring the sensory deficit over time, VEGF-B186 had a clear inhibitory effect on the development of the neuropathy (P=0.038; Fig. 5C), suggesting that VEGF-B186 induces resistance of sensory axons to degeneration. For instance, one day after the 4th paclitaxel injection, 86% of the mice receiving VEGF-B186 still responded to a pinprick challenge compared to only 29% of PBS-treated mice (P<0.05; χ2). To show that this therapeutic effect was not due to vasculoprotective effects of VEGF-B, we quantified the number of perfused vessels in mice receiving paclitaxel and recombinant VEGF-B186 after the fourth paclitaxel injection. A similar number of vessels was perfused in VEGF-B186 vs. PBS-treated mice (perfused vessel area: 5.8±0.4 vs. 4.7±1.2% in VEGF-B186- vs. PBS-injected mice; P=NS) and a comparable number of vessel-branching points were detected (branching points of perfused vessels: 8.8±0.7 vs. 7.7±1.8 in VEGF-B186- vs. PBS-injected mice; P=NS). Notably, a similar concentration of recombinant VEGF, which was used as a positive control to induce angiogenic effects, had a clear vessel-promoting effect. For instance, the perfused vessel area in VEGF-treated mice was 2.6-fold higher, and the number of branching points was 1.8-fold increased relative to untreated mice. Altogether, these findings indicate that VEGF-B offers therapeutic perspectives for the prevention and treatment of distal neuropathies by directly affecting the sensory nerves.
DISCUSSION
Even though VEGF-B has been considered a putative angiogenic agent, it might have originated as a neuronal factor during evolution. Indeed, a genomic survey of the nematode Caenorhabditis elegans, which is devoid of a vascular system, predicts a single ancestral VEGF/PDGF ligand and 4 genes resembling the VEGF receptors, named ver-1 to ver-4 (26). Intriguingly, these 4 receptors are expressed in specialized cells of neural origin, such as support glial cells of amphid and phasmid neurons, as well as chemosensorial neurons (26). Vessels that developed later in evolution than nerves thus seem to have coopted the ancestral VEGF/PDGF family as a critical regulator of their formation. Since VEGF family members originated from a single ancestral ligand, it is possible that VEGF-B has kept its original neuronal function, and still exerts neuroprotective, rather than angiogenic, activities in higher organisms (11).
Indeed, a recent study in mice confirmed that VEGF-B down-regulates, through tyrosine-kinase activity of FLT1, cell death and cell death-related pathways in different injury models of the nervous system, such as the optic nerve crush and middle cerebral artery occlusion models (8, 27, 28). Intracerebroventricular delivery of VEGF-B also induces neurogenesis (27), and protective effects of VEGF-B are also observed in studies with motor neurons expressing the mutant SOD1G93A protein (7). Our present study provides unprecedented evidence that VEGF-B also exerts neuroprotective effects in the sensory nervous system. Interestingly, we found that the effects of VEGF-B were restricted to pathological conditions. Indeed, in healthy conditions, mice lacking VEGF-B did not display any sensory abnormalities, indicating that VEGF-B was not required for the survival of sensory neurons. However, DRG neurons isolated from adult VEGF-B−/− mice exhibited increased neuronal stress under cultured conditions and were also more sensitive to neuronal stress. Furthermore, when challenged in a model of retrograde degeneration of sensory axons, VEGF-B-deficient mice displayed a more severe sensory deficit. When added to primary DRG cultures, VEGF-B protein reduced neuronal stress, increased the survival of primary DRG neurons, and maintained the mitochondrial membrane potential. Another finding from our study is that these effects of VEGF-B were mediated through its FLT1 receptor. Indeed, primary DRG neurons isolated from mice lacking the tyrosine-kinase domain of the FLT1 receptor failed to respond to recombinant VEGF-B186. A neutralizing anti-FLT1 antibody also blocked the neuroprotective effects of VEGF-B186. Neuronal overexpression of FLT1 further protected mice from a paclitaxel-induced distal neuropathy, confirming the direct protective effect of FLT1 on the sensory neurons. Altogether, these data convincingly show that VEGF-B and FLT1 have potent neuroprotective activities.
Intriguingly, VEGF-B did not seem to act as a vasculoprotective factor in the nervous system. Indeed, the number of perfused vessels in VEGF-B−/− mice challenged with paclitaxel was similar to that of paclitaxel-treated WT mice. In contrast, VEGF-B−/− mice exhibited significantly less PGP9.5+ axons after paclitaxel compared to WT littermates. Local recombinant VEGF-B186 delivery was also protective against paclitaxel by preserving axonal stability, without eliciting any obvious effect on the vasculature. Notably, when adding a similar concentration of VEGF, effects on both the vascular and nervous system were noticed. Rather than acting as an angiogenic and neuroprotective factor, like VEGF, the effects of VEGF-B in the sensory nervous system are thus only neuroprotective in nature. The hypothesis that VEGF-B has kept its original neuronal function, and still exerts neuroprotective rather than angiogenic activities in higher organisms, is thus supported by this study.
VEGF has previously been implicated in cytotoxic chemotherapy-induced neuropathies. Indeed, studies in animal models have demonstrated that systemic therapy with VEGF restores nerve function in paclitaxel- or cisplatin-induced neuropathies (16, 29). The effects of VEGF in these studies were mainly attributed to the vasculoprotective effects of VEGF, thereby explaining its regenerative effects on nerve function as a secondary effect to improved neural perfusion. There are, however, some serious risks associated with a proangiogenic VEGF therapy for chemotherapy-induced neuropathies. Indeed, the role of VEGF-driven angiogenesis in tumor growth is well established, as illustrated by the increasing clinical application of antiangiogenic compounds targeting VEGF in cancer (30, 31). The delivery of VEGF to relieve chemotherapy-induced neuropathy in cancer patients, therefore, does not present a realistic option. Similar to VEGF, erythropoietin has been reported to exert beneficial effects in neuropathies (32), but recent analyses revealed that it also accelerates tumor growth (33). The nerve-growth factor (NGF) also exerts potent therapeutic effects in various models of neuropathy, but its therapeutic usefulness is restricted due to undesired side-effects, such as thermal hyperalgesia (34). Finding an effective cure for these neuropathies thus seems challenging (35).
VEGF-B therapy may offer a feasible and more promising approach for the treatment of paclitaxel-induced neuropathies. Indeed, the angiogenic effects of VEGF-B are questionable and still subject to debate (4, 36, 37). A recent study reported that its angiogenic activities are restricted to the ischemic heart (2). Evidence for a role of VEGF-B in stimulating tumor growth is also surprisingly limited. In fact, there is only a single study so far, in which it is reported that VEGF-B levels are slightly increased in advanced or aggressive tumors (38). There is thus very little biological evidence for a role of VEGF-B in directly stimulating tumor vascularization or growth, and the absence of such biological effects provides a clear rationale to further assess the therapeutic possibilities of VEGF-B for the treatment of paclitaxel-induced neuropathies.
In summary, our data thus indicate that VEGF-B counteracts paclitaxel-induced neuropathies and stimulate further research to investigate the therapeutic utility of VEGF-B in the sensory nervous system.
Supplementary Material
Acknowledgments
D.L. is supported by the Research Foundation Flanders, Belgium; J.D. and K.P. are supported by the Institute for the Promotion of Innovation by Science and Technology in Flanders. P.C. is supported by grants from the Geneeskundige stichting Koningin Elisabeth, and by the Muscular Dystrophy Association. This project is partly funded by a research and development grant of the governmental Institute for Science and Technology (IWT) for the promotion of research between universities (D.L., Katholieke Universiteit Leuven) and industry (T.M. and R.N., Johnson & Johnson Pharmaceutical Research and Development, Beerse, Belgium).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Lahteenvuo J. E., Lahteenvuo M. T., Kivela A., Rosenlew C., Falkevall A., Klar J., Heikura T., Rissanen T. T., Vahakangas E., Korpisalo P., Enholm B., Carmeliet P., Alitalo K., Eriksson U., Yla-Herttuala S. (2009) Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation 119, 845–856 [DOI] [PubMed] [Google Scholar]
- 2. Li X., Tjwa M., Van Hove I., Enholm B., Neven E., Paavonen K., Jeltsch M., Juan T. D., Sievers R. E., Chorianopoulos E., Wada H., Vanwildemeersch M., Noel A., Foidart J. M., Springer M. L., von Degenfeld G., Dewerchin M., Blau H. M., Alitalo K., Eriksson U., Carmeliet P., Moons L. (2008) Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler. Thromb. Vasc. Biol. 28, 1614–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zentilin L., Puligadda U., Lionetti V., Zacchigna S., Collesi C., Pattarini L., Ruozi G., Camporesi S., Sinagra G., Pepe M., Recchia F. A., Giacca M. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J. 24, 1467–1478 [DOI] [PubMed] [Google Scholar]
- 4. Mould A. W., Tonks I. D., Cahill M. M., Pettit A. R., Thomas R., Hayward N. K., Kay G. F. (2003) Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 48, 2660–2669 [DOI] [PubMed] [Google Scholar]
- 5. Hagberg C. E., Falkevall A., Wang X., Larsson E., Huusko J., Nilsson I., van Meeteren L. A., Samen E., Lu L., Vanwildemeersch M., Klar J., Genove G., Pietras K., Stone-Elander S., Claesson-Welsh L., Yla-Herttuala S., Lindahl P., Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464, 917–921 [DOI] [PubMed] [Google Scholar]
- 6. Falk T., Zhang S., Sherman S. J. (2009) Vascular endothelial growth factor B (VEGF-B) is up-regulated and exogenous VEGF-B is neuroprotective in a culture model of Parkinson's disease. Mol. Neurodegener. 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poesen K., Lambrechts D., Van Damme P., Dhondt J., Bender F., Frank N., Bogaert E., Claes B., Heylen L., Verheyen A., Raes K., Tjwa M., Eriksson U., Shibuya M., Nuydens R., Van Den Bosch L., Meert T., D'Hooge R., Sendtner M., Robberecht W., Carmeliet P. (2008) Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J. Neurosci. 28, 10451–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y., Zhang F., Nagai N., Tang Z., Zhang S., Scotney P., Lennartsson J., Zhu C., Qu Y., Fang C., Hua J., Matsuo O., Fong G. H., Ding H., Cao Y., Becker K. G., Nash A., Heldin C. H., Li X. (2008) VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J. Clin. Invest. 118, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carmeliet P., Tessier-Lavigne M. (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 [DOI] [PubMed] [Google Scholar]
- 10. Ruiz de Almodovar C., Lambrechts D., Mazzone M., Carmeliet P. (2009) Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 89, 607–648 [DOI] [PubMed] [Google Scholar]
- 11. Zacchigna S., Lambrechts D., Carmeliet P. (2008) Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 9, 169–181 [DOI] [PubMed] [Google Scholar]
- 12. Valable S., Bellail A., Lesne S., Liot G., Mackenzie E. T., Vivien D., Bernaudin M., Petit E. (2003) Angiopoietin-1-induced PI3-kinase activation prevents neuronal apoptosis. FASEB J. 17, 443–445 [DOI] [PubMed] [Google Scholar]
- 13. Mukouyama Y. S., Shin D., Britsch S., Taniguchi M., Anderson D. J. (2002) Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705 [DOI] [PubMed] [Google Scholar]
- 14. Schratzberger P., Schratzberger G., Silver M., Curry C., Kearney M., Magner M., Alroy J., Adelman L. S., Weinberg D. H., Ropper A. H., Isner J. M. (2000) Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat. Med. 6, 405–413 [DOI] [PubMed] [Google Scholar]
- 15. Schratzberger P., Walter D. H., Rittig K., Bahlmann F. H., Pola R., Curry C., Silver M., Krainin J. G., Weinberg D. H., Ropper A. H., Isner J. M. (2001) Reversal of experimental diabetic neuropathy by VEGF gene transfer. J. Clin. Invest. 107, 1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirchmair R., Tietz A. B., Panagiotou E., Walter D. H., Silver M., Yoon Y. S., Schratzberger P., Weber A., Kusano K., Weinberg D. H., Ropper A. H., Isner J. M., Losordo D. W. (2007) Therapeutic angiogenesis inhibits or rescues chemotherapy-induced peripheral neuropathy: taxol- and thalidomide-induced injury of vasa nervorum is ameliorated by VEGF. Mol. Ther. 15, 69–75 [DOI] [PubMed] [Google Scholar]
- 17. Sondell M., Sundler F., Kanje M. (2000) Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 12, 4243–4254 [DOI] [PubMed] [Google Scholar]
- 18. Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. George S. H., Gertsenstein M., Vintersten K., Korets-Smith E., Murphy J., Stevens M. E., Haigh J. J., Nagy A. (2007) Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 104, 4455–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tschachler E., Reinisch C. M., Mayer C., Paiha K., Lassmann H., Weninger W. (2004) Sheet preparations expose the dermal nerve plexus of human skin and render the dermal nerve end organ accessible to extensive analysis. J. Invest. Dermatol. 122, 177–182 [DOI] [PubMed] [Google Scholar]
- 21. Tsujino H., Kondo E., Fukuoka T., Dai Y., Tokunaga A., Miki K., Yonenobu K., Ochi T., Noguchi K. (2000) Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol. Cell. Neurosci. 15, 170–182 [DOI] [PubMed] [Google Scholar]
- 22. Peters C. M., Jimenez-Andrade J. M., Jonas B. M., Sevcik M. A., Koewler N. J., Ghilardi J. R., Wong G. Y., Mantyh P. W. (2007) Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp. Neurol. 203, 42–54 [DOI] [PubMed] [Google Scholar]
- 23. Wilson K., Geenen F., Biermans R., Nuydens R., Meert T. (2006) Sensory dysfunctions after topical application of cytostastic: a new model for the study of peripheral neuropathies. Int. J. Neuroprot. Neurodegen. 3, 31–36 [Google Scholar]
- 24. Bernardi P., Scorrano L., Colonna R., Petronilli V., Di Lisa F. (1999) Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 264, 687–701 [DOI] [PubMed] [Google Scholar]
- 25. Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 26. Popovici C., Isnardon D., Birnbaum D., Roubin R. (2002) Caenorhabditis elegans receptors related to mammalian vascular endothelial growth factor receptors are expressed in neural cells. Neurosci. Lett. 329, 116–120 [DOI] [PubMed] [Google Scholar]
- 27. Sun Y., Jin K., Childs J. T., Xie L., Mao X. O., Greenberg D. A. (2006) Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev. Biol. 289, 329–335 [DOI] [PubMed] [Google Scholar]
- 28. Sun Y., Jin K., Childs J. T., Xie L., Mao X. O., Greenberg D. A. (2004) Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J. Cereb. Blood Flow. Metab. 24, 1146–1152 [DOI] [PubMed] [Google Scholar]
- 29. Kirchmair R., Walter D. H., Ii M., Rittig K., Tietz A. B., Murayama T., Emanueli C., Silver M., Wecker A., Amant C., Schratzberger P., Yoon Y. S., Weber A., Panagiotou E., Rosen K. M., Bahlmann F. H., Adelman L. S., Weinberg D. H., Ropper A. H., Isner J. M., Losordo D. W. (2005) Antiangiogenesis mediates cisplatin-induced peripheral neuropathy: attenuation or reversal by local vascular endothelial growth factor gene therapy without augmenting tumor growth. Circulation 111, 2662–2670 [DOI] [PubMed] [Google Scholar]
- 30. Kerbel R. S. (2008) Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature 438, 932–936 [DOI] [PubMed] [Google Scholar]
- 32. Lipton S. A. (2004) Erythropoietin for neurologic protection and diabetic neuropathy. N. Engl. J. Med. 350, 2516–2517 [DOI] [PubMed] [Google Scholar]
- 33. Bennett C. L., Silver S. M., Djulbegovic B., Samaras A. T., Blau C. A., Gleason K. J., Barnato S. E., Elverman K. M., Courtney D. M., McKoy J. M., Edwards B. J., Tigue C. C., Raisch D. W., Yarnold P. R., Dorr D. A., Kuzel T. M., Tallman M. S., Trifilio S. M., West D. P., Lai S. Y., Henke M. (2008) Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 299, 914–924 [DOI] [PubMed] [Google Scholar]
- 34. Shu X. Q., Mendell L. M. (1999) Neurotrophins and hyperalgesia. Proc. Natl. Acad. Sci. U. S. A. 96, 7693–7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Argyriou A. A., Koltzenburg M., Polychronopoulos P., Papapetropoulos S., Kalofonos H. P. (2008) Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit. Rev. Oncol. Hematol. 66, 218–228 [DOI] [PubMed] [Google Scholar]
- 36. Silvestre J. S., Tamarat R., Ebrahimian T. G., Le-Roux A., Clergue M., Emmanuel F., Duriez M., Schwartz B., Branellec D., Levy B. I. (2003) Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ. Res. 93, 114–123 [DOI] [PubMed] [Google Scholar]
- 37. Li Y., Zhang F., Nagai N., Tang Z., Zhang S., Scotney P., Lennartsson J., Zhu C., Qu Y., Fang C., Hua J., Matsuo O., Fong G. H., Ding H., Cao Y., Becker K. G., Nash A., Heldin C. H., Li X. (2008) VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J. Clin. Invest. 118, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanda M., Nomoto S., Nishikawa Y., Sugimoto H., Kanazumi N., Takeda S., Nakao A. (2008) Correlations of the expression of vascular endothelial growth factor B and its isoforms in hepatocellular carcinoma with clinico-pathological parameters. J. Surg. Oncol. 98, 190–196 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.