Abstract
The mammalian embryo relies on maternal circulating retinoids (vitamin A derivatives) for development. β-Carotene is the major human dietary provitamin A. β-Carotene-15,15′-oxygenase (CMOI) has been proposed as the main enzyme generating retinoid from β-carotene in vivo. CMOI is expressed in embryonic tissues, suggesting that β-carotene provides retinoids locally during development. We performed loss of CMOI function studies in mice lacking retinol-binding protein (RBP), an established model of embryonic vitamin A deficiency (VAD). We show that, unexpectedly, lack of CMOI in the developing tissues further exacerbates the severity of VAD and thus the embryonic malformations of RBP−/− mice. Since β-carotene was not present in any of the mouse diets, we unveiled a novel action of CMOI independent from its β-carotene cleavage activity. We also show for the first time that CMOI exerts an additional function on retinoid metabolism by influencing retinyl ester formation via modulation of lecithin:retinol acyltransferase (LRAT) activity, at least in developing tissues. Finally, we demonstrate unequivocally that β-carotene can serve as an alternative vitamin A source for the in situ synthesis of retinoids in developing tissues by the action of CMOI.—Kim, Y.-K., Wassef, L., Chung, S., Jiang, H., Wyss, A., Blaner, W. S., Quadro, L. β-Carotene and its cleavage enzyme β-carotene-15,15′-oxygenase (CMOI) affect retinoid metabolism in developing tissues.
Keywords: provitamin A, lecithin:retinol acyltransferase, LRAT, embryonic vitamin A deficiency
Vitamin A is an essential nutrient for the mammalian embryo, which depends on maternal circulating retinoids (vitamin A and its derivatives) to fulfill its requirements (1, 2). In the unfed state, the bulk of circulating retinoids is in the form of retinol bound to its sole specific carrier protein, retinol-binding protein (RBP) (3). RBP mobilizes hepatic retinoid stores to deliver retinol to the target tissues. However, upon dietary vitamin A intake, retinyl ester packaged in chylomicrons might account for the majority of circulating retinoids (3). Although at lower concentrations, other forms of retinoids, such as provitamin A carotenoids in lipoproteins and chylomicrons, retinyl ester in lipoproteins, and glucuronide retinoids and retinoic acid bound to albumin, also circulate in the bloodstream (2). Overall, the levels of all these forms reflect the maternal vitamin A status, which is determined by both the concentration of retinoids within the stores and their recent dietary intake.
Most of the world's population relies mainly on provitamin A carotenoids from plants as a source of vitamin A (4). β-Carotene is the most abundant carotenoid present in the human diet, blood, and tissues, and it is the best characterized retinoid precursor (4). To generate retinoids, β-carotene needs to be cleaved enzymatically. Symmetric cleavage by β-carotene-15,15′-oxygenase (CMOI) gives rise to 2 molecules of retinaldehyde, which can then be either oxidized to retinoic acid, the active form of vitamin A that acts as a transcriptional regulator (5), or reduced to retinol. Esterification of retinol generates retinyl ester, the storage form of vitamin A in tissues (6). In vivo, 95% of retinoids arising from β-carotene are produced by this pathway (4). Asymmetric cleavage at double bonds other than the central 15,15′ double bond of the polyene chain produces apocarotenals with different chain lengths, which in turn might yield 1 molecule of retinaldehyde upon chain shortening (7). An asymmetric cleavage enzyme, β-carotene-9′,10′-oxygenase (CMOII), has been identified and characterized from mice (8). Both CMOI and CMOII are expressed in mouse and human maternal and embryonic tissues (9–11). Furthermore, β-carotene is present in the mammalian circulation and in the vertebrate egg yolk (12). Human studies have shown a direct correlation between the amount of carotenoids, including β-carotene, in the maternal circulation and those in the infant plasma, suggesting a net flow of these compounds across the placenta in amounts controlled by the maternal concentration (13, 14). Also, Dimenstein et al. (15) reported that in women with a subadequate vitamin A status, maternal serum β-carotene levels correlate with placental and cord serum retinol concentration. Taken together, these data suggest that besides maternal preformed vitamin A, local de novo biosynthesis of retinoic acid from provitamin A might be an important source of retinoid during development. Nevertheless, whether and how transplacental transfer of β-carotene occurs and whether its cleavage contributes to the vitamin A demand of the mammalian embryo have yet to be established. In the present study, we show that CMOI is expressed in developing mouse tissues from very early stages of embryogenesis. Furthermore, by analyzing the embryonic development of mice lacking both CMOI and RBP (CMOI−/−RBP−/−) under different regimens of maternal vitamin A intake, we show that, unexpectedly, the absence of CMOI in a model of embryonic vitamin A deficiency (VAD; RBP−/− mice) further affects embryonic development. The severe developmental defects of the double-knockout mice on a vitamin A-deficient diet during pregnancy are due to the lack of CMOI in the developing tissues. Our study also reveals that CMOI deficiency manifests itself in an autosomal dominant fashion but with different degrees of penetrance depending on the gene copy number. Given the absence of β-carotene from the mouse diet used in our study, these data unveil a novel effect of CMOI on embryonic development that is independent from its major function to cleave β-carotene. This effect is most likely due to additional functions that CMOI exerts on retinoid metabolism. Indeed, we also provide compelling evidence that CMOI might control the formation of vitamin A stores, at least in the developing tissues. Finally, we demonstrate for the first time that maternal circulating β-carotene can cross the placenta intact and reach the developing tissues to serve as a source of vitamin A for the in situ synthesis of retinoids by the action of CMOI.
MATERIALS AND METHODS
Knockout mice
A mouse strain lacking CMOI and RBP (CMOI−/−RBP−/−) was established by crossing RBP−/− (16) and CMOI−/− (17) mice. The resulting double-heterozygous mice of the F1 generation were crossed (CMOI+/−RBP+/−×CMOI+/−RBP+/−) and the double-knockout animals (CMOI−/−RBP−/−) were obtained in the F2 generation at the expected Mendelian ratio. After being generated, CMOI−/−RBP−/− mice where maintained as an inbred line fed a regular chow diet until the beginning of the pregnancy (see below). Genotypes were confirmed as published (16, 17). All mice used for this study were from a mixed C57Bl/6 × sv129 genetic background.
Nutritional manipulation
Female mice were maintained on a standard nutritionally complete vitamin A-sufficient chow diet (vitamin A, 25–28 IU/g diet; β-carotene, from trace to 3.8 ppm) until 3 mo of age. At the time of vaginal plug detection [set as 0.5 days post coitum (dpc), the onset of gestation], females were assigned randomly to one of two purified diets, either a vitamin A-sufficient diet (25 IU vitamin A/g diet) or a vitamin A-deficient diet (<0.22 IU vitamin A/g diet), until the day of sacrifice (14.5 dpc). These latter diets did not contain β-carotene (see representative HPLC profiles in Supplemental Fig. S1) and were prepared based on the AIN-93 formulations (ref. 18; LabDiet, Somerville, NJ, USA). Their nutrient composition was identical except for the concentration of vitamin A. Note that RBP−/− mice, which rely on dietary vitamin A to support normal embryonic development (16, 19), do not breed if maintained on a diet containing <22 IU vitamin A/g diet. Therefore, we maintained our mouse colony on diets containing vitamin A levels higher than those recommended (18). Diet and water were available to all animals on an ad libitum basis until the time of sacrifice. Mice were maintained on a 12 h dark-light cycle between 7:00 PM and 7:00 AM. All animals were sacrificed by CO2 inhalation between 9:30 and 11:30 AM when maternal serum, liver, placenta, and embryos were collected. All animal experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Rutgers University Institutional Committee on Animal Care.
Intraperitoneal injection of β-carotene
Following the study performed by Glise et al. (20), we added β-carotene in a mixture of ethanol, cremophor and PBS (1:11:18 ratio) at a final concentration of 1 μg/μl, under red light (reagents from Sigma, St. Louis, MO, USA). We administered this β-carotene emulsion to pregnant female mice by intraperitoneal (i.p.) injection, in a single dose either at 13.5 dpc or for 4 consecutive days from 6.5 to 9.5 dpc (1 injection daily). We used two different doses of β-carotene in our experiments, 1× and 10×. The 10× dose corresponds approximately to 10 μg β-carotene/g body weight (∼200–250 μg/mouse of 20–25 g body weight). This dose gives rise to ∼20 μg of retinol activity equivalent (RAE; 1 μg RAE=12 μg β-carotene from foods). This unit, adopted in 2001, takes into account the poor intestinal absorption of carotenoids and the fact that the bioactivity of β-carotene in foods is less than previously thought (18). This dose is comparable to the daily dietary vitamin A amount that a mouse ingests from food in the mouse facility at Rutgers; i.e., ∼22 μg of retinol equivalent. This amount is calculated considering that 1 μg RAE = 1 μg all-trans-retinol; that the breeder chow diet used in the mouse facility at Rutgers contains ∼25 IU vitamin A/g diet; and that a mouse eats ∼3 g food/d.
HPLC analysis of retinol, retinyl ester, and β-carotene
Reverse-phase HPLC analysis of retinoids (21) and β-carotene (17) was performed as described previously. Briefly, tissues (100–200 mg) were homogenized in 1 ml of PBS. Half of the homogenate was used to extract retinoids (21). The other half was used to extract β-carotene by adding 0.5 ml of methanol and 1 ml of acetone and then performing the extraction with 1 ml of petroleum ether. This latter extraction was repeated twice, and the supernatant was treated as for retinoid analysis (21). Retinoids and β-carotene were separated on a Ultrasphere C18 column (Beckman Coulter, Fullerton, CA, USA) with acetonitrile, methanol, and methylene chloride (70:15:15) used as the running solvent at 1.8 ml/min. Retinol, retinyl esters (retinyl palmitate, oleate, linoleate, and stearate) and β-carotene were identified by comparing retention times and spectral data with those of authentic standards. Retinyl acetate (for retinoids) and echinenone (for β-carotene) were added as internal standards. Detection limits are as follows: for retinoids, serum <0.1 ng/dl and tissues <1 ng/g; for β-carotene, serum <1 ng/dl, and tissues <10 ng/g. Serum and tissues, including intestine, never showed any detectable peak corresponding to intact β-carotene, regardless of the type of diet of the mice (Supplemental Fig. S2). Also, a subgroup of animals was maintained on a purified diet from weaning until 3 mo of age, when they were sacrificed to collect serum and tissues for HPLC analysis of β-carotene levels. No β-carotene was detected.
Determination of tissue levels of all-trans-retinoic acid
Tissue levels of all-trans-retinoic acid were determined by ultra performance liquid chromatography tandem mass spectrometry (UPLC/MS/MS). We used LC/MS-grade acetonitrile and water (Fisher Scientific, Pittsburgh, PA, USA). Pentadeuterated all-trans-retinoic acid (atRA-d5) was used as an internal standard (Toronto Research Chemicals, North York, ON, Canada). Tissue homogenates were extracted using a 2-step acid-base extraction method as described by Kane et al. (22) with minor modifications. Briefly, 1 embryo (200 mg) was homogenized in 500 μl ethanol containing 5 ng atRA-d5 in a glass tube; 12.5 μl of 1 M KOH was added to the homogenate and mixed well. The mixture was extracted with 6 ml of hexane. The organic phase containing nonpolar retinoids (retinol and retinyl esters) was removed. HCl (30 μl, 4 M) was added to the aqueous phase, and polar retinoids (retinoic acid) were removed on extraction with 6 ml hexane. The organic phases was evaporated under nitrogen gas. Retinoic acid extracts were resuspended in 70 μl of acetonitrile. Only glass containers, pipettes, and calibrated syringes were used to handle retinoic acid. UPLC/MS/MS analyses were performed on a Waters Xevo TQ MS Acquity UPLC system (Waters, Milford, MA, USA). The system was controlled by MassLynx 4.1 software (Waters). Samples were maintained at 4°C in the autosampler, and a volume of 5 μl was loaded onto a Waters Acquity UPLC HHS C18 column (2.1 mm inner diameter × 100 mm with 1.8-μm particles), and a 2.1- × 5-mm guard column with the same packing material. The column was maintained at 40°C. The flow rate was 300 μl/min in binary gradient mode with the following mobile phase gradient: initiated with 32% mobile phase A (H2O containing 0.1% formic acid) and 68% mobile phase B (acetonitrile containing 0.1% formic acid). The gradient was maintained for 6.3 min. Acetonitrile content was increased linearly to 85% over 6.4 min and maintained for 9.5 min. Next, acetonitrile was increased to 100% to wash the column for 2 min and then decreased to 68%. atRA was eluted between 8.2 and 8.4 min. Positive electrospray ionization MS/MS was performed using the following parameters: capillary voltage 3.8 kV, source temperature 150°C, desolvation temperature 500°C, desolvation gas flow 800 L/h, collision gas flow 0.15 ml/min. Optimized cone voltage was 16 V, collision energy for multiple reactions monitoring mode (MRM) was 18 eV, and use the following transitions: atRA for quantification 301.16 → 123.00 m/z, atRA for verification 301.16 → 205.03 m/z, atRA-d5 306.15 → 127.03 m/z. Detection limit was 10 pg/g embryo tissue.
Reverse-transcription (RT)-PCR analysis
Total RNA from mouse tissues was extracted as described previously (23). cDNA was synthesized using Roche Transcriptor First Strand cDNA Synthesis (Roche Diagnostics, Indianapolis, IN, USA). Expression of CMOI and CMOII genes was assessed as previously reported (23) using the following primers: CMOI: Fw 5′GAGCAAGTACAACCATTGGT3′, Rev1 5′AACTCAGACACCAGGATTC3′, or Rev2 5′CTCCAAAGCTGTGGTAGTAGC3′; CMOII: Fw 5′CCGATTCAATGGCAAAAAGT3′, Rev 5′GCTTTCACTCTGGTTGGGAG3′; β-actin: Fw 5′CGGAGGGAAAGATTCCTCTG3′, Rev 5′AGGGCCGGCACATTGAAGGT3′.
Western blot analysis
Embryonic and extraembryonic tissues were analyzed. A rabbit polyclonal anti-mouse CMOI antiserum and a mouse monoclonal anti-mouse CMOII (gift of Dr. Johannes Von Lintig, Case Western Reserve University, Cleveland, OH, USA) were used for immunodetection. Signals were detected by using an imager system. β-Actin, detected by a mouse monoclonal anti-actin antibody (Sigma), was used as a loading control for tissue samples.
Real-time RT-PCR analysis
A Roche Lightcycler 480 was used with the Lightcycler 480 SYBR green I master mix (Roche Diagnostics). Primer sequences were as follows: LRAT: Fw 5′GCAGTTGGGACTGACTCCAT3′, Rev 5′CAGATTGCAGGAAGGGTCAT3′; β-actin: as described above. Detailed method was as described previously (23).
Preparation of embryonic microsomes and LRAT enzymatic activity assay
Microsomes were isolated from 1.5 g of embryo by differential centrifugation. Tissues were homogenized in 8 ml of 10 mM Tris-HCl buffer containing 25% sucrose and protease inhibitors, pH 7.4, and centrifuged at 800 g for 10 min. Supernatants were centrifuged at 12,000 g for 30 min, followed by ultracentrifugation at 100,000 g for 1 h. All the steps were performed at 4°C. Next, pellets were resuspended in 200 μl of the homogenization buffer without sucrose and stored at −80°C until further use. Prior to performing the LRAT activity assay, optimal reaction time and concentration of microsomal proteins were determined. LRAT enzymatic activity was confirmed by adding 3 mM phenylmethanesulfonyl fluoride (PMSF), the serine protease inhibitor that selectively inhibits LRAT activity (24, 25), to a subset of the reactions (data not shown). Assay conditions were as follows: 60 μg of microsomal proteins was incubated for 12 min at 37°C with 5 μM [3H]-retinol in 150 mM potassium phosphate buffer (pH 7.25) containing 20 μM bovine serum albumin (BSA) and protease inhibitors. Enzymatic reaction was stopped by adding an equal volume of ice-cold ethanol containing 0.1 mg/ml butylated hydroxytoluene (BHT). As a control, ethanol-BHT was mixed with microsomes before adding the reaction mix. Radiolabled retinoids were analyzed by HPLC (21), and for each run, the peak corresponding to retinyl palmitate was collected, dried, and mixed with scintillation solution.
Statistical analyses
The Shapiro-Wilk test was used to test for normality of variables. For HPLC analysis, when retinol levels were not normally distributed, statistical analysis was performed by Kruskal-Wallis test followed by Mann-Whitney test. When retinyl esters or β-carotene levels were not normally distributed, values were logarithmically transformed prior to statistical analysis and reported as geometric means. Normally distributed values were statistically analyzed by t test or ANOVA with correction for multiple comparisons using the Fisher's least significant difference test. For the real-time PCR analysis, normality of 2(−ΔΔCT) values for each sample was confirmed by Shapiro-Wilk test. Data analyzed by Student's t test to determine significant differences of gene expression from the calibrator. Analyses were performed with SPSS statistical software (SPSS 10; Release 10.0.7a; SPSS Inc., Chicago, IL, USA).
RESULTS
Temporal expression of CMOI and CMOII during mouse development
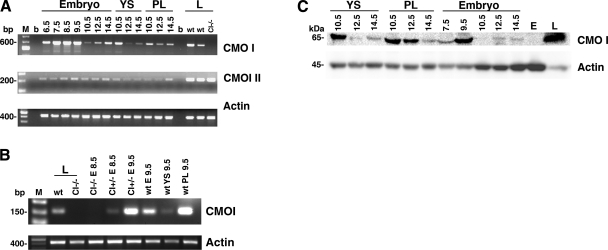
RT-PCR analysis for CMOI and CMOII was performed on wild-type embryos, placenta, and yolk sac collected from 6.5 to 14.5 dpc (Fig. 1A). Expression of both enzymes in the developing tissues is shown from 6.5 dpc in Fig. 1. More specifically, Fig. 1B shows that CMOI is expressed in the embryonic tissues at early developmental stages. In this experiment, in addition to wild-type, CMOI−/− mice were used (17). CMOI mRNA was detected in wild-type embryonic day (E)9.5 embryos and in CMOI+/− E8.5 and E9.5 embryos from CMOI−/− mothers. These latter embryos were dissected as a whole developmental unit and therefore contained tissues of both embryonic and maternal origin. However, since the mother lacked the CMOI gene, the detected mRNA was synthesized in the embryonic tissues (CMOI+/−). This result shows early embryonic CMOI expression from 8.5 dpc. From 10.5 to 14.5 dpc, mRNA of both enzymes was detected in embryo, placenta, and yolk sac. While CMOI mRNA levels showed a very distinct pattern of expression depending on the developmental stage, CMOII mRNA levels appeared steadier (Fig. 1A). The pattern of CMOI protein expression mirrored that of its mRNA (Fig. 1C). In contrast, in the developing tissues, CMOII protein was below our Western blot detection limits (data not shown). These data support the hypothesis that developing tissues might rely on local retinoid synthesis from β-carotene via CMOI activity and thus suggest a role for β-carotene and its cleavage enzyme in mouse embryogenesis. This might be true, for instance, during times of insufficient maternal dietary vitamin A intake.
Figure 1.
CMOI and CMOII expression in wild-type embryos, yolk sacs, and placentas at different stages of development. A) RT-PCR analysis. Wild type females were sacrificed at different times during gestation from 6.5 to 14.5 dpc. From 6.5 to 9.5 dpc, the whole embryo, including maternal, intraembryonic and extraembryonic tissues, was used for RNA or protein extraction. At 10.5, 12.5, and 14.5 dpc, embryo, placenta (PL), and yolk sac (YS) were dissected separately, and RNA or proteins were extracted. Livers (L) from wild-type (wt) and CMOI-knockout mice (CI−/−) were used as positive and negative controls for CMOI expression, respectively. Same liver samples were used as positive controls for CMOII expression. M, 100-bp molecular size marker; b, blank. Molecular size of marker bands is indicated at left. Expected molecular size of products: CMOI, 613 bp; CMOII, 228 bp; β-actin, 430 bp. Primers used to amplify CMOI were Fw and Rev2 (see Materials and Methods). B) RT-PCR analysis. CMOI+/− E8.5 and CMOI+/− E9.5 from CMOI−/− mothers were dissected as a whole and therefore contained both embryonic and extraembryonic tissues. CMOI−/− E8.5 from CMOI−/− mothers were used as negative control. At 9.5 dpc, wt embryo (E), yolk sac, and placenta were dissected separately. Livers from wt and CI−/− mice were used as further positive and negative controls for CMOI expression, respectively. Expected molecular size of products: CMOI, 147 bp; β-actin, 430 bp. Primers used to amplify CMOI were Fw and Rev1 (see Materials and Methods). C) Western blot analysis. Molecular mass of detected proteins is indicated at left. L, protein extracts from wt liver; E, protein extracts from CMOI−/− embryo at 14.5 dpc.
Effects of the absence of both CMOI and RBP on mammalian embryonic development
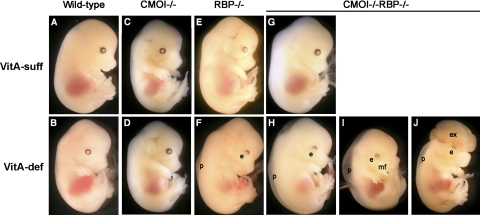
To explore this hypothesis further, we performed loss of CMOI function studies. Specifically, we wanted to verify whether providing intact β-carotene to the developing tissues of a model of embryonic VAD would improve or prevent the VAD-associated embryonic malformations. CMOI−/− mice are viable and fertile (17), and even when maintained on the vitamin A-deficient diet during pregnancy, no embryonic defects were observed, as in wild-type animals (Supplemental Table S1 and Fig. 2C, D). Indeed, on the one hand, in the absence of retinyl ester of dietary origin, CMOI−/− (and wild-type) dams can still provide sufficient vitamin A to the developing embryo through the retinol-RBP pathway (16, 19). On the other hand, we have shown previously that the absence of the retinol-RBP pathway makes the mice predominantly dependent on retinyl ester of dietary origin to support embryonic development (16, 19). As a consequence, when deprived of dietary vitamin A during pregnancy, RBP−/− females generate RBP−/− embryos with the typical features of VAD syndrome, such as malformed eye and peripheral edema (Fig. 2F and ref. 16). We therefore generated mutant mice lacking both CMOI and RBP (CMOI−/−RBP−/−), an indispensable tool to address our question.
Figure 2.
Gross morphology of embryos from dams maintained on vitamin A-sufficient (VitA-suff) dietary regimen (A, C, E, G) or vitamin A-deficient (VitA-def) dietary regimen (B, D, F, H–J) during pregnancy. Wild-type (A, B), CMOI−/− (C, D), RBP−/− (E, F), and CMOI−/−RBP−/− (G–J) embryos from dams of the same genotype, respectively, were collected at 14.5 dpc. e, abnormal eye (reduced pigmentation in the ventral region); p, peripheral edema; mf, abnormal midfacial region (snout foreshortened and divided by a sagittal median cleft, prolabium absent, maxillary process bearing whiskers separated by a larger than normal distance); ex, exencephaly (exteriorized brain). Same magnification was used for all panels. Panels A, B, E, F were previously published (23).
CMOI−/−RBP−/− mice are viable and fertile, as progeny from the crosses of these mutants could be obtained. However, in order to investigate further viability and fertility of this strain, groups of CMOI−/−RBP−/− females were maintained on the vitamin A-sufficient diet until ∼3 mo of age, when they were mated with either CMOI−/−RBP−/− or RBP−/− males. From 0.5 dpc, the females were maintained on either vitamin A-sufficient or -deficient diet. Pregnant age-matched RBP−/−, CMOI−/−, and wild-type females as well as nonpregnant females for each of the 4 genotypes were maintained on the same dietary regimens and used as controls. Dams were sacrificed at 14.5 dpc, and embryos were dissected, genotyped, and analyzed for external gross morphology. CMOI−/−RBP−/− embryos developing from CMOI−/−RBP−/− dams fed the vitamin A-sufficient diet were grossly normal (Supplemental Table S1 and Fig. 2G). In contrast, both CMOI+/−RBP−/− and CMOI−/−RBP−/− embryos from CMOI−/−RBP−/− dams fed the vitamin A-deficient diet during pregnancy were malformed, and the severity of these malformations was greater than those shown by RBP−/− embryos from RBP−/− dams fed a similar dietary regimen. Indeed, in addition to malformed eyes and peripheral edema, as seen in RBP−/− embryos (50% of the embryos; Fig. 2F; ref. 16), 47% of the CMOI−/−RBP−/− embryos also showed abnormal midfacial region (cleft face and palate; Fig. 2I and Supplemental Fig. S3B), and 3% displayed abnormal midfacial region and exencephaly (Fig. 2J). CMOI+/−RBP−/− embryos from double-knockout mothers fed the vitamin A-deficient diet displayed the same malformations as the double-knockout embryos, although with a different frequency (Supplemental Fig. S3D). Specifically, they showed a greater number of embryos with malformed eyes and peripheral edema (62%) and a reduced number of those with abnormal midfacial region (36%), with no significant differences in the percentage of embryos with abnormal midfacial region and exencephaly (2%). We also noticed a milder and wider degree of midfacial malformations in the heterozygous embryos (data not shown). Interestingly, CMOI−/−RBP−/− embryos from a CMOI+/−RBP−/− mother deprived of dietary vitamin A during pregnancy also displayed malformed eyes, peripheral edema, and abnormal midfacial region (Supplemental Fig. S3C).
Taken together, our data indicate a role for CMOI during embryonic development that is revealed only under limiting maternal vitamin A intake. They also suggest that CMOI deficiency can manifest itself with a gene-dosage effect.
Embryonic tissue retinoid levels
We next asked whether the severity of the embryonic malformations correlate with a more severe embryonic VAD. We first measured retinol and retinyl ester levels by HPLC (21) in wild-type, RBP−/−, CMOI−/−, and CMOI−/−RBP−/− embryos from wild-type, RBP−/−, CMOI−/−, and CMOI−/−RBP−/− dams, respectively (Table 1). Pregnant females were maintained on the vitamin A-sufficient or -deficient diet throughout gestation. Retinol and retinyl ester levels for wild-type and RBP−/− embryos were as previously reported (23). In agreement with the severity of their developmental defects, CMOI−/−RBP−/− and CMOI+/−RBP−/− embryos from double-knockout dams fed the vitamin A-deficient diet displayed the lowest retinol and retinyl ester levels among the different genotypes analyzed (Table 1). Most important, levels of retinoic acid, the active form of vitamin A, measured by LC-MS analysis in CMOI−/−RBP−/− embryos from dams fed the vitamin A-deficient diet were also significantly reduced compared to those of RBP−/− embryos from dams fed a similar dietary regimen (2.4±0.1 vs. 3.3±0.4 ng/g, respectively; n=5 or 6/group; P=0.004). Overall, the reduced levels of retinoids of the CMOI−/−RBP−/− embryos from dams deprived of dietary vitamin A correlate with the severity of their malformations.
Table 1.
Retinol and retinyl ester levels of E14.5 embryos from dams maintained under different regimens of dietary vitamin A intake
| Genotype | VitA-suff |
VitA-def |
||||
|---|---|---|---|---|---|---|
| Retinol | Retinyl ester | n | Retinol | Retinyl ester | n | |
| WT | 126.3 ± 48.5 | 304.9 ± 64.3 | 8 | 94.4 ± 8.0 | 181.6 ± 29.4 | 5 |
| RBP−/− | 51.5 ± 5.38* | 215.0 ± 32.0* | 4 | 33.6 ± 10.6* | 77.2 ± 34.9* | 4 |
| CMOI−/− | 181.7 ± 8.3* | 52.4 ± 18.0* | 4 | 109.0 ± 11.7* | 34.8 ± 11.6* | 4 |
| CMOI−/−RBP−/− | 45.0 ± 7.0* | 50.7 ± 19.5* | 4 | 14.9 ± 3.2* | 26.6 ± 8.9* | 7 |
| CMOI+/−RBP−/− | 57.7 ± 20.5* | 141.6 ± 35.7*,# | 7 | 12.6 ± 2.3* | 28.8 ± 16.5* | 7 |
Retinol and retinyl ester levels (ng/g) in embryos collected at 14.5 dpc were determined by reverse-phase HPLC. VitA-suff, vitamin A-sufficient maternal dietary regimen; VitA-def, vitamin A-deficient maternal dietary regimen; WT, wild type. See text and Supplemental Table S1 for details. VitA-def CMOI+/−RBP−/− and CMOI−/−RBP−/− embryos were those displaying malformed eyes and peripheral edema only. Values represent means ± sd; n = total embryos/group. Statistical analysis was performed as described in Materials and Methods. Values of P < 0.05 were considered statistically significant.
P < 0.05 vs. corresponding WT;
P < 0.05 vs. corresponding CMOI−/−.
Interestingly, embryos lacking CMOI always displayed lower retinyl ester levels compared to wild-type embryos, regardless of the presence or absence of RBP and irrespective of the dietary regimen (Table 1). The absence of one copy of the enzyme (CMOI+/−RBP−/−) significantly reduced embryonic retinyl ester levels compared to wild type when the dams were fed the vitamin A-sufficient diet. These levels declined further when both copies of CMOI were absent, showing an obvious gene-dosage effect. This effect was no longer evident when the dams were maintained on the vitamin A-deficient diet, even though embryonic retinyl ester levels were still significantly lower compared to embryos that expressed CMOI (Table 1). In summary, the reduction in embryonic retinyl ester levels in mice lacking CMOI was independent of the maternal dietary regimen and the genetic background of the mouse strain.
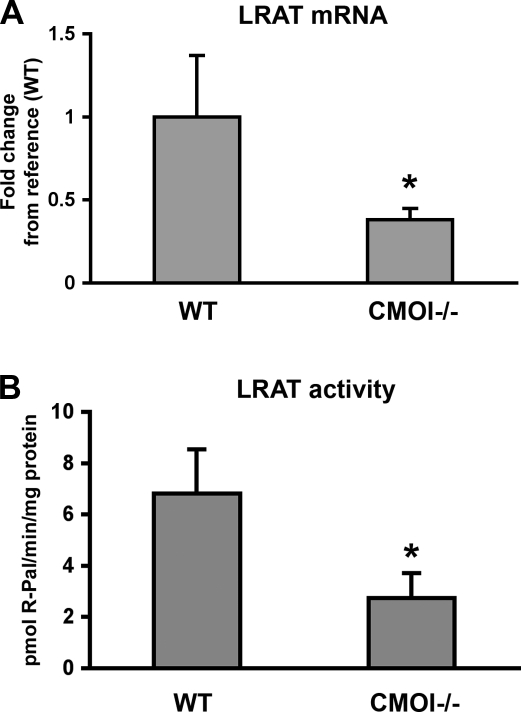
Formation of retinyl esters, the storage form of vitamin A, occurs through esterification of retinol, primarily by the action of the enzyme LRAT (26, 27). We therefore measured LRAT mRNA levels in embryos from dams fed the vitamin A-sufficient diet by real time RT-PCR. LRAT mRNA levels were reduced significantly in embryos lacking CMOI, compared to wild-type embryos (Fig. 3A). Accordingly, LRAT enzymatic activity was also significantly decreased in CMOI−/− embryos (Fig. 3B). These results provide the first indication that CMOI might exert an additional function on retinoid metabolism by controlling the formation of vitamin A stores, at least in developing tissues, when β-carotene is not present in the diet.
Figure 3.
Embryonic LRAT mRNA levels and enzymatic activity in the absence of CMOI. A) Expression levels of LRAT mRNA measured by real-time RT-PCR in wild-type and CMOI−/− embryos from dams maintained on the vitamin A-sufficient diet throughout life and gestation. Values are expressed as means ± sd using the 2−ΔΔCT method; n = 8/group. Statistical analysis was performed as described in Materials and Methods. B) LRAT activity in microsomes isolated from wild-type (WT) and CMOI−/− embryos from dams maintained on the vitamin A-sufficient diet throughout life and gestation. Reactions were run for 12 min at 37°C; 3 pools/group (7–8 embryos/pool) were averaged. Results are expressed as means ± sd. R-Pal, retinyl palmitate. *P < 0.05 vs. WT.
Maternal serum and tissue retinol and retinyl ester levels
To investigate whether a severe VAD status of the CMOI−/−RBP−/− dams could also contribute to the more severe VAD of their embryos, retinol and retinyl ester levels were assessed in maternal serum and tissues by HPLC (21). Retinoid levels were measured in the bloodstream of nonpregnant females to better estimate the amount of vitamin A available to the embryos. Serum retinol and retinyl ester levels for wild-type and RBP−/− females prior to pregnancy under different regimens of dietary vitamin A intake were as expected (Table 2; ref. 23). When compared to wild-type on the vitamin A-sufficient diet, CMOI−/− females showed slightly reduced levels of serum retinol, as reported (Table 2; ref. 17). However, their serum retinol levels were similar to those of wild-type females when maintained on the vitamin A-deficient diet (Table 2). Serum retinol levels of CMOI−/−RBP−/− females were comparable to those of the RBP−/−, regardless of the dietary regimen (Table 2). In addition, CMOI−/−RBP−/− hepatic retinol and retinyl ester levels were not affected by the 2-wk dietary vitamin A deprivation but were slightly increased compared to the other strains, irrespective of the diet (Table 2).
Table 2.
Maternal serum and liver retinol and retinyl ester levels under different regimens of dietary vitamin A intake
| Tissue and genotype | VitA-suff |
VitA-def |
||||
|---|---|---|---|---|---|---|
| Retinol | Retinyl ester | n | Retinol | Retinyl ester | n | |
| Serum | ||||||
| WT | 20.7 ± 3.4 | 1.1 (nd–9.3) | 6 | 32.2 ± 3.7 | nd | 4 |
| RBP−/− | 2.2 ± 0.3* | 0.5 (nd–10.0) | 8 | 1.4 ± 0.4* | nd | 7 |
| CMOI−/− | 17.2 ± 2.8* | 0.3 (nd–12.0) | 10 | 35.2 ± 4.4 | nd | 5 |
| CMOI−/−RBP−/− | 2.0 ± 0.3* | 0.3 (nd–2.5) | 17 | 1.5 ± 0.3* | nd | 11 |
| Liver | ||||||
| WT | 3.7 ± 1.2 | 270.3 ± 116.6 | 6 | 7.8 ± 4.5 | 295.7 ± 90.5 | 7 |
| RBP−/− | 6.9 ± 2.4* | 387.5 ± 100.0 | 7 | 2.6 ± 1.4* | 380.4 ± 78.5 | 6 |
| CMOI−/− | 10.1 ± 0.5* | 326.6 ± 50.1 | 4 | 9.4 ± 4.2 | 375.6 ± 68.3 | 6 |
| CMOI−/−RBP−/− | 4.5 ± 1.0 | 490.3 ± 144.3* | 6 | 4.2 ± 0.9 | 474.2 ± 162.7* | 6 |
Retinol and retinyl ester levels in serum (μg/dl) and liver (μg/g) were determined by reverse-phase HPLC. VitA-suff, vitamin A-sufficient maternal dietary regimen; VitA-def, vitamin A-deficient maternal dietary regimen; WT, wild type; nd, not detectable. See text and Supplemental Table S1 for details. Values are expressed as means ± sd, except for serum retinyl ester levels, which are expressed as geometric means with range of absolute values in parentheses. Statistical analysis as described in Materials and Methods.
P < 0.05 vs. corresponding WT.
Overall, CMOI−/−RBP−/− and RBP−/− females fed the vitamin A-deficient diet for 2 wk showed a similar vitamin A status and comparable levels of circulating retinoids available to the developing embryos. Nevertheless, the two strains displayed distinct embryonic phenotypes when bred on a diet deprived of vitamin A, strengthening the hypothesis that the developmental defects of the CMOI−/−RBP−/− embryos are due to the lack of CMOI in the developing tissues.
Is intact β-carotene an alternative source for local synthesis of retinoic acid in the developing tissues?
Finally, we used CMOI−/−RBP−/− mice to investigate whether maternal circulating intact β-carotene could prevent the VAD status and the associated malformations of the CMOI+/−RBP−/− embryos from dams fed the vitamin A-deficient diet. Firstly, we validated a method to deliver intact β-carotene in the maternal bloodstream and verified whether this circulating intact β-carotene could cross the placental barrier and subsequently be taken up by the embryonic tissues. To circumvent all the issues related to the intestinal absorption of β-carotene, especially in murine models (4, 28, 29), and avoid further modification of the dietary regimen of the dams, we supplemented CMOI−/− females, maintained on the vitamin A-sufficient diet throughout life and gestation, with a single dose of β-carotene by i.p. injection at 13.5 dpc. The dams were then sacrificed 24 h later to collect serum, embryos, and placenta for β-carotene analysis by HPLC. Two different β-carotene doses were used: 1× and 10× (see Materials and Methods). Intact β-carotene was detected in the maternal bloodstream 24 h after the i.p. injection, with the highest concentration identified upon administration of the highest dose. In addition, intact β-carotene was also detected in placental and embryonic tissues, in a similar dose-dependent manner (Table 3).
Table 3.
Maternal serum, embryo, and placenta levels of carotenoids in pregnant CMOI−/− females supplemented with β-carotene
| Tissue | Vehicle | bC-1× | bC-10× |
|---|---|---|---|
| Serum | nd | 14.3 (3.9–45.7) | 152.0 (130.2–180.0) |
| Placenta | nd | 109.5 (71.3–208.2) | 445.0 (114.0–2169.0) |
| Embryo | nd | 2.5 (nd–28.5) | 19.9 (nd–178.5) |
CMOI−/− females were maintained on the vitamin A-sufficient diet during pregnancy and supplemented with 1× or 10× β-carotene (bC) emulsion (see Materials and Methods) at 13.5 dpc. Dams were sacrificed at 14.5 dpc. β-Carotene levels were determined by reverse-phase HPLC in maternal serum (μg/dl), placenta (ng/g), and embryo (ng/g). Values are expressed as geometric means with range of absolute values in parentheses; n = 3–8 samples/group. nd, not detectable.
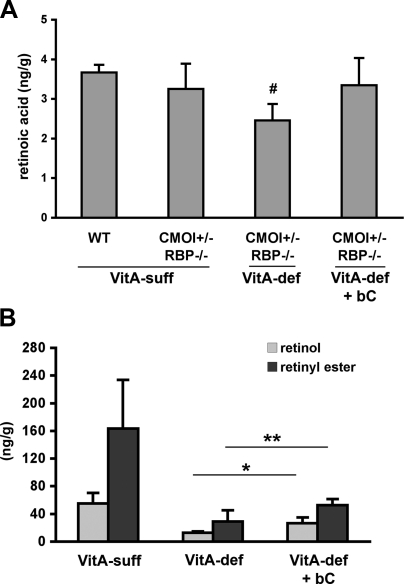
After establishing the efficacy of the supplementation method, we mated 3-mo-old CMOI−/−RBP−/− females, raised on the vitamin A-sufficient diet, with RBP−/− males. These females were maintained on the vitamin A-deficient diet throughout gestation and supplemented with the 10× dose of β-carotene once daily from 6.5 to 9.5 dpc. This is a crucial developmental window during which organogenesis begins (1); retinoid levels rise sharply to remain steady until later stages (30); maternal retinoic acid supplementation prevents or partially rescues the lethality or the developmental abnormalities of numerous knockout strains showing the typical features of VAD (31, 32); and CMOI is expressed in embryonic tissues (Fig. 1; refs. 10, 11). At 14.5 dpc, dams were sacrificed, and maternal serum and embryos were collected. First, maternal circulating retinoid and carotenoid levels were measured by HPLC. While the supplementation of the double-knockout females fed the vitamin A-deficient diet during pregnancy did not alter circulating levels of retinol, intact β-carotene could still be detected in the maternal bloodstream 5 d after the last i.p. injection (i.e., at 14.5 dpc; Table 4). We next analyzed the effects of the maternal supplementation on external morphology and retinoid and carotenoid levels of the CMOI+/−RBP−/− embryos, which were expected to cleave β-carotene taken up from the maternal circulation. Table 5 shows the distribution of the phenotypes of CMOI+/−RBP−/− embryos from double-knockout dams fed the vitamin A-deficient diet during pregnancy with or without β-carotene supplementation. About 60% of the embryos from the supplemented dams appeared grossly phenotypically normal, and only 32% showed minor eye defects and peripheral edema. None of them had cleft face and/or palate. The exencephaly was not improved. These data suggest that maternal circulating β-carotene reaching the embryos can ameliorate the defects of the heterozygous embryos, likely through the action of embryonic CMOI, generating adequate retinoid to support normal development. In addition, LC-MS analysis of embryonic retinoic acid levels confirmed our interpretation of the results. Indeed, retinoic acid levels, which were significantly reduced in CMOI+/−RBP−/− embryos from dams fed the vitamin A-deficient diet, were restored to normal levels when their mothers were supplemented with β-carotene (Fig. 4A). Consistently, retinol and retinyl ester levels also increased in CMOI+/−RBP−/− embryos on maternal β-carotene supplementation when compared to those of CMOI+/−RBP−/− embryos from dams fed an unsupplemented vitamin A-deficient diet (Fig. 4B).
Table 4.
Serum levels of retinoids and carotenoids in pregnant CMOI−/−RBP−/− females supplemented with β-carotene
| Component | VitA-def | n | VitA-def + bC | n |
|---|---|---|---|---|
| Retinol | 1.4 ± 0.2 | 9 | 1.4 ± 0.3 | 4 |
| Retinyl ester | nd | 9 | nd | 5 |
| β-Carotene | nd | 9 | 7.0 ± 2.3 | 4 |
CMOI−/−RBP−/− females were maintained on the vitamin A-deficient diet during pregnancy and supplemented with 10 μg/g of β-carotene (bC) from 6.5 to 9.5 dpc (1 i.p. injection/d). Dams were sacrificed at 14.5 dpc. Serum retinol, retinyl ester, and β-carotene levels (μg/dl) were determined by reverse phase HPLC. Values are expressed as means ± sd; n = dams/group. nd, not detectable.
Table 5.
Distribution of phenotypes of CMOI+/−RBP−/− embryos from double knockout dams on the vitamin A-deficient diet during pregnancy with and without β-carotene supplementation
| Dietary regimen | n | Embryonic phenotype (%) |
|||
|---|---|---|---|---|---|
| Normal | RBP-like | Cleft | Exencephaly | ||
| VitA-def | 89 | 0 | 62 | 36 | 2 |
| VitA-def + bC | 82 | 61 | 32 | 0 | 7 |
CMOI−/−RBP−/− females, mated with RBP−/− males, were maintained on the vitamin A-deficient (VitA-def) diet during pregnancy, with or without supplementation with 10× β-carotene (bC) emulsion (see Materials and Methods) from 6.5 to 9.5 dpc (1 i.p. injection/d). Dams were sacrificed at 14.5 dpc, and the gross morphology of CMOI+/−RBP−/− embryos was analyzed. Normal, embryos looked like wild-type embryos from wild-type dams bred on vitamin A-sufficient diet; RBP-like, embryos showed peripheral edema and malformed eyes; Cleft, embryos showed peripheral edema, malformed eyes and abnormal mid-facial region; Exencephaly, embryos showed peripheral edema, malformed eyes, and exteriorized brain. n = total number of embryos.
Figure 4.
Retinoid levels in CMOI+/−RBP−/− embryos from CMOI−/−RBP−/− dams fed the vitamin A-deficient diet with β-carotene supplementation. A) Retinoic acid levels in CMOI+/−RBP−/− and wild-type (WT) embryos from dams fed the vitamin A-sufficient diet (VitA-suff), and from dams fed the vitamin A-deficient diet (VitA-def) with or without β-carotene supplementation (bC). Analysis was performed by LC-MS; n = 3 to 7 embryos/group. #P < 0.05 vs. VitA-suff WT; ANOVA. B) Retinol and retinyl ester levels in CMOI+/−RBP−/− embryos from CMOI−/−RBP−/− dams fed the VitA-suff or VitA-def diet with or without bC supplementation. Retinol and retinyl ester levels from VitA-suff and VitA-def CMOI+/−RBP−/− embryos are as in Table 1. Supplementation was performed as described in Materials and Methods. Analysis was performed by HPLC; n = 4 to 7 embryos/group. Statistical analysis performed by ANOVA. CMOI+/−RBP−/− embryos from dams fed VitA-def diet showed peripheral edema and eye malformations; CMOI+/−RBP−/− embryos from dams fed VitA-def diet + bC appeared normal. *P < 0.05 vs. VitA-def retinol; **P < 0.05 vs. VitA-def retinyl ester; ANOVA.
DISCUSSION
It is well documented that retinoids are indispensable for maintaining normal embryonic development (1) and that the embryo relies on maternal circulating retinoid for its supply of vitamin A (2). However, much of the current knowledge in this field pertains to the role of maternal circulating preformed retinoids. The function of provitamin A carotenoids has remained elusive.
CMOI is expressed in the embryo from 8.5 dpc
Studies in the late 1980s and early 1990s proposed that β-carotene metabolism could generate, directly or indirectly, through the production of retinol, physiologically significant amounts of retinoic acid in tissues, independently of retinol mobilization from the liver (33, 34). This pathway could take place not only in intestine (34) but also in tissues where β-carotene was shown to accumulate, such as liver and adipose, or in those with an elevated demand of retinoids, such as developing tissues. Here we demonstrate that the mRNA of CMOI, the main enzyme generating retinoids from β-carotene in adult tissues, is also expressed in the embryonic tissues from early developmental stages, i.e., from 8.5 dpc. The homogenous mRNA and protein levels of actin, as well as the correlation between the expression pattern of CMOI protein and mRNA at different stages of development, indicate that the variation of the expression of this enzyme throughout development is physiological. These data strongly favor the hypothesis of an important role of CMOI during embryogenesis. In contrast, the generally lower and steadier levels of CMOII mRNA and the failure to detect CMOII protein imply a less prominent function of this enzyme in embryonic retinoid and β-carotene metabolism, at least in wild-type mice with adequate levels of vitamin A.
Embryonic CMOI deficiency impairs retinoid metabolism and causes malformations under certain conditions
The absence of CMOI in the developing tissues impaired embryonic development by worsening VAD in the RBP-knockout background. Indeed, CMOI−/−RBP−/− embryos showed more severe phenotypes compared to RBP−/− when the dams were maintained on the vitamin A-deficient diet. These developmental defects were associated with a more severe vitamin A-deficient status of the embryos themselves, as indicated by the lower levels of retinol, retinyl ester and, most of all, retinoic acid compared to RBP−/− embryos. We exclude that the malformations of the CMOI−/−RBP−/− embryos were caused by a more severe vitamin A-deficient status of the double-knockout mothers, as both maternal serum and tissue levels of retinoids were similar in RBP−/− and CMOI−/−RBP−/− dams, regardless of the dietary regimen. In other words, the amounts of retinoids available to the developing embryos were similar in the double-knockout and RBP−/− strains, and yet they displayed distinct embryonic phenotypes. In addition, the fact that CMOI−/−RBP−/− embryos from dams fed the vitamin A-deficient diet showed the same phenotype regardless of whether they developed from double-knockout or CMOI+/−RBP−/− dams further strengthens our conclusion that CMOI is required by the embryo.
Overall, our data show that lack of CMOI in developing tissues causes a severe VAD and its associated embryonic malformations. This result was unexpected, given that β-carotene is absent or below the limit of detection in the diets used for our experiments. Therefore, we unveiled a novel effect of CMOI on embryonic development, which is independent from its ability to cleave the provitamin A.
CMOI performs an additional function in retinoid metabolism
Embryos lacking CMOI always showed significantly reduced retinyl ester levels compared to wild-type embryos, regardless of the maternal dietary regimens and the genetic background of the mouse strain. Formation of retinyl esters is mediated by LRAT (26, 27), an enzyme expressed throughout mouse embryonic development (23). In agreement with the reduced levels of retinyl ester, both LRAT mRNA and enzymatic activity were significantly lower in CMOI−/− embryos compared to wild type (Fig. 3). The impaired ability to form vitamin A stores could likely contribute to the severe VAD and, hence, the phenotype of the double-knockout embryos from dams fed the vitamin A-deficient diet. Indeed, retinyl ester formation is a key step in the complex regulatory mechanisms that maintain retinoic acid homeostasis in developing tissues (23). The fact that LRAT gene is a well-known target for retinoic acid action (26) raises the possibility that CMOI might control LRAT expression via retinoic acid signaling. However, consistent with their normal phenotype, retinoic acid levels of embryos lacking CMOI from dams fed the vitamin A-sufficient diet did not differ from those of wild-type embryos from dams fed the same dietary regimen (CMOI−/− vs. WT, 3.6±05 vs. 3.8±0.5 ng/g, P=0.5). Yet, on this dietary regimen, mRNA levels of LRAT (and retinyl ester formation) are reduced in embryos lacking CMOI. Therefore, we exclude that CMOI influences LRAT expression via retinoic acid, at least when the mice are maintained on the vitamin A-sufficient diet. In contrast, consistent with their embryonic malformations, retinoic acid levels were reduced in the double-knockout embryos from dams fed the vitamin-A deficient diet compared to RBP−/− embryos. Therefore, we speculate that, under this condition, reduced retinoic acid synthesis is likely the effect and not the cause of reduced retinyl ester formation.
These results provide a strong indication that, by influencing the formation of vitamin A stores, CMOI exerts an additional function in retinoid metabolism. This alternative activity of CMOI seems to be specific to the developing tissues, as it has not been observed in other tissues, like liver or lung (35), for instance. The molecular mechanisms through which CMOI might regulate LRAT mRNA, and consequently retinyl ester formation, is still unclear, and investigations to address this question are ongoing. One possibility is that, in the absence of (and/or in addition to) β-carotene, CMOI might cleave an alternative substrate, generating signaling molecules that, in turn, can influence, directly or indirectly, LRAT transcription, and consequently its activity. Alternatively, the effect of CMOI on retinyl ester formation could take place through protein–protein interactions. The concept that formation of protein complexes can facilitate multistep reactions has been proposed, for example, in vision (36). Retinal pigment epithelium-specific protein 65 kDa (RPE 65), an enzyme that catalyzes the conversion of all-trans-retinyl ester to 11-cis-retinol in the eye (36), and a member of the family of proteins that includes CMOI and CMOII (37), has shown the tendency to associate with other proteins involved in the visual cycle (36). Interestingly, not only RPE 65 colocalizes with LRAT in the retinal-pigment epithelium of the eye (37), but recently also CMOI and LRAT have been shown to colocalize in the same cell type in some tissues (38).
CMOI deficiency manifests itself in an autosomal dominant fashion, with a different degree of penetrance depending on the gene copy number
Although CMOI+/−RBP−/− embryos developing from CMOI−/−RBP−/− dams deprived of dietary vitamin A displayed the same type of developmental defects of the CMOI−/−RBP−/− embryos, the frequency of these malformations was different. Specifically, CMOI+/−RBP−/− embryos showed a greater number of embryos with milder defects. Moreover, the ability of CMOI to influence retinyl ester formation also appeared to be gene-dosage dependent, at least when the dams were maintained on the vitamin A-sufficient diet. Notably, haploinsufficiency of CMOI has been reported recently in a patient with hypercarotenemia consuming a diet containing carotenoid but with inadequate amount of retinol (39).
Developing tissues might rely on localized retinoid synthesis from β-carotene via CMOI
The double-knockout mouse model we generated was instrumental to investigate whether maternal supplementation of β-carotene could improve or prevent the VAD-associated developmental defects of the embryos. We showed that maternal circulating β-carotene could indeed ameliorate the embryonic defects of the CMOI+/−RBP−/− embryos from double-knockout dams bred on the vitamin A-deficient diet. Consistently, retinoic acid levels of the rescued embryos were restored to normal levels and their retinol and retinyl ester levels increased compared to those of CMOI+/−RBP−/− embryos from unsupplemented dams. We were not expecting that the β-carotene supplementation of the double-knockout mothers would restore embryonic retinol and retinyl ester levels to those of wild-type embryos, because the CMOI−/−RBP−/− dams were maintained on the vitamin A-deficient diet for 2 wk, and the supplementation was performed only for 4 d. At the moment, we cannot exclude the possibility that a longer regimen of maternal β-carotene administration would perhaps further increase retinol and retinyl ester levels of the CMOI+/−RBP−/− embryos, but we doubt that they will reach the levels of the wild-type embryos. Indeed, due to their genotype, CMOI+/−RBP−/− embryos have lower retinol and retinyl ester levels compared to wild type, even when the dams were maintained on the vitamin A-sufficient diet. Based on this evidence, we hypothesize that β-carotene is as efficient as preformed vitamin A in restoring retinoid levels. Interestingly, we did not observe improvement of the exencephaly on β-carotene supplementation. Currently, we cannot rule out whether our experimental conditions might not be appropriate to achieve this rescue or whether this is a vitamin A-independent phenotype. Indeed, although the malformations observed in the CMOI−/−RBP−/− embryos have been described previously employing other models of retinoid-deficiency (1) or mice bearing retinoid receptor gene disruptions (40), these defects are not exclusive of VAD (41, 42).
β-carotene in maternal-fetal nutrition
The nutritional status of the mother is a key factor for the development and growth of the fetus, as the embryo relies on nutrients supplied by the mother through the bloodstream. This condition is particularly true for an essential nutrient like vitamin A, whose requirement for normal development has been well established. Embryonic VAD as well as vitamin A excess might result in fetal malformations, representing a serious problem in both the developing world and the industrialized countries. β-Carotene is the best-characterized vitamin A precursor and the most abundant supply of this nutrient in the human diet. These studies contribute to expand our knowledge of maternal-fetal nutrition and dietary contribution to embryonic development, thus providing new insight into appropriate dietary practices during pregnancy. Understanding β-carotene metabolism in the developing tissues and its role in maternal-fetal nutrition has a significant impact on human health, fulfilling the need to reduce the incidence of fetal malformations associated with an abnormal maternal intake of micronutrients, such as vitamins.
Supplementary Material
Acknowledgments
This work was supported by grants R01HD057493 and R01HD057493-02S1 from the U.S. National Institutes of Health (NIH) and partially by National Research Initiative grant 2006-35200-16580 from the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service, Bioactive Food Components for Optimal Health (31.0) Program to L.Q. In addition, W.S.B. and H.J were supported by grants R01DK079221 and RC2AA019413 from NIH.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Clagett-Dame M., DeLuca H. F. (2002) The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 22, 347–381 [DOI] [PubMed] [Google Scholar]
- 2. Marceau G., Gallot D., Lemery D., Sapin V. (2007) Metabolism of retinol during mammalian placental and embryonic development. Vitam. Horm. 75, 97–115 [DOI] [PubMed] [Google Scholar]
- 3. Vogel S., Gamble M. V., Blaner W. S. (1999) Biosynthesis, absorption, metabolism and transport of retinoids. In Handbook of Experimental Pharmacology. Retinoids: The Biochemical and Molecular Basis of Vitamin A and Retinoid Action (Nau H., Blaner W. S. eds.) Vol. 139, pp. 31–95, Springer Verlag, Heidelberg, Germany [Google Scholar]
- 4. Von Lintig J. (2010) Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 30, 35–56 [DOI] [PubMed] [Google Scholar]
- 5. Balmer J. E., Blomhoff R. (2002) Gene expression regulation by retinoic acid. J. Lipid Res. 43, 1773–1808 [DOI] [PubMed] [Google Scholar]
- 6. O'Byrne S. M., Wongsiriroj N., Libien J., Vogel S., Goldberg I. J., Baehr W., Palczewski K., Blaner W. S. (2005) Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J. Biol. Chem. 280, 35647–35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang X. D., Russell R. M., Liu C., Stickel F., Smith D. E., Krinsky N. I. (1996) Beta-oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from beta-apocarotenoic acids. J. Biol. Chem. 271, 26490–26498 [PubMed] [Google Scholar]
- 8. Kiefer C., Hessel S., Lampert J. M., Vogt K., Lederer M. O., Breithaupt D. E., von Lintig J. (2001) Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 276, 14110–14116 [DOI] [PubMed] [Google Scholar]
- 9. Von Lintig J., Hessel S., Isken A., Kiefer C., Lampert J. M., Voolstra O., Vogt K. (2005) Towards a better understanding of carotenoid metabolism in animals. Biochim. Biophys. Acta 1740, 122–131 [DOI] [PubMed] [Google Scholar]
- 10. Redmond T. M., Gentleman S., Duncan T., Yu S., Wiggert B., Gantt E., Cunningham F. X., Jr. (2001) Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15′-dioxygenase. J. Biol. Chem. 276, 6560–6565 [DOI] [PubMed] [Google Scholar]
- 11. Paik J., During A., Harrison E. H., Mendelsohn C. L., Lai K., Blaner W. S. (2001) Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J. Biol. Chem. 276, 32160–32168 [DOI] [PubMed] [Google Scholar]
- 12. Lampert J. M., Holzschuh J., Hessel S., Driever W., Vogt K., von Lintig J. (2003) Provitamin A conversion to retinal via the beta, beta-carotene-15,15′-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 130, 2173–2186 [DOI] [PubMed] [Google Scholar]
- 13. Scaife A. R., McNeill G., Campbell D. M., Martindale S., Devereux G., Seaton A. (2006) Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br. J. Nutr. 95, 771–778 [DOI] [PubMed] [Google Scholar]
- 14. Yeum K. J., Ferland G., Patry J., Russell R. M. (1998) Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J. Am. Coll. Nutr. 17, 442–447 [DOI] [PubMed] [Google Scholar]
- 15. Dimenstein R., Trugo N. M., Donangelo C. M., Trugo L. C., Anastacio A. S. (1996) Effect of subadquate maternal vitamin A status on placental transfer of retinol and β-carotene to the human fetus. Biol. Neonate. 69, 230–234 [DOI] [PubMed] [Google Scholar]
- 16. Quadro L., Hamberger L., Gottesman M. E., Wang F., Colantuoni V., Blaner W. S., Mendelsohn C. L. (2005) Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 146, 4479–4490 [DOI] [PubMed] [Google Scholar]
- 17. Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., Elste V., Hunziker W., Goralczyk R., Oberhauser V., von Lintig J., Wyss A. (2007) CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J. Biol. Chem. 282, 33553–33561 [DOI] [PubMed] [Google Scholar]
- 18. Ross A. C. (2010) Diet in vitamin A research. Retinoids. Methods Mol. Biol. 652, 295–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quadro L., Hamberger L., Gottesman M. E., Colantuoni V., Ramakrishnan R., Blaner W. S. (2004) Transplacental delivery of retinoid: the role of retinol-binding protein and lipoprotein retinyl ester. Am. J. Physiol. Endocrinol. Metab. 286, E844–E851 [DOI] [PubMed] [Google Scholar]
- 20. Glise D., Riondel J., Favier A. (1998) Comparative distribution of beta-carotene and lycopene after intraperitoneal administration in mice. In Vivo 12, 447–454 [PubMed] [Google Scholar]
- 21. Kim Y. K., Quadro L. (2010) Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 652, 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kane M. A., Napoli J. L. (2010) Quantification of endogenous retinoids. Methods Mol. Biol. 652, 1–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y. K., Wassef L., Hamberger L., Piantedosi R., Palczewski K., Blaner W. S., Quadro L. (2008) Retinyl ester formation by lecithin: retinol acyltransferase (LRAT) is a key regulator of retinoid homeostasis in mouse embryogenesis. J. Biol. Chem. 283, 5611–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shingleton J. L., Skinner M. K., Ong D. E. (1989) Retinol esterification in Sertoli cells by lecithin-retinol acyltransferase. Biochemistry 28, 9647–9653 [DOI] [PubMed] [Google Scholar]
- 25. Fortuna V. A., Trugo L. C., Borojevic R. (2001) Acyl-CoA: retinol acyltransferase (ARAT) and lecithin:retinol acyltransferase (LRAT) activation during the lipocyte phenotype induction in hepatic stellate cells. J. Nutr. Biochem. 12, 610–621 [DOI] [PubMed] [Google Scholar]
- 26. Zolfaghari R., Ross A. C. (2000) Lecithin:retinol acyltransferase from mouse and rat liver. CDNA cloning and liver-specific regulation by dietary vitamin a and retinoic acid. J. Lipid Res. 41, 2024–2034 [PubMed] [Google Scholar]
- 27. Batten M. L., Imanishi Y., Maeda T., Tu D. C., Moise A. R., Bronson D., Possin D., Van Gelder R. N., Baehr W., Palczewski K. (2004) Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem. 279, 10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeum K. J., Russell R. M. (2002) Carotenoid bioavailability and bioconversion. Annu. Rev. Nutr. 22, 483–504 [DOI] [PubMed] [Google Scholar]
- 29. Erdman J. W., Jr., Bierer. T.L., Gugger E. T. (1993) Absorption and transport of carotenoids. Ann. N. Y. Acad. Sci. 691, 76–85 [DOI] [PubMed] [Google Scholar]
- 30. Ulven S. M., Gundersen T. E., Weedon M. S., Landaas V. O., Sakhi A. K., F. S. H., Geronimo B. A., Moskaug J. O., Blomhoff R. (2000) Identification of endogenous retinoids, enzymes, binding proteins and receptors during early postimplantation development in mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans retinoic acid. Dev. Biol. 220, 379–391 [DOI] [PubMed] [Google Scholar]
- 31. Niederreither K., Vermot J., Fraulob V., Chambon P., Dolle P. (2002) Retinaldehyde dehydrogenase 2 (RALDH2)- independent patterns of retinoic acid synthesis in the mouse embryo. Proc. Natl. Acad. Sci. U. S. A. 99, 16111–16116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pasqualetti M., Neun R., Davenne M., Rijli F. (2001) Retinoic acid rescues inner ear defects in Hoxa1 deficient mice. Nat. Genet. 29, 34–39 [DOI] [PubMed] [Google Scholar]
- 33. Napoli J. L., Race K. R. (1988) Biogenesis of retinoic acid from β-carotene. J. Biol. Chem. 263, 17372–17377 [PubMed] [Google Scholar]
- 34. Wang X. D., Krinsky N. I., Tang G. W., Russell R. M. (1992) Retinoic acid can be produced from excentric cleavage of beta-carotene in human intestinal mucosa. Arch. Biochem. Biophys. 293, 298–304 [DOI] [PubMed] [Google Scholar]
- 35. van Helden Y. G., Heil S. G., van Schooten F. J., Kramer E., Hessel S., Amengual J., Ribot J., Teerds K., Wyss A., Lietz G., Bonet M. L., von Lintig J., Godschalk R. W., Keijer J. (2010) Knockout of the Bcmo1 gene results in an inflammatory response in female lung, which is suppressed by dietary beta-carotene. Cell. Mol. Life Sci. 67, 2039–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiser P. D., Palczewski K. (2010) Membrane-binding and enzymatic properties of RPE65. Prog. Retin. Eye Res. 29, 428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shmarakov I., Fleshman M. K., D'Ambrosio D. N., Piantedosi R., Riedl K. M., Schwartz S. J., Curley R. W., Jr., von Lintig J., Rubin L. P., Harrison E. H., Blaner W. S. (2010) Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch. Biochem. Biophys. 504, 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lindqvist A., Sharvill J., Sharvill D. E., Andersson S. (2007) Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J. Nutr. 137, 2346–2350 [DOI] [PubMed] [Google Scholar]
- 40. Mark M., Ghyselinck N. B., Chambon P. (2009) Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 7, e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Engelking L. J., Evers B. M., Richardson J. A., Goldstein J. L., Brown M. S., Liang G. (2006) Severe facial clefting in Insig-deficient mouse embryos caused by sterol accumulation and reversed by lovastatin. J. Clin. Invest. 116, 2356–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Willnow T. E., Hilpert J., Armstrong S. A., Rohlmann A., Hammer R. E., Burns D. K., Herz J. (1996) Defective forebrain development in mice lacking gp330/megalin. Proc. Natl. Acad. Sci. U. S. A. 93, 8460–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.