Abstract
Aquaporin-4 (AQP4) deficiency in mice reduces neuroinflammation in experimental autoimmune encephalomyelitis (EAE) produced by active immunization with myelin oligodendrocyte glycoprotein peptide (MOG). Potential mechanisms for the protective effect of AQP4 deficiency were investigated, including AQP4-dependent leukocyte and microglia cell function, immune cell entry in the central nervous system (CNS), intrinsic neuroinflammation, and humoral immune response. As we found with active-immunization EAE, neuroinflammation was greatly reduced in AQP4-knockout mice in adoptive-transfer EAE. AQP4 was absent in immune cells, including activated T lymphocytes. The CNS migration of fluorescently labeled, MOG-sensitized T lymphocytes was comparable in wild-type and AQP4-knockout mice. Microglia did not express AQP4. Serum anti-AQP4 antibodies were absent in EAE. Remarkably, intracerebral injection of LPS produced much greater neuroinflammation in wild-type than in AQP4-knockout mice, and cytokine (TNF-α and IL-6) secretion was reduced in astrocyte cultures from AQP4-knockout mice. Adenovirus-mediated expression of AQP4, or of an unrelated aquaporin, AQP1, increased cytokine secretion in astrocyte and nonastrocyte cell cultures, supporting the involvement of aquaporin water permeability in cytokine secretion. Our data suggest an intrinsic proinflammatory role of AQP4 involving AQP4-dependent astrocyte swelling and cytokine release. Reduction in AQP4 water transport may be protective in neuroinflammatory CNS diseases.—Li, L., Zhang, H., Varrin-Doyer, M., Zamvil, S. S., Verkman, A. S. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation.
Keywords: water channel, neuromyelitis optics, astrocyte, EAE
Experimental autoimmune encephalomyelitis (EAE) is an extensively used model to study the pathogenesis of neuroinflammatory demyelinating diseases, such as multiple sclerosis, and to evaluate potential therapies (1). Many EAE variants using different animal models and immunization approaches have been developed that produce, to differing extents, characteristic neuroinflammatory lesions in the central nervous system (CNS) with demyelination and clinical motor dysfunction. EAE is mediated by myelin-specific Th1 or Th17 cells (2, 3), although a humoral response is also important in certain models (4, 5).
Motivated by the discovery that aquaporin-4 (AQP4) is the target antigen in the multiple sclerosis variant neuromyelitis optica (NMO; ref. 6) and that AQP4 autoantibodies (NMO-IgG) are involved in NMO disease pathogenesis (7–9), we recently investigated the AQP4 dependence of EAE severity. AQP4 is a water-selective membrane transport protein expressed in astrocytes throughout the CNS that is involved in brain water balance, neuroexcitation and astrocyte migration (10). We found that compared with wild-type mice, AQP4-knockout mice showed remarkably attenuated EAE following active immunization with myelin oligodendrocyte glycoprotein (MOG35–55) peptide, with reduced motor dysfunction, CNS inflammation, and demyelination (11).
The purpose of this study was to investigate potential mechanisms responsible for the neuroprotective effect of AQP4 deficiency in EAE. We systematically investigated the various steps in EAE pathogenesis involving immune cell function, T-lymphocyte penetration into the CNS, and the consequent CNS response, as well as AQP4-dependent humoral responses. Our studies were done using wild-type and AQP4-null mice bred on the C57BL/6 genetic background. AQP4-null mice are not different from wild-type mice in their survival, growth, and behavior, CNS gross and microscopic anatomy, and basal intracranial pressure and blood-brain barrier integrity (12–14). Biophysical studies indicated a mildly expanded extracellular space in the brain cortex of AQP4-null mice at baseline (15, 16). AQP4-null mice manifest significant phenotypes in response to stresses, including reduced cytotoxic brain swelling in water intoxication, stroke, and bacterial meningitis (12, 17); increased vasogenic brain swelling in tumor, abscess, and hydrocephalus (13, 18); reduced astrocyte migration and glial scarring following injury (19, 20); and prolonged seizure and cortical spreading neuroexcitation following mechanical or chemical stimuli (21). Each of these phenotypes is likely explicable on the basis of reduced astrocyte cell osmotic water permeability in AQP4 deficiency but provides no useful clues about the mechanisms of reduced EAE in AQP4 deficiency.
We found here that the neuroprotective effect of AQP4 deficiency in EAE cannot be explained by AQP4-dependent differences in immune cell function or CNS penetration, or microglia cell function or humoral response. We found evidence, however, for an intrinsic proinflammatory role for AQP4 in the CNS, which may have broad implications to CNS diseases associated with inflammation.
MATERIALS AND METHODS
Mice
AQP4-null mice in a C57BL/6 genetic background were obtained by >10 back-crosses of AQP4-null mice generated originally by targeted gene disruption in a CD1 background (22). Studies were performed on 8-to-10-wk-old weight-matched, female C57BL/6 wild-type and AQP4-null mice. Mice were maintained in air-filtered cages and fed normal mouse chow in the University of California, San Francisco (UCSF) Animal Care facility. All procedures were approved by the UCSF Committee on Animal Research.
Active-immunization and adoptive-transfer EAE
MOG35–55 peptide (NH2-MEVGWYRSPFSRVVHLYRNGK-COOH), >98% pure, was synthesized by Biomatik (Wilmington, DE, USA). For active-immunization EAE, MOG35–55 was dissolved in PBS at 4 mg/ml, then 1:1 emulsified with complete Freund's adjuvant supplemented with 4 mg/ml heat-inactivated Mycobacterium tuberculosis H37Ra (Sigma, St. Louis, MO, USA). According to standard procedures (11), mice were injected with 0.1 ml of the emulsion subcutaneously, distributed over 3 sites along the midline of the back between the shoulders. Mice received 200 ng pertussis toxin (Sigma) in 200 μl PBS intraperitoneally at the time of immunization and 2 d later. Mice were assessed daily for clinical signs using the following scoring: 0, normal mouse, no signs of disease; 1, limp tail or hindlimb weakness, but not both; 2, limp tail and hindlimb weakness; 3, partial hindlimb paralysis; and 4, complete hindlimb paralysis. For adoptive-transfer EAE, mice were immunized as described above. At 10–14 d, the draining lymph nodes were isolated, and collected cells were resuspended at 6 × 106 cells/ml in RPMI 1640 medium supplemented with 10% FBS, penicillin/streptomycin, l-glutamine, sodium pyruvate, nonessential amino acids, and β-mercaptoethanol, according to standard procedures (23). MOG35–55 was added at 10 μg/ml together with recombinant murine IL-12 (Sigma) at 5 ng/ml and recombinant human IL-2 (Sigma) at 50 U/ml. After 3 d in culture (37°C, 5% CO2), collected cells were suspended in HBSS for transfer. Mice received 2 × 107 viable cells by retro-orbital sinus injection. Pertussis toxin (200 ng) in 200 μl PBS was given intraperitoneally just after cell transfer and again 48 h later. Clinical score was assessed daily as described above. After adoptive transfer, at d 15, brain and spinal cord were removed for staining with hematoxylin and eosin (H&E), Luxor fast blue (for myelin), and CD45 immunoreactivity. H&E and CD45-stained sections were scored for the severity of inflammation using the following scale: 0, no inflammation; 1, mild inflammation with few mononuclear cells infiltrates; 2, marked inflammation with multiple infiltrates per ×100 field; and 3, severe inflammation with extensive infiltrates in both white and gray mater.
Intracerebral LPS
Mice were anesthetized by intraperitoneal 2,2,2-tribromoethanol (125 mg/kg, Sigma), and the head was immobilized in a stereotactic frame (Benchmark, Neurolab, St. Louis, MO, USA). Core temperature was maintained at 37–38°C using a heating lamp and rectal temperature probe. The skin above the brain was shaved and disinfected with betadine. A midline scalp incision was made to expose the bregma and lambda. A burr hole was made on the right side, at a location 1.2 mm posterior to bregma and 1 mm right lateral to midline using a high-speed drill (0.7 mm burr; Foredom, Bethel, CT, USA). LPS (Escherichia coli 0111:B4; Sigma) at 1 μg/μl in sterile PBS was injected at a depth of 2.5 mm through a 30-gauge needle attached to a 10-μl Hamilton syringe (Hamilton, Reno, NV, USA). The injection volume was 2 μl, and the needle was kept in place for 5–10 min after injection. The scalp was closed with 5-0 silk suture. Mice were killed 24 h later by anesthetic overdose and underwent transcardiac perfusion with PBS followed by 4% paraformaldehyde. Brains were removed, fixed in 4% paraformaldehyde for 24 h, and embedded in paraffin. In some experiments, at 2 h after LPS injection, brains were homogenized in ice-cold lysis buffer containing 25 mM HEPES (pH 7.4), 0.1% 3-[(3-cholamidopropyl) dimethyl-ammonio]1-propanesulfonate; 5 mM MgCl2; 1.3 mM EDTA; 1 mM EGTA; 10 μg/ml pepstatin, aprotinin and leupeptin; and 1 mM PMSF. Homogenates were centrifuged (15 min at 50,000 rpm) and stored at −80°C for mouse TNF-α ELISA assay (Invitrogen, Carlsbad, CA, USA).
RT-PCR and immunostaining
For RT-PCR, mice (control and MOG sensitized) were killed by anesthetic overdose, and lymph nodes and spleen were removed. Total RNA was isolated by homogenization in TRIzol reagent (Invitrogen). cDNA was reverse-transcribed from 5 μg mRNA with oligo(dT) (Super-Script first-strand synthesis system for RT-PCR; Invitrogen), using kidney as a positive control. After reverse transcription, PCR was carried out using gene-specific primers designed to amplify the AQP4 coding sequence. Fluorescence-based real-time reverse transcription-PCR (RT-PCR) using 2 μg cDNA from astrocyte cultures was carried out using a LightCycler with FastStart DNA MasterPLUS SYBR Green I kit (Roche Diagnostics, Indianapolis, IN, USA). PCR conditions comprised an initial step at 95°C for 5 min followed by 40 cycles at 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s. Primers were as follows: β-actin: 5′-TGTATGCCTCTGGTCGTACC-3′ (sense), 5′-CAGGTCCAGACGCAGGATG-3′ (antisense); TLR4: 5′-CAAGTTTAGAGAATCTGGTGGCTGTGG-3′ (sense), 5′-TGAAAGGCTTGGTCTTGAAT GAAGTCA-3′ (antisense); TNF-α: 5′-CTGTAGCCCACGTCGTAGC-3′ (sense), 5′-TTGAGATCCATGCCGTTG-3′ (antisense); NF-κB: 5′-CAGCTCTTCTCAAAGCAGCA-3′ (sense), 5′-TCCAGGTCATAGAGAGGCTCA-3′ (antisense). β-Actin was used as the reference gene, and pooled wild-type and AQP4-null astrocyte cDNA was used as the calibrator. Results are reported as normalized, calibrated ratios.
For immunofluorescence, tissues and astrocyte cultures were fixed in the 4% paraformaldehyde. Paraffin sections were stained with H&E or Luxol fast blue. For CD45 immunocytochemistry, paraffin-embedded sections were cut at 5-μm thickness and deparaffinized, then treated with citrate buffer using microwave antigen retrieval and 3% hydrogen peroxide. Sections were incubated for 2 h with a primary CD45 antibody (1:200; Abcam, Cambridge, MA, USA) at room temperature, then incubated with biotinylated secondary goat anti-rabbit antibody (1:1000; Vector Laboratories, Burlingame, CA, USA), followed by avidin-biotin peroxidase complex (1:1000). Peroxidase labeling was visualized with diaminobenzidine to yield a brown color. For Iba-1, AQP4, and glial fibrillary acidic protein (GFAP) immunofluorescence, sections or cells were incubated with rabbit anti-Iba-1 (1:500; Wako, Osaka, Japan), rabbit anti-AQP4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:200) or mouse anti-GFAP (1:500; Millipore, Billerica, MA, USA) primary antibody and goat anti-rabbit or anti-mouse secondary antibody (1:200; Molecular Probes, Eugene, OR, USA). Cell nuclei were stained blue with DAPI.
CNS penetration of fluorescently labeled, MOG-sensitized T lymphocytes
To fluorescently label lymphocytes, a stock solution of carboxyfluorescein succinimidyl ester (CFSE; 10 mM in DMSO, Invitrogen) was diluted in PBS to 25 μM. Lymphocytes, as cultured and activated for adoptive-transfer EAE, were suspended in PBS containing 1% BSA at 2 × 106 cells/ml and incubated with CFSE for 15 min at 37°C. Cells were washed and resuspended in culture medium for 15 min, and then resuspended in PBS on ice. Gentle trituration was used to minimize cell clumping. Cells were injected into wild-type and AQP4-null mice as done for adoptive-transfer EAE. Brains were harvested at 24 and 48 h after intracardiac perfusion with PBS and 10-μm-thick frozen sections were cut for counting of fluorescent cells.
Blood-brain barrier permeability
Control and MOG-immunized mice (after 12 d) were injected intravenously with Evans blue dye (4% in PBS, 160 mg/kg, Sigma). After 90 min, mice were anesthetized, and the left cardiac ventricle was perfused with 20 ml PBS. Brains were removed and immersed in 1 ml formamide (Sigma) at 55°C overnight to extract the Evans blue dye. Extracted dye was quantified by optical absorbance at 610 nm against Evans blue/formamide standards.
Lymphocyte proliferation assay
Splenic CD3+ T lymphocytes from MOG35–55-specific T-lymphocyte receptor transgenic (2D2) mice were purified (>95%) by MACS (Miltenyi Biotec, Bergisch Gladbach, Germany), then cultured with irradiated splenocytes from naive wild-type or AQP4-deficient mice with different concentrations of MOG35–55. At 72 h, cultures were pulsed with 1 μCi [3H]-thymidine and harvested 18 h later for measurement of [3H]-thymidine incorporation.
Anti-AQP4 autoantibody assay
Wild-type mice were immunized with MOG35–55 peptide. At specified times, blood was collected for serum isolation. For immunofluorescence, FRT-AQP4 cells were fixed in 4% paraformaldehyde for 15 min, and blocked with 5% BSA for 1 h. Cells were incubated with EAE (or control) serum at 1:50 or 1:200 for 2 h at room temperature, then incubated with goat anti-mouse secondary antibody (1:200, Molecular Probes). As a positive control, cells were immunostained with human serum from a seropositive NMO patient who met the established criteria for diagnosis of NMO, using anti-human secondary antibody (1:200; Molecular Probes).
Cell culture and adenovirus infection
Primary astrocyte cultures were prepared from cortex of wild-type and AQP4-null neonatal mice, as described previously (24). Briefly, the cerebral hemispheres were isolated, the meninges were dissected away, and the hippocampus, basal ganglia, and olfactory bulb were removed. Cortical tissues were minced with forceps and incubated for 15 min at 37°C in DMEM containing 0.25% trypsin-EDTA. Dissociated cells were centrifuged at 500 g for 5 min and resuspended in DMEM containing 10% FBS and 1% penicillin/streptomycin in polylysine-coated 75 cm2 flasks and grown at 37°C in a 5% CO2 incubator with a change of medium 2×/wk. After cell confluence (d 8–10), flasks were shaken in rotator at 180 rpm overnight, then at 220 rpm for 4 h to purify astrocyes. At confluence (d 12–15), cultures were treated with 10 μM cytosine arabinoside for 48 h to prevent proliferation of other cell types, and the medium was replaced with DMEM containing 3% FBS and 0.15 mM dibutyryl cAMP to induce differentiation. Cultures were maintained for up to 2 wk longer with a change of medium 2×/wk. Immunocytochemistry showed that >95% of the cells stained positively for the astrocytic marker, GFAP. T24 cells (human bladder carcinoma cell line, HTB-4; American Type Culture Collection, Manassas, VA, USA) were cultured at 37°C in 5% CO2 in complete DMEM medium containing 10% FBS, 100 U/ml penicillin, and l00 μg/ml streptomycin. Fisher rat thyroid (FRT) cells stably transfected with AQP4 were cultured at 37°C (5% CO2) with Coon's F-12 growth medium containing 10% FBS, 10 U/ml penicillin, 100 μg/ml streptomycin, 4 mM L-glutamine, and 0.6 mg/ml zeocin.
Adenoviruses containing full-length coding sequences of AQP1, M1-AQP4, M23-AQP4, and green fluorescent protein (GFP) were generated by Vector Biolaboratories. For virus infection, AQP4-null astrocyte cell cultures were seeded on 24-well plates. After 1 wk, cells were incubated in 1 ml DMEM medium with 5% FBS containing adenovirus at a multiplicity of infection (MOI) of 20, which was determined to give strong expression without affecting cell viability. The virus-containing medium was removed 3 h later and replaced with fresh DMEM medium containing 10% FBS. Cells were used 2 or 3 d later. For infection of T24 cells, cells on 24-well plates at 50% confluence were incubated for 36 h in 1 ml DMEM medium with 5% FBS containing adenovirus at a MOI of 500, then the medium was replaced with fresh DMEM/H-16 medium containing 10% FBS.
In some experiments, cytokine secretion was induced in cell cultures by a 2-h incubation with LPS (100 ng/ml) with or without 20 mM d-mannitol, or with manganese (III) acetate dihydrate (Sigma). Supernatants and/or cells were collected and kept at −80°C for cytokine assay. Protein content was assayed by the bicinchoninic (BCA) procedure, and tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) were assayed by ELISA (BD Bioscience, San Jose, CA, USA).
RESULTS
Adoptive-transfer EAE
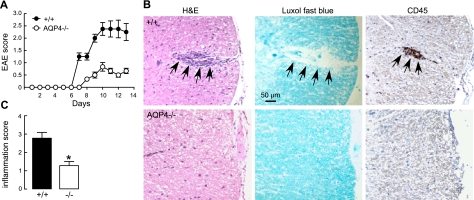
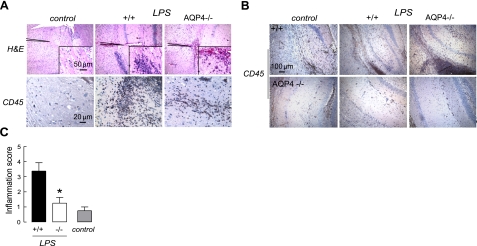
Our previous study of EAE in AQP4 deficiency involved EAE production by active immunization with MOG35–55 oligopeptide. Here, we first determined whether AQP4 deficiency is also protective in an adoptive-transfer model of EAE in which sensitized T lymphocytes from MOG-treated wild-type mice were cultured and sensitized in vitro, and then introduced intravenously into naive wild-type or AQP4-null recipient mice. As shown in Fig. 1A, clinical signs of EAE were seen by 6 d after intravenous transfer. EAE disease severity, as assessed by clinical score, was much reduced in AQP4-null vs. wild-type mice. Representative histology in Fig. 1B (left panels) shows greater inflammation in spinal cord of wild-type mice at 10 d after T-lymphocyte transfer. Figure 1B shows corresponding significant loss of myelin (middle panels), and that the inflammatory cell infiltrates are primarily CD45-positive mononuclear cells (right panels), as expected in EAE. Histology scores are summarized in Fig. 1C. These experiments show that AQP4 deficiency in recipient mice is protective in adoptive-transfer EAE.
Figure 1.
Attenuated adoptive-transfer EAE in AQP4-null mice. A) Activated, MOG-sensitized T lymphocytes from wild-type mice were transferred to naive wild-type (+/+) or AQP4-null (−/−) mice. Means ± se of clinical scores (4 mice/group). B) Representative spinal cord H&E staining (left panels), Luxol fast blue staining (middle panels), and CD45 immunocytochemistry (right panels) at 15 d after T-lymphocyte transfer into wild-type or AQP4-null mice. Arrows denote EAE lesion. C) Inflammatory score determined by masked assessment of H&E and CD45 sections (se, 3 mice/group, 5 sections examined/mouse). *P < 0.001.
AQP4 expression in immune cells
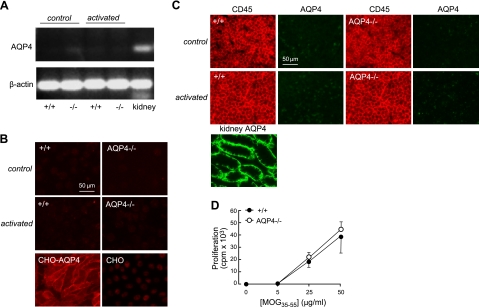
We investigated whether AQP4 expression in immune cells might account for the reduced severity of EAE in AQP4-null mice. AQP4 expression was studied by RT-PCR and immunofluorescence. Figure 2A shows no detectable AQP4 transcript by RT-PCR of T lymphocytes before or after in vitro culture, as done in adoptive-transfer EAE. Mouse kidney cDNA was the positive control. Figure 2B shows no detectable AQP4 protein in T lymphocytes by immunofluorescence, with AQP4-expressing Chinese hamster ovary (CHO) cells as a positive control. Figure 2C shows no AQP4 immunofluorescence in lymph nodes from control or MOG-immunized mice (as harvested for adoptive-transfer EAE). AQP4 immunofluorescence of mouse kidney was the positive control. Lymph node size in untreated wild-type and AQP4-null mice was comparable, as was the increase in lymph node size after MOG treatment (data not shown). Further, splenic antigen-presenting cells from wild-type and AQP4-null mice presented MOG35–55 to naive 2D2 T lymphocytes with comparable efficiency (Fig. 2D). These results indicate that AQP4 expression in immune cells is not responsible for the attenuated EAE in AQP4 deficiency.
Figure 2.
Absence of AQP4 expression in immune cells. A) RNA was isolated from T lymphocytes, without (control) or after MOG sensitization (as done for adoptive-transfer EAE), and from mouse kidney (positive control). AQP4 and β-actin were amplified by RT-PCR using specific primers. Representative of 3 sets of amplifications on different cultures. B) AQP4 immunofluorescence in T lymphocytes, without or after MOG sensitization. AQP4-expressing Chinese hamster ovary (CHO) cells (CHO-AQP4) as the positive control and nontransfected CHO cells (CHO) as the negative control. C) CD45 and AQP4 immunofluorescence in lymph nodes of control mice and at 15 d after MOG immunization. Mouse kidney shown as a positive control for AQP4. D) Splenocytes from wild-type and AQP4-deficient mice stimulate proliferation of MOG35–55-specific T lymphocyte in a similar manner. MACS-separated 2D2 T lymphocytes were cultured with irradiated splenic antigen-presenting cells from naive wild-type or AQP4-deficient mice in the presence of MOG35–55. Proliferation was measured by [3H]-thymidine incorporation (sd, 3 sets of cultures, differences not significant).
CNS penetration of MOG-sensitized T lymphocytes
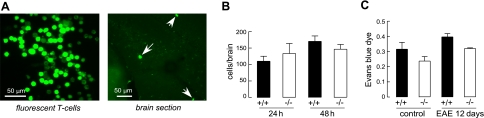
The possibility was tested that reduced immune cell penetration into the CNS might be responsible for the reduced severity of EAE in AQP4-null mice. Activated T lymphocytes are thought to efficiently permeate the blood-brain barrier by mechanisms involving specific receptors (25). We generated MOG-sensitized T lymphocytes from wild-type mice in vitro, as done for adoptive-transfer EAE, which were labeled with a green fluorescent dye and injected intravenously in wild-type and AQP4-null mice. The presence of fluorescently labeled T lymphocytes in brain was measured at 24 and 48 h after injection, examining 10-μm-thick frozen brain sections. Figure 3A shows the fluorescent, MOG-activated T lymphocytes used for injection, along with a representative frozen section of brain containing the fluorescent cells. Figure 3B shows no significant difference in the number of fluorescent T lymphocytes counted in brain slices at 24 or 48 h after injection.
Figure 3.
AQP4 deletion does not alter penetration of T lymphocytes into the CNS. MOG-sensitized T lymphocytes (as prepared for adoptive-transfer EAE) were fluorescently labeled with CFSE. Naive wild-type and AQP4-null mice were injected intravenously with 2 × 107 labeled lymphocytes, and brains were perfused and harvested at 24 and 48 h. A) Left panel: fluorescently labeled MOG-activated T lymphocytes. Right panel: representative frozen section of brain, showing fluorescent T lymphocytes that crossed the blood-brain barrier. B) Number of fluorescent T lymphocytes counted in 10-μm-thick brain sections per mouse at 24 and 48 h (se, 3 mice/group at each time point, differences not significant). C) Extravasated Evans blue dye in brain in control mice and at 12 d after MOG immunization (se, 4 mice/group at each time point, differences not significant).
Blood-brain barrier integrity was also examined. The blood-brain barrier at baseline was comparably tight in wild-type and AQP4-null mice as assessed by Evan's blue dye extravasation (Fig. 3C), in agreement with prior results for wild type vs. AQP4-null mice in a CD1 genetic background (12, 14). Blood-brain barrier permeability was also not different in wild-type vs. AQP4-null mice when measured at a relatively early time (12 d) after active immunization with MOG35–55 oligopeptide, prior to development of clinical weakness. These studies suggest that differences in blood-brain barrier properties cannot account for the reduced EAE severity in AQP4 deficiency.
AQP4 humoral response
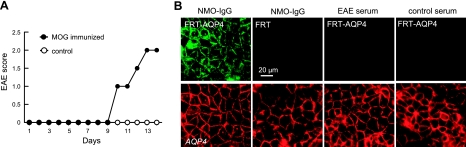
On the basis of the finding that AQP4 autoantibodies produce NMO-like neuroinflammatory lesions when injected directly into brain of naive mice (9) and exacerbate neuroinflammatory lesions in rats with preexisting EAE (7, 8), we tested whether AQP4 autoantibodies are produced in EAE in mice. EAE-associated AQP4 autoantibodies in wild-type mice could contribute to their greater EAE disease severity. Serum from mice was obtained at 13 d after active immunization with MOG, at which time significant neurological impairment was seen (Fig. 4A). The presence of AQP4 autoantibodies was assayed using FRT cells overexpressing mouse AQP4 by transient transfection. Figure 4B shows no detectable AQP4 autoantibodies in control or EAE sera. All serum samples from immunized and control mice were negative, tested up to a 1:50 dilution. As a positive control a 1:200 dilution of serum from an NMO patient produced strong immunofluorescence in the AQP4-expressing cells but not in nontransfected cells.
Figure 4.
Absence of anti-AQP4 autoantibodies in EAE. A) Active-immunization EAE was produced in wild-type mice by MOG35–55 peptide. Clinical score. B) AQP4-expressing FRT cells (FRT-AQP4) and control nontransfected cells (FRT) stained with serum from control and EAE mice, and green fluorescent secondary anti-mouse antibody. Positive control is human serum from patient with NMO (EAE serum). AQP4 stained red. Representative of 3 sets of staining studies.
Intrinsic CNS inflammatory response
We tested whether intrinsic differences in the CNS host response to inflammatory stimuli might be responsible for the reduced severity of EAE in AQP4 deficiency. Neuroinflammation was produced by injection of LPS directly into brain parenchyma in order to bypass the blood-brain barrier penetration step. Neuroinflammation was assessed at 24 h after LPS injection. Figure 5A shows brain H&E staining and CD45 immunocytochemistry in control (saline-injected) and LPS-injected mice. Extensive leukocyte infiltrates were seen in LPS-injected wild-type mice, which were relatively infrequent in AQP4-null mice. Figure 5B shows a gallery of brain sections from 3 wild-type and 3 AQP4-null mice. Figure 5C summarizes inflammation scores obtained by two independent observers who assessed the H&E and CD45-stained sections in a masked procedure. There was significantly greater inflammation in the LPS-treated wild-type mice than the LPS-treated AQP4-null mice. A small amount of inflammation was seen in control mice as a consequence of the needle insertion.
Figure 5.
Reduced brain inflammation in AQP4-null mice after intracerebral LPS injection. Mice were injected intracerebrally with PBS (control) or LPS. A) Brain histology at 1 d by H&E staining (top panels) and CD45 immunocytochemistry (bottom panels). B) Gallery of CD45-immunostained sections of hippocampus from 3 wild-type and 3 AQP4-null mice. C) Quantification of inflammation done using H&E and CD45-stained sections (se). *P < 0.01 vs. +/+.
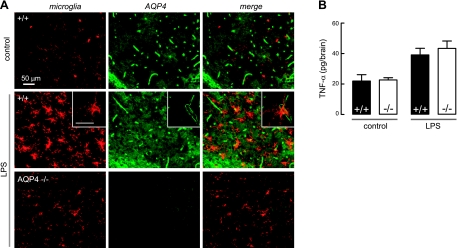
A prior report suggested the expression of AQP4 in microglia after LPS administration (26), which, if correct, would raise the possibility of AQP4-dependent microglia cell function as a determinant of EAE severity. Using Iba-1 as a selective marker of microglia, we found microglial cell activation in LPS-treated mice, greater in wild-type than AQP4-null mice (Fig. 6A), as expected from their greater neuroinflammatory response. However, the expression patterns of AQP4 and Iba-1 were nonoverlapping, indicating absence of AQP4 expression in microglia. Absence of AQP4 expression in microglia was also found in mice following active-immunization and adoptive-transfer EAE (data not shown). The absence of AQP4 in microglia indicates that AQP4-dependent differences in intrinsic microglia cell responses cannot account for the reduced EAE severity in AQP4 deficiency. This conclusion was supported by measurements of the release of a cytokine (TNF-α) in brain at 3 h after LPS administration. At this short time, TNF-α release is primarily from microglial cells and is minimally confounded by secondary effects of neuroinflammation, such as astrocyte activation, local blood-brain barrier breakdown, and cell extravasation. Figure 6B shows significant TNF-α elevation in LPS vs. saline-injected brain, but no differences in wild type vs. AQP4-null mice.
Figure 6.
Microglial cells do not express AQP4. A) Absence of AQP4 expression in reactive microglia following intracerebral injection of LPS. Immunofluorescence of brain sections stained for microglial marker anti-Iba1 (red) and AQP4 (green). Representative of 3 sets of experiments. Scale bars = 50 μm. B) TNF-α in whole-brain homogenates at 2 h after intracerebral injection of saline (control) or LPS (se, n=4, differences between +/+ and −/− not significant).
Cytokine secretion in cell cultures
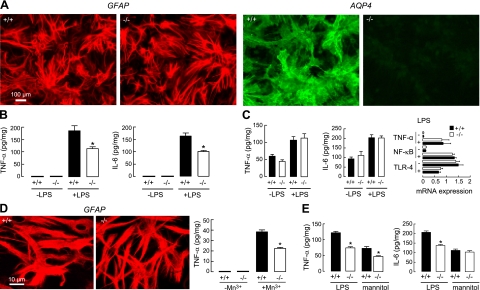
To investigate the possible involvement of AQP4 in the inflammatory response of astrocytes, measurements of cytokine release were made on differentiated primary astrocyte cultures from brain cortex of neonatal wild-type and AQP4-null mice. Figure 7A shows similar morphology and GFAP immunoreactivity of the cultures (left panels), with strong AQP4 expression in the cultures from wild-type mice (right panels). Cultures were judged to be free of microglia by Iba-1 staining (not shown). Initial screening of a panel of 12 cytokines showed robust elevations in TNF-α and IL-6 at 2 h after LPS addition in astrocyte cultures from wild-type mice. TNF-α and IL-6 were, therefore, measured in subsequent experiments. Figure 7B shows significantly greater release of both TNF-α and IL-6 into the medium of cultures from wild-type vs. AQP4-null mice at 2 h after LPS addition, with the levels of these cytokines near the limit of detection in the absence of LPS. This was a robust finding seen in 5 separate sets of cultures. However, cell-associated TNF-α and IL-6 were not different in cultures from wild-type vs. AQP4-null mice at 2 h after LPS (Fig. 7C, left and middle panels). The AQP4-dependent difference in cytokine release was not related to reduced TLR-4 expression in the AQP4-deficient astrocytes, as demonstrated by quantitative RT-PCR (Fig. 7C, right panel) and immunofluorescence (not shown). Figure 7C (right panel) also shows comparable increases in TNF-α and NF-κB transcript expression following LPS. These data suggest AQP4 involvement in cytokine release/secretion rather than in transcription or translation. Reduced TNF-α release was also found at 24 h after addition of Mn3+, which causes cytokine release in a TLR-4-independent manner (Fig. 7D, right panel). We found greater swelling in Mn3+-treated astrocytes from wild-type vs. AQP4-null mice (Fig. 7D, left panels), suggesting a possible mechanism for AQP4-dependent cytokine release involving astrocyte water permeability and cell swelling. In support of a water permeability/cell-swelling mechanism was the finding of reduced TNF-α and IL-6 release from astrocytes when the culture medium was made mildly hyperosmolar with mannitol to minimize cell swelling (Fig. 7E).
Figure 7.
Reduced cytokine release by astrocyte cultures in AQP4 deficiency. A) GFAP (left panels) and AQP4 (right panels) immunofluorescence of differentiated, primary cultures of astrocytes from neonatal mouse brain cortex. B) TNF-α and IL-6 in culture medium at 2 h after LPS (100 ng/ml) or saline addition (se, n=6). Representative of 5 sets of cultures. C) Cell-associated TNF-α and IL-6 at 2 h after LPS (se, n=4, differences not significant comparing −LPS and +LPS). Representative of 3 sets of cultures. (right) Quantitative real-time RT-PCR of indicated transcripts from astrocyte cultures (se, n=4, differences not significant comparing −LPS and +LPS). D) Right panel: TNF-α in culture medium at 24 h after Mn3+ exposure (se, n=4). Left panels: GFAP immunofluorescence of astrocytes after 24 h Mn3+ exposure. E) TNF-α and IL-6 in culture medium at 2 h after LPS in control medium (290 mosmol) or hyperosmolar (310 mosmol) medium containing excess 20 mM mannitol (se, n=4). *P < 0.01 vs. corresponding +/+.
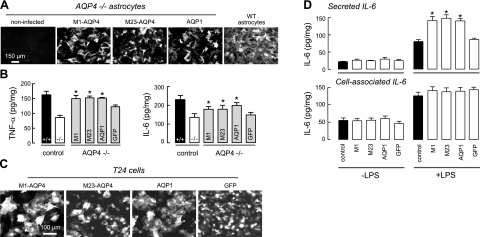
To investigate whether the increase in cytokine secretion is related to the water permeability function of AQP4, we measured LPS-induced secretion of TNF-α and IL-6 in AQP4-null astrocytes after adenovirus-mediated expression of aquaporins. Figure 8A shows expression of M1-AQP4, M23-AQP4, and AQP1 at 3 d after adenovirus infection. AQP1 was studied as an unrelated aquaporin with similar water permeability function to AQP4. At an MOI of 20, expression of aquaporins was seen in most astrocytes, without evidence of cytotoxicity. By immunofluorescence, the expression of M1 and M23 AQP4 was comparable to that seen in astrocyte cultures from wild-type mice. Figure 8B show significantly increased TNF-α and IL-6 secretion at 2 h after LPS in the AQP4-null astrocytes after adenoviral expression of M1-AQP4, M23-AQP4, or AQP1, compared to noninfected or GFP adenovirus-infected cultures. These results suggest the involvement of AQP4 water permeability in cytokine release. To test the generality of aquaporin-facilitated cytokine secretion, we identified a nonastrocyte cell line that showed robust LPS-induced IL-6 secretion and does not express aquaporins, T24 urinary bladder cells. Figure 8C shows expression of M1-AQP4, M23-AQP4, AQP1, and GFP in T24 cells after adenovirus infection. Figure 8D (top panel) shows significantly increased LPS-stimulated IL-6 secretion in the aquaporin-expressing T24 cells compared with control or GFP-expressing cells, without increased cell-associated IL-6 (Fig. 8D, bottom panel). These data suggest that aquaporin-facilitated cytokine secretion is not cell type specific.
Figure 8.
Evidence for aquaporin-facilitated cytokine secretion. A) Immunofluorescence of AQP4 (M1 and M23 isoforms) and AQP1 in adenovirus-treated primary astrocyte cultures from AQP4-null mice (AQP4−/− astrocytes). AQP4 immunofluorescence of culture from wild-type mice shown at right. B) TNF-α and IL-6 in culture medium at 2 h after LPS (100 ng/ml) or saline addition (se, n=4). Representative of 3 sets of cultures/infections. *P < 0.01 vs. −/− control. C) AQP4 and AQP1 immunofluorescence, and GFP fluorescence, of adenovirus-treated T24 (bladder) cells. D) Secreted IL-6 (in culture medium, top panel) and cell-associated IL-6 (bottom panel) at 2 h after addition of LPS (se, n=4). *P < 0.01 vs. LPS-treated control.
DISCUSSION
Neuroinflammation and demyelination in MOG-induced EAE in mice involves a series of steps, including T-lymphocyte sensitization and activation, passage into the CNS across the blood-brain barrier, and initiation of a neuroinflammatory cascade involving multiple inflammatory factors and immune cell actions. There is also a potential role for humoral factors, as antibodies against various CNS targets have been identified in EAE (27). Here, we systematically dissected these processes in order to determine the AQP4-dependent steps that might account for the reduced severity in AQP4 deficiency in our original study of EAE produced by active immunization with MOG35–55 oligopeptide (11), and, as found here, in EAE produced adoptive-transfer of MOG-sensitized T lymphocytes. AQP4 expression was absent in the relevant immune cells, as well as in microglia. The CNS penetration of intravenously administered, MOG-sensitized T lymphocytes was comparable in wild-type and AQP4-null recipient mice, as was Evan's blue dye extravasation. Anti-AQP4 antibodies were not found in the EAE model. The remarkable positive finding was a greater inflammatory response in brain of wild-type vs. AQP4-null mice following LPS challenge. Our data implicate AQP4 as a novel determinant of neuroinflammation that likely accounts for the reduced EAE severity in AQP4 deficiency, and for the greatly reduced neuroinflammatory response in AQP4 deficiency in a mouse model of bacterial meningitis (17).
The known biology of AQP4 and the findings here in astrocyte cultures suggest the cellular mechanisms responsible for the involvement of AQP4 in neuroinflammation. AQP4 facilitates water movement into the brain through an intact blood-brain barrier in cytotoxic brain edema, as seen in water intoxication and ischemic stroke (12); as a bidirectional water transporter AQP4 also facilitates exit of excess water from the brain in vasogenic brain edema, as seen in brain tumor, abscess, and obstructive hydrocephalus (13). AQP4-facilitated water transport by astrocytes at the blood-brain and cerebrospinal fluid-brain barriers likely accounts for the AQP4-dependent water movement in brain. AQP4 plays a similar role in spinal cord, as seen in studies of clinical and histological outcomes following compression (28) and impact (29) spinal cord injury. Astrocyte water permeability is at least 5–10 times reduced in AQP4 deficiency, as demonstrated in astrocyte cultures and in vivo (30). We found here that cytokine release from astrocyte cultures was reduced in AQP4 deficiency in response to very different stimuli, including LPS and Mn3+. The increased swelling of astrocytes from wild-type mice and the reduced cytokine release in hyperosmolar media suggest a mechanism involving AQP4-dependent astrocyte water permeability and consequent cell swelling to account for the reduced cytokine release in AQP4 deficiency. The increased cytokine release following astrocyte and nonastrocyte cell transfections with AQP4, or an unrelated aquaporin, AQP1, supports the involvement of aquaporin water permeability in cytokine secretion. Evidence for involvement of aquaporins in an analogous process, secretory vesicle exocytosis, has been reported (31, 32), though the biophysical mechanisms remain speculative on how aquaporin water transport facilitates fusion of secretory vesicles with the cell plasma membrane. Although other mechanisms to account for the proneuroinflammatory effect of AQP4 cannot be ruled out, such as differences in expression of various genes in AQP4 deficiency, our proposed mechanism provides a direct link between the unique molecular function of AQP4, osmotic water transport, and neuroinflammation. We propose that the proinflammatory role of AQP4 involves a positive-feedback cycle of local brain swelling (cytotoxic edema) and secretion of proinflammatory cytokines, which, at the molecular level, hinges on AQP4-dependent osmotic water permeability and astrocyte swelling.
A second major role of AQP4 in brain is in astrocyte migration, which we have proposed involves AQP4-facilitated water transport in lamellipodia at the leading edge of migrating cells. AQP4-null astrocytes migrate much slower than wild-type astrocytes in vitro (20) and in vivo (19), and glial scarring is reduced in AQP4-null mice (20). AQP4-dependent astrocyte migration is unlikely to play a role in LPS-induced neuroinflammation here, as little astrocyte migration occurs in 24–48 h following a neuroinflammatory stimulus. A third role of AQP4 is in neuroexcitation, where AQP4-facilitated water transport in astrocytes modulates extracellular space water and K+ handling. Mice lacking AQP4 manifest increased seizure severity (21), delayed K+ reuptake from the extracellular space following neuroexcitation (33), and impaired visual (34), auditory (35), and olfactory (36) signal transduction. AQP4-dependent neuroexcitation is unlikely to play a role in neuroinflammation, as AQP4 deficiency would be predicted to increase rather than reduce neuroexcitotoxicity and downstream neuroinflammatory responses. Finally, the mild extracellular space expansion in AQP4 deficiency (15, 16) is unlikely to be responsible for reduced neuroinflammation because the CNS diffusion of inflammatory cells and soluble factors would be increased, rather than reduced, in AQP4 deficiency.
Our original motivation for studying EAE in AQP4 deficiency was to obtain insight into the pathogenesis of NMO, where AQP4 autoantibodies are found in the majority of NMO patients (37). A central question in NMO has been the role of NMO-IgG in disease pathogenesis, versus, or perhaps in addition to, activated T lymphocytes as in EAE. Evidence was reported recently that NMO-IgG, when injected together with human complement into brain parenchyma of naive mice, produced, in 10 d, characteristic NMO lesions with perivascular neuroinflammation and complement deposition, demyelination, and loss of AQP4 and GFAP immunoreactivity (9). Neuroinflammatory lesions were not produced in AQP4-null mice. Our findings here suggest a second, AQP4-dependent mechanism for modulation of neuroinflammation in NMO and other neuroinflammatory diseases. We note that though AQP4 expression is generally low within active NMO lesions, it is increased in surrounding brain in NMO; in contrast, AQP4 expression remains intact within plaque lesions in multiple sclerosis (38, 39).
In summary, our results establish a novel role for AQP4 in neuroinflammation, which we propose at the cellular level involves AQP4-dependent differences in astrocyte water permeability and consequent cell swelling and cytokine release. Pharmacological inhibition of AQP4 water permeability or reduction in AQP4 plasma membrane expression may thus be of benefit in the therapy of neuroinflammatory diseases.
Acknowledgments
This work was supported by grants from the Guthy-Jackson Charitable Foundation (A.S.V. and S.S.Z.); U.S. National Institutes of Health (NIH) grants DK35124, EY13574, HL73856, DK72517, DK86125, and EB00415 (A.S.V.); and NIH grants AI73737 and NS63008, National Multiple Sclerosis Society grant RG4124, and the Maisin Foundation (S.S.Z.).
REFERENCES
- 1. Ransohoff R. M. (2006) A mighty mouse: building a better model of multiple sclerosis. J. Clin. Invest. 116, 2313–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009) IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 [DOI] [PubMed] [Google Scholar]
- 3. Kroenke M. A., Carlson T. J., Andjelkovic A. V., Segal B. M. (2008) IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 205, 1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lyons J. A., Ramsbottom M. J., Cross A. H. (2002) Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur. J. Immunol. 32, 1905–1913 [DOI] [PubMed] [Google Scholar]
- 5. Weber M. S., Prod'homme T., Patarroyo J. C., Molnarfi N., Karnezis T., Lehmann-Horn K., Danilenko D. M., Eastham-Anderson J., Slavin A. J., Linington C., Bernard C. C., Martin F., Zamvil S. S. (2010) B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann. Neurol. 68, 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lennon V. A., Kryzer T. J., Pittock S. J., Verkman A. S., Hinson S. R. (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett J. L., Lam C., Kalluri S. R., Saikali P., Bautista K., Dupree C., Glogowska M., Case D., Antel J. P., Owens G. P., Gilden D., Nessler S., Stadelmann C., Hemmer B. (2009) Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 66, 617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinoshita M., Nakatsuji Y., Kimura T., Moriya M., Takata K., Okuno T., Kumanogoh A., Kajiyama K., Yoshikawa H., Sakoda S. (2009) Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem. Biophys. Res. Commun. 386, 623–627 [DOI] [PubMed] [Google Scholar]
- 9. Saadoun S., Waters P., Bell B. A., Vincent A., Verkman A. S., Papadopoulos M. C. (2010) Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verkman A. S., Binder D. K., Bloch O., Auguste K., Papadopoulos M. C. (2006) Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim. Biophys. Acta 1758, 1085–1093 [DOI] [PubMed] [Google Scholar]
- 11. Li L., Zhang H., Verkman A. S. (2009) Greatly attenuated experimental autoimmune encephalomyelitis in aquaporin-4 knockout mice. BMC Neurosci. 10, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manley G. T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A. W., Chan P., Verkman A. S. (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 6, 159–163 [DOI] [PubMed] [Google Scholar]
- 13. Papadopoulos M. C., Manley G. T., Krishna S., Verkman A. S. (2004) Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 18, 1291–1293 [DOI] [PubMed] [Google Scholar]
- 14. Saadoun S., Tait M. J., Reza A., Davies D. C., Bell B. A., Verkman A. S., Papadopoulos M. C. (2009) AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience 161, 764–772 [DOI] [PubMed] [Google Scholar]
- 15. Yao X., Hrabetova S., Nicholson C., Manley G. T. (2008) Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J. Neurosci. 28, 5460–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H., Verkman A. S. (2010) Microfiberoptic measurements of extracellular space volume in brain and tumor slices based on fluorescent dye partitioning. Biophys. J. 99, 1284–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Papadopoulos M. C., Verkman A. S. (2005) Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 280, 13906–13912 [DOI] [PubMed] [Google Scholar]
- 18. Bloch O., Auguste K. I., Manley G. T., Verkman A. S. (2006) Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J. Cereb. Blood Flow Metab. 26, 1527–1537 [DOI] [PubMed] [Google Scholar]
- 19. Auguste K. I., Jin S., Uchida K., Yan D., Manley G. T., Papadopoulos M. C., Verkman A. S. (2007) Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 21, 108–116 [DOI] [PubMed] [Google Scholar]
- 20. Saadoun S., Papadopoulos M. C., Watanabe H., Yan D., Manley G. T., Verkman A. S. (2005) Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell Sci. 118, 5691–5698 [DOI] [PubMed] [Google Scholar]
- 21. Binder D. K., Yao X., Zador Z., Sick T. J., Verkman A. S., Manley G. T. (2006) Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia 53, 631–636 [DOI] [PubMed] [Google Scholar]
- 22. Ma T., Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. (1997) Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Invest. 100, 957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller S. D., Karpus W. J., Davidson T. S. (2010). Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. Chap. 15, unit 15–11 [DOI] [PubMed] [Google Scholar]
- 24. Swanson R. A., Liu J., Miller J. W., Rothstein J. D., Farrell K., Stein B. A., Longuemare M. C. (1997) Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci. 17, 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bartholomaus I., Kawakami N., Odoardi F., Schlager C., Miljkovic D., Ellwart J. W., Klinkert W. E., Flugel-Koch C., Issekutz T. B., Wekerle H., Flugel A. (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 [DOI] [PubMed] [Google Scholar]
- 26. Tomas-Camardiel M., Venero J. L., de Pablos R. M., Rite I., Machado A., Cano J. (2004) In vivo expression of aquaporin-4 by reactive microglia. J. Neurochem. 91, 891–899 [DOI] [PubMed] [Google Scholar]
- 27. Martin Mdel P., Monson N. L. (2007) Potential role of humoral immunity in the pathogenesis of multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). Front. Biosci. 12, 2735–2749 [DOI] [PubMed] [Google Scholar]
- 28. Saadoun S., Bell B. A., Verkman A. S., Papadopoulos M. C. (2008) Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 131, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 29. Kimura A., Hsu M., Seldin M., Verkman A. S., Scharfman H. E., Binder D. K. (2010) Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann. Neurol. 67, 794–801 [DOI] [PubMed] [Google Scholar]
- 30. Solenov E., Watanabe H., Manley G. T., Verkman A. S. (2004) Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am. J. Physiol. Cell Physiol. 286, C426–C432 [DOI] [PubMed] [Google Scholar]
- 31. Cho S. J., Sattar A. K., Jeong E. H., Satchi M., Cho J. A., Dash S., Mayes M. S., Stromer M. H., Jena B. P. (2002) Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc. Natl. Acad. Sci. U. S. A. 99, 4720–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugiya H., Matsuki M. (2006) AQPs and control of vesicle volume in secretory cells. J. Membr. Biol. 210, 155–159 [DOI] [PubMed] [Google Scholar]
- 33. Padmawar P., Yao X., Bloch O., Manley G. T., Verkman A. S. (2005) K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat. Meth. 2, 825–827 [DOI] [PubMed] [Google Scholar]
- 34. Li J., Patil R. V., Verkman A. S. (2002) Mildly abnormal retinal function in transgenic mice without Muller cell aquaporin-4 water channels. Invest. Ophthalmol. Vis. Sci. 43, 573–579 [PubMed] [Google Scholar]
- 35. Li J., Verkman A. S. (2001) Impaired hearing in mice lacking aquaporin-4 water channels. J. Biol. Chem. 276, 31233–31237 [DOI] [PubMed] [Google Scholar]
- 36. Lu D. C., Zhang H., Zador Z., Verkman A. S. (2008) Impaired olfaction in mice lacking aquaporin-4 water channels. FASEB J. 22, 3216–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weinshenker B. G., Wingerchuk D. M., Pittock S. J., Lucchinetti C. F., Lennon V. A. (2006) NMO-IgG: a specific biomarker for neuromyelitis optica. Dis. Markers 22, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roemer S. F., Parisi J. E., Lennon V. A., Benarroch E. E., Lassmann H., Bruck W., Mandler R. N., Weinshenker B. G., Pittock S. J., Wingerchuk D. M., Lucchinetti C. F. (2007) Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130, 194–205 [DOI] [PubMed] [Google Scholar]
- 39. Misu T., Fujihara K., Kakita A., Konno H., Nakamura M., Watanabe S., Takahashi T., Nakashima I., Takahashi H., Itoyama Y. (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130, 1224–1234 [DOI] [PubMed] [Google Scholar]